Detection of occult tumor cells in regional lymph nodes is associated with poor survival in pN0 non-small cell lung cancer: a meta-analysis
Introduction
Lung cancer remains the leading cause of cancer deaths in the United States, accounting for about 27% of all estimated cancer death in 2014 (1). The current staging of lung cancer plays a critical role in the prognosis of this disease. Lymph node (LN) metastasis is the vital prognostic factor in regional lung cancer. However, the accurate identification of the status of LNs remains an elusive goal (2). Data are available indicating the modest 5-year survival (73%) reported in the earliest stage of non-small cell lung cancer (NSCLC) (denoted as stage IA by the International Association for the Study of Lung Cancer) (3). These documents suggest that operable patients might have occult tumor cells (OTCs) by the time of operation and a proportion of these patients are understaged by the routine hematoxylin-eosin staining staging procedure (4). At any rate, OTCs in regional LNs are identified more frequently than expected in those patients diagnosed as N0 by conventional methods.
At present, immunohistochemistry staining (IHC) and reverse transcription-polymerase chain reaction (RT-PCR) analysis for epithelial markers can be used to identify OTCs in NSCLC (2), serving as morphological and non-morphological methods respectively. Variable terminology and definitions have used to define such small metastatic foci, including micrometastasis, isolated tumor cell, disseminated tumor cell, minimal residual disease and OTC. We chose the term of “OTC” because it globally depicts metastases that are not diagnosed by standard hematoxylin-eosin pathologic methods. Hermanek and his colleagues (5) proposed that, by using IHC, micrometastases are clusters of tumor cells with greatest diameter between 0.2 and 2 mm, while isolated tumor cells are defined as single tumor cells or small clusters of cells measuring small than 0.2 mm in greatest diameter. In addition, RT-PCR is also utilized for the detection of OTCs from solid tumors and to quantify the expression of tumor markers and genes associated with tumors (6).
Up to now, studies specifically focusing on the prognostic importance of OTCs in pN0 lung cancer are limited. Some previous investigations have stated that the presence of OTCs in LNs are clinically relevant in NSCLC and confer worse prognosis (7-9). However, others documented that OTC has no negative impact on pN0 NSCLC patients with regards to overall survival (OS) or disease-free survival (DFS) (10). Hence, whether, or to what extent, the hypothesis of the relatively poor prognosis in pN0 NSCLC patients correlating with the existence of OTCs in regional LNs requires to be verified. This study aims to provide meta-estimates comparing pN0 NSCLC patients with OTCs to those without OTCs in regional LNs in terms of OS and DFS.
Materials and methods
Search strategy and selection criteria
We systematically searched PubMed and Chinese Biological Medicine databases up to Dec. 12th 2014 using the following key words: “micrometastasis” or “occult tumor cell” or “skip metastasis” or “microdissemination” or “isolate tumor cell” or “small tumor deposit”, “lung cancer” “NSCLC” and “lymph node”. We also searched the Cochrane library (http://www.cochrane.org) with the same terms within the same period. Furthermore, we reviewed all reference lists of relevant articles and review articles for additional studies that met our inclusion criteria. No language restrictions and time limits were applied to the initial search.
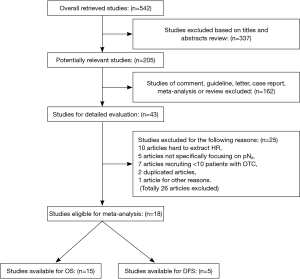
Two reviewers (Z. He and Y. Xia) independently screened all the titles and abstracts for eligibility and retrieved full articles for further assessments. We defined eligible studies as patients with complete clinical records, data on follow-up and pathologically conformed and completely removed primary pN0 NSCLC. We also required documented validation of molecular detection of OTC using any form of IHC or RT-PCR. Adequate documents of the compared groups (OTC group, representing patients of pN0 with OTC and control group, representing those without OTC) were also required to extract hazard ratios (HRs) and 95% confidence intervals (CIs). We excluded studies that totally enrolled less than 20 patients or pooled less than 10 in the OTC group. Furthermore, we also excluded studies that focused on small-cell lung cancer (SCLC) or mixed with SCLC. Additionally, studies that mainly documented OTC in distant sites of body like blood circulation or bone marrow were not eligible for this analysis. In case of multiple publications from the same author or institution with identical or overlapping study population recruitment more than 30%, the most informative and recent study was included. The details of selection flow were summarized in Figure 1.
In the assessment of risk of bias, the Newcastle-Ottawa Scale was utilized to evaluate all the cohort studies. This scale uses a maximum of nine stars to assess a study in three domains (section of participants, comparability of both groups, and the ascertainment of outcomes).
Data extraction
Two authors (Z. He and Y. Xia) independently extracted the following data from the pooled articles. Discrepancies were resolved by discussion with two senior authors (Y. Chen and L. Chen). A standardized form was developed to extract data from each enrolled studies, including author, publication year, study year, study type (prospective or retrospective), numbers of patients participating in each study, population ethnicity, numbers of patients with OTC, mean numbers of LNs dissected (MLND), incidence of positive LNs in all harvested ones, detection method (IHC or RT-PCT), definition of OTC using a certain assay and prognostic outcome (OS and/or DFS).
Statistical methods
Time to event data were synthesized using HRs and 95% CIs. In all HR calculations, the group without OTC was regarded as the reference. Primary outcomes included OS and/or DFS, which were used to analyze the prognosis of patients with OTC compared to those without OTC. As summary statistics were not directly given in any of the articles to allow direct calculation of the HRs and CIs, we extracted statistics from Kaplan-Meier curves and estimated HRs and 95% CIs according to the algorithm proposed by Tierney (11). When the P value was available, the HR and variance were estimated using P value (Mantel Hansel, log rank, or Cox regression) and events in each arm observed-expected.
When the P value was absent, the HR was calculated using survival curve, and the curve was divided into time periods as suggested by Parmar et al. (12). Less than 20% of total events were included in each time period, and time periods were different between each study depending on event rates. From the curve data, variance, observed minus expected (O-E) value, and HR were calculated. Minimum and maximum follow-up data, which were either identified directly from data reported or estimated from the survival curve using methods suggested by Tierney et al. (11), were collected to estimated HRs accurately.
For each meta-analysis result, I-squared statistic was performed to assess the heterogeneity among the included trials. When the heterogeneity was statistically significant (I-squared >50%), a random effect model was applied for analysis. Otherwise, a fixed effect model was used. In the subgroup analysis, heterogeneity between groups was absent when P value was more than 0.05.
Both the Begg’s and Egger’s bias indicators were used to test the effect of publication bias. A two-tailed P value less than 0.05 was considered statistically significant. All the statistical tests used in our meta-analysis were performed using STATA version 11.5 (Stata Corporation, College Station, TX, USA).
This meta-analysis was in consistence with the PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluated healthcare interventions (13).
Results
We pooled 542 potentially relevant articles from our research of the published literatures. Totally, 499 articles were excluded, resulting in 43 for detailed evaluations (Figure 1). Of the 43 articles, 10 presented insufficient information for extracting HRs, 5 not exclusively recruiting pN0 patients (14-18). Moreover, 7 studies enrolled less than 10 cases in the OTC group (19-25). Two duplicated articles (26,27) with the same authors were less informative than 2 enrolled articles (7,28). Additionally, 1 article presenting an obscure definition in the OTC identification was excluded (29), of which, microarray analysis software was used to identify a variety of gene patterns that specifically correlated with LNs metastases and eventually the top 50 most significant genes were used to distinguish the OTC-positive and OTC-negative in LNs. Finally, 18 articles were eligible for meta-analysis and only 2 studies (7,8) documented sufficient data for extracting HRs for both OS and DFS, 13 and 3 studies merely for OS and DFS analysis, respectively.
The 18 enrolled articles used as data sources for the present meta-analysis included 1,951 patients: 481 in the OTC group (24.65%). Study size ranged from 31 to 580 patients. The incidence of OTC in the LNs that were negative by routine histopathology reached as high as 29.1% (9). However, another study presented the lowest incidence of OTC of 1.7% from the same population ethnicity (Japanese) (30). Fourteen pooled studies were based on the retrospective data except for 4 prospective ones. While IHC method was used in 14 studies with RT-PCT technique in 4 studies. In addition, 13 studies provided sufficient data for analyzing HRs of OS in the subgroup analyses (LNs/Pts. <12 and ≥12). The detailed characteristics of the included clinical trials were summarized in the Table 1.
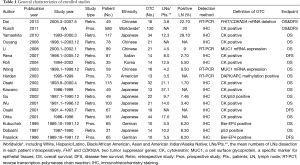
Full table
Impact of OTC on OS/DFS of enrolled pN0 NSCLC patients
There were totally 15 studies involved in the analysis of the OS with 5 studies available in DFS. The combined HR of OS was 2.22 (95% CI, 1.87 to 2.64), while the combined HR of DFS was 2.4 (95% CI, 1.71 to 3.36) (Figure 2). These data indicated that the prognosis of patients with OTC was inferior to those without OTC with regard to OS and DFS.
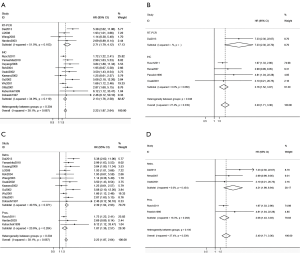
The statistics for heterogeneity showed results of I-squared were 38.1% and 27.4% in OS and DFS analysis accordingly (Figure 2). Thus the fixed-effects model was use for HR of OS and DFS.
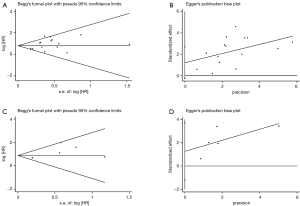
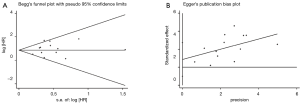
There was no publication bias detected using both the Begg’s and Egger’s bias indicators at both sections of analyses (P=0.100 for OS including 15 articles, and P=0.199 for DFS including 5 articles, respectively, seeing Figure 3).
Impact of OTC on OS/DFS of pN0 NSCLC patients using IHC or RT-PCR technique
With the intention of reducing the heterogeneity among studies, we initially divided them into two groups according to the different methods in the detection of OTC: IHC and RT-PCR analysis. In 11 studies for OS analysis using IHC method, the combined HR was 2.13 (95% CI, 1.76 to 2.58) and these combined studies were homogeneous (I-squared =34.9%). While in 4 studies for OS using RT-PCT technique, the combined HR for OS was 2.71 (95% CI, 1.79 to 4.12) with moderate heterogeneity (I-squared =51.5%). Notably, Between-group heterogeneity was lower (P=0.304) (Figure 2A). Likewise, of 5 studies available for DFS analysis, the same trend was obtained in both IHC and RT-PCR subgroups, but presenting significant heterogeneity between subgroups (P=0.048) (Figure 2B).
Impact of OTC on OS/DFS of pN0 NSCLC patients based on different study type
Of all the 15 studies available for OS analysis, 12 retrospective studies presenting the HR of 2.39 (95% CI, 1.94 to 2.93) agreed with 3 prospective ones with HR of 1.87 (95% CI, 1.36 to 2.57). Both of these combined studies were of homogeneity (I-squared =40.5% and 20.6%, respectively) (Figure 2C). Five studies provided valuable data for DFS analysis illustrating the same trend in 3 retrospective and 2 prospective studies. Detailed information was available in Figure 2D.
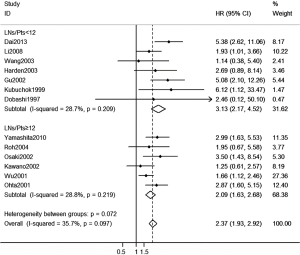
Impact of OTC on OS of pN0 NSCLC patients undergoing different MLND (LNs/Pts. <12 or ≥12)
Totally, 13 studies documented sufficient information for HR of OS analysis regarding MLND (Figure 4). Patients with OTC were more than three times likely to have the probability to death comparing with those without OTC in LNs/Pts. <12 subgroup (HR: 3.13, 95% CI, 2.17 to 4.52). Interestingly, the general trend for lower HR favored LNs/Pts. ≥12 subgroup (HR: 2.09, 95% CI, 1.63 to 2.68), but this was not significantly different between subgroups (P=0.072). Neither of the subgroups illustrated heterogeneity in each pooled estimates (I-squared: 28.7% and 28.8%, respectively).
In this section involving 13 studies, significant difference was absent in the publication bias analysis (P=0.330) (Figure 5).
Discussion
We review 18 cohort studies, most of which showed the evidence that pN0 patients with OTCs in regional LNs, even receiving complete tumor resection, had an associated higher risk of recurrence or death compared with those without OTCs (7-9,28,30-40). This seemingly undisputed conclusion was challenged by several documents which indicated that the presence of OTCs had no significant effect on the OS or DFS (10,41,42). Hence, we could not arbitrarily reach the extent of the impact of OTCs in regional LNs on survival of pN0 NSCLC prior to the meta-analysis.
In the meta-analysis, the prognosis of patients with OTCs was inferior to those without OTCs in the terms of OS and DFS regardless of detection methods, study types and MLND per patients intraoperatively. This emphasizes the importance of the status of regional LNs, which has a primary impact on the management of NSCLC patients (43). The detection of LN occult micrometastatic tumors cells provides a precise assessment of tumor staging and has powerful clinical implications for completely resected pN0 NSCLC patients. Currently, postoperative adjuvant chemotherapy is a not accepted as a routine standard therapy for those patients because of its uncertain results for the improvement of patients’ prognosis. Additionally, nodal micrometastasis does not only reflect lymphogenous spread but also it may be a signal of early phase of hematogenous systemic tumor cells dissemination (7). Finally, the incidence OTC as high as 29.1% in LNs highlights that many of these cancers could not be curable by operation alone. This identification of subgroups of patients with different outcomes could help physicians tailor accurate postoperative management strategies and stratify patients who would probably benefit most from aggressive chemotherapy (44).
One previously published systematic review has estimated the relationship between the presence of OTCs and the prognosis of the early stage lung cancer based on eight included studies but mixed with IHC and RT-PCT detection methods (45). Concerns have been addressed that RT-PCT may be overly sensitive and may include false-positive cases and consequently magnify the role of OTCs in the prognosis of NSCLC (2). Thus we imperatively conducted the meta-analysis to stratify the potentially distinctive roles of RT-PCR and IHC in OTCs detection. In the subgroup analysis, HR in the RT-PCR subgroup was comparable to that in IHC in term of OS. However, the statistically significant difference of HRs for DFS was obtained in both groups. These probably reflected diversity in case selection, heterogeneity in histopathology, or the fact that studies have been small and retrospective.
In view of surgical procedure for these patients potentially bearing OTCs in regional LNs, the ACOSOG Z0030 study group recommends that the mean numbers of LNs resected during lobectomy be ≥12 (46). Likewise, removal of 11 to 15 LNs confers better prognostic outcomes in the early stage NSCLC patients undergoing lobectomy (47). In the current meta-analysis, MLND varied from 3.5 to 37.1 based on different cohorts of patients. To clarify the potential heterogeneity in LNs dissection in the meta-analysis, we conducted a subgroup analysis to assess HRs of OS according to MLND (LNs/Pts. <12, or ≥12). The trend of worse prognostic outcome was presented in the LNs/Pts. <12 subgroup compared to that in the LNs/Pts. ≥12, despite of lacking statistically significant difference. Furthermore, both of the subgroup analyses showed a robust evidence for the prognostic value of OTCs in the early stage operable NSCLC.
It is universally accepted that retrospective studies are prone to bias as they particularly rely on data collected for another purpose (48), while the prospective cohort studies are relatively reliable and robust. However, in this study, patients with OTCs in LNs deemed higher risk of death or recurrence than those without OTCs regardless of the study design.
Despite of the conclusion we have obtained above, our research also had several limitations. Firstly, the selection of the controls varied between studies. It excluded the studies that totally enrolled less than 20 patients or less than 10 in the OTC group and those lacking sufficient survival data (e.g., HR, CI or survival curve). Secondly, there was statistical heterogeneity, probably originating from the differences in the characteristics of patients, normalization controls, technical platforms, the cut-off values or any other technical issues. Thirdly, the present meta-analysis was limited to the articles published up to December 2014, indicating the possibility that some relevant unpublished studies, which may have met the inclusion criteria, were missed. Finally, the available data included in this meta-analysis were primarily originated from certain ethnicities like Japanese, Chinese and German. Thus, a study of large cohort, multicenter and long follow-up may be required to clarify the prognostic value of OTCs in LNs in resected pN0 NSCLC patients.
In conclusion, the correlation of OTCS in LNs and survival of pN0 NSCLC has direct clinical implications. The controversy of the extent of LNs dissection in the procedures of pN0 stage NSCLC has be clearer. Furthermore, improved detection techniques could be expected and finally a subgroup of patients who will potentially most benefit from adjuvant therapy might be identified.
Acknowledgements
This research was partly supported by Dr. Meilin Wang for language revision.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Tan W, et al. Lung cancer lymph node micrometastasis detection using real-time polymerase chain reaction: correlation with vascular endothelial growth factor expression. J Thorac Cardiovasc Surg 2013;145:702-7; discussion 707-8. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Gilmore DM, Khullar OV, Colson YL. Developing intrathoracic sentinel lymph node mapping with near-infrared fluorescent imaging in non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:S80-4. [Crossref] [PubMed]
- Hermanek P, Hutter RV, Sobin LH, et al. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer 1999;86:2668-73. [Crossref] [PubMed]
- Zhang X, Xie J, Yu C, et al. mRNA expression of CK19, EGFR and LUNX in patients with lung cancer micrometastasis. Exp Ther Med 2014;7:360-4. [PubMed]
- Dai CH, Li J, Yu LC, et al. Molecular diagnosis and prognostic significance of lymph node micrometastasis in patients with histologically node-negative non-small cell lung cancer. Tumour Biol 2013;34:1245-53. [Crossref] [PubMed]
- Rusch VW, Hawes D, Decker PA, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol 2011;29:4313-9. [Crossref] [PubMed]
- Yamashita T, Uramoto H, Onitsuka T, et al. Association between lymphangiogenesis-/micrometastasis- and adhesion-related molecules in resected stage I NSCLC. Lung Cancer 2010;70:320-8. [Crossref] [PubMed]
- Kawano R, Hata E, Ikeda S, et al. Micrometastasis to lymph nodes in stage I left lung cancer patients. Ann Thorac Surg 2002;73:1558-62. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Ge MJ, Wu QC, Wang M, et al. Detection of disseminated lung cancer cells in regional lymph nodes by assay of CK19 reverse transcriptase polymerase chain reaction and its clinical significance. J Cancer Res Clin Oncol 2005;131:662-8. [Crossref] [PubMed]
- Marchevsky AM, Qiao JH, Krajisnik S, et al. The prognostic significance of intranodal isolated tumor cells and micrometastases in patients with non-small cell carcinoma of the lung. J Thorac Cardiovasc Surg 2003;126:551-7. [Crossref] [PubMed]
- Ono T, Minamiya Y, Ito M, et al. Sentinel node mapping and micrometastasis in patients with clinical stage IA non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2009;9:659-61. [Crossref] [PubMed]
- Passlick B, Pantel K, Kubuschok B, et al. Expression of MHC molecules and ICAM-1 on non-small cell lung carcinomas: association with early lymphatic spread of tumour cells. Eur J Cancer 1996;32A:141-5. [Crossref] [PubMed]
- Maruyama R, Sugio K, Fukuyama Y, et al. Evaluation of p53 alterations in occult lymph node metastases. J Surg Oncol 2000;73:143-7. [Crossref] [PubMed]
- Ayabe T, Tomita M, Matsuzaki Y, et al. Micrometastasis and expression of nm23 messenger RNA of lymph nodes from lung cancer and the postoperative clinical outcome. Ann Thorac Cardiovasc Surg 2004;10:152-9. [PubMed]
- Franco MR, Parra ER, Takagaki TY, et al. Detection of micrometastases in pN0 non-small cell lung cancer: an alternative method combining tissue microarray and immunohistochemistry. J Bras Pneumol 2008;34:129-35. [Crossref] [PubMed]
- Tezel C, Ersev AA, Kiral H, et al. The impact of immunohistochemical detection of positive lymph nodes in early stage lung cancer. Thorac Cardiovasc Surg 2006;54:124-8. [Crossref] [PubMed]
- Ahrendt SA, Yang SC, Wu L, et al. Molecular assessment of lymph nodes in patients with resected stage I non-small cell lung cancer: preliminary results of a prospective study. J Thorac Cardiovasc Surg 2002;123:466-73; discussion 473-4. [Crossref] [PubMed]
- Goldstein NS, Mani A, Chmielewski G, et al. Immunohistochemically detected micrometastases in peribronchial and mediastinal lymph nodes from patients with T1, N0, M0 pulmonary adenocarcinomas. Am J Surg Pathol 2000;24:274-9. [Crossref] [PubMed]
- Hashimoto T, Kobayashi Y, Ishikawa Y, et al. Prognostic value of genetically diagnosed lymph node micrometastasis in non-small cell lung carcinoma cases. Cancer Res 2000;60:6472-8. [PubMed]
- Benlloch S, Galbis-Caravajal JM, Alenda C, et al. Expression of molecular markers in mediastinal nodes from resected stage I non-small-cell lung cancer (NSCLC): prognostic impact and potential role as markers of occult micrometastases. Ann Oncol 2009;20:91-7. [Crossref] [PubMed]
- Izbicki JR, Passlick B, Hosch SB, et al. Mode of spread in the early phase of lymphatic metastasis in non-small-cell lung cancer: significance of nodal micrometastasis. J Thorac Cardiovasc Surg 1996;112:623-30. [Crossref] [PubMed]
- Li J, Li ZN, Yu LC, et al. Gene diagnosis of micrometastases in regional lymph nodes of patients with stage I non-small cell lung cancer: impact on staging and prognosis. Clin Transl Oncol 2013;15:882-8. [Crossref] [PubMed]
- Passlick B, Izbicki JR, Kubuschok B, et al. Detection of disseminated lung cancer cells in lymph nodes: impact on staging and prognosis. Ann Thorac Surg 1996;61:177-82; discussion 183. [Crossref] [PubMed]
- Xi L, Lyons-Weiler J, Coello MC, et al. Prediction of lymph node metastasis by analysis of gene expression profiles in primary lung adenocarcinomas. Clin Cancer Res 2005;11:4128-35. [Crossref] [PubMed]
- Osaki T, Oyama T, Gu CD, et al. Prognostic impact of micrometastatic tumor cells in the lymph nodes and bone marrow of patients with completely resected stage I non-small-cell lung cancer. J Clin Oncol 2002;20:2930-6. [Crossref] [PubMed]
- Ouyang WW, Lu B, Fu HY, et al. Detection of regional lymph node micrometastasis and its impact on long-term survival of non-small cell lung cancer (NSCLC) patients. Ai Zheng 2008;27:756-60. [PubMed]
- Li SH, Wang Z, Liu XY, et al. Gene diagnosis and prognostic significance of lymph node micrometastasis after complete resection of histologically node-negative non-small cell lung cancer. World J Surg 2008;32:1651-6. [Crossref] [PubMed]
- Roh MS, Lee JI, Choi PJ, et al. Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology 2004;45:580-6. [Crossref] [PubMed]
- Wang Z, Liu XY, Liu FY, et al. Lymph node occult micrometastasis in patients with non-small cell lung carcinoma: genetic diagnosis and its impact on prognosis. Ai Zheng 2003;22:1204-8. [PubMed]
- Gu CD, Osaki T, Oyama T, et al. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: impact on recurrence and Survival. Ann Surg 2002;235:133-9. [Crossref] [PubMed]
- Wu J, Ohta Y, Minato H, et al. Nodal occult metastasis in patients with peripheral lung adenocarcinoma of 2.0 cm or less in diameter. Ann Thorac Surg 2001;71:1772-7; discussion 1777-8.
- Osaki T, Oyama T, Inoue M, et al. Molecular biological markers and micrometastasis in resected non-small-cell lung cancer. Prognostic implications. Jpn J Thorac Cardiovasc Surg 2001;49:545-51. [Crossref] [PubMed]
- Ohta Y, Oda M, Wu J, et al. Can tumor size be a guide for limited surgical intervention in patients with peripheral non-small cell lung cancer? Assessment from the point of view of nodal micrometastasis. J Thorac Cardiovasc Surg 2001;122:900-6. [Crossref] [PubMed]
- Kubuschok B, Passlick B, Izbicki JR, et al. Disseminated tumor cells in lymph nodes as a determinant for survival in surgically resected non-small-cell lung cancer. J Clin Oncol 1999;17:19-24. [PubMed]
- Dobashi K, Sugio K, Osaki T, et al. Micrometastatic P53-positive cells in the lymph nodes of non-small-cell lung cancer: prognostic significance. J Thorac Cardiovasc Surg 1997;114:339-46. [Crossref] [PubMed]
- Rena O, Carsana L, Cristina S, et al. Lymph node isolated tumor cells and micrometastases in pathological stage I non-small cell lung cancer: prognostic significance. Eur J Cardiothorac Surg 2007;32:863-7. [Crossref] [PubMed]
- Harden SV, Tokumaru Y, Westra WH, et al. Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clin Cancer Res 2003;9:1370-5. [PubMed]
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5. [Crossref] [PubMed]
- Li ZM, Ding ZP, Luo QQ, et al. Prognostic significance of the extent of lymph node involvement in stage II-N1 non-small cell lung cancer. Chest 2013;144:1253-60. [Crossref] [PubMed]
- Zheng Z, Pan TC, Li J, et al. Meta-analysis of relationship between lymph node micrometastasis and prognosis in stage I non-small cell lung cancer patients. Ai Zheng 2004;23:185-8. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. [Crossref] [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [Crossref] [PubMed]
- Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies--report of the ISPOR Task Force on Retrospective Databases. Value Health 2003;6:90-7. [Crossref] [PubMed]


