Moderate hypothermic circulatory arrest in total arch repair for acute type A aortic dissection: clinical safety and efficacy
Introduction
During the past decades, deep hypothermic circulatory arrest (HCA) has been widely used and is considered by many to be an effective modality in surgical repair of acute type A aortic dissection (TAAD) (1-4). Although the use of HCA in adjunction with selective antegrade cerebral perfusion (SACP) has improved the surgical results (5-8), these complex procedures are still associated with significantly high operative mortality and morbidity and there has been no consensus on the optimal temperature during HCA.
Due to the adverse effects of deep HCA (DHCA), a recent development in TAAD repair has been a departure from the use of deep hypothermia and excellent results have been achieved with the use of warmer temperatures in aortic arch repair (5-11). This has raised the question of whether DHCA affords any additional benefits to justify its adverse effects (12).
In the past decade, the strategy of temperature management in our center has evolved from the use of deep HCA to moderate HCA (MHCA) for repair of acute TAAD. In this study we seek to examine whether MHCA provides equivalent early outcomes as DHCA in patients with acute TAAD undergoing total arch replacement and frozen elephant trunk implantation under unilateral SACP (uSACP).
Methods
Patients
The present study is part of a prospective clinical trial. The Institutional Review Board of Beijing Anzhen Hospital, Capital Medical University approved the study protocol (File # 2014019). Written consent was obtained from the patients and their relatives before the operation.
Between August 2014 and July 2015, we enrolled 74 patients who underwent emergency aortic arch repair for acute TAAD. The median time from symptom onset to surgery was 39 hours (mean 59.0±59.5, range 1–270). Mean age was 47.7±9.8 years and 54 were male (73.0%). Mean body mass index was 26.2±3.6 kg/m2. Chest pain was present in 69 patients (93.2%). Based on the nasopharyngeal temperature at the initiation of HCA, patients were divided into groups with lower and higher temperatures, defined as the DHCA and MHCA groups (13): (I) the DHCA group: <20 °C (n=35, 47.3%); (II) the MHCA group: 20–28 °C (n=39, 52.7%).
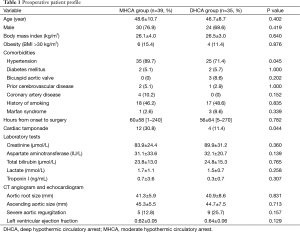
As shown in Table 1, the two groups were similar in age, body mass index and other preoperative variables (P>0.05), except the MHCA group had a higher percentage of hypertension (89.7% vs. 71.4%, P=0.045) and cardiac tamponade (30.8% vs. 11.4%, P=0.044). Preoperative visceral functions as indicated by the concentrations of creatinine (kidney), aspartate aminotransferase and total bilirubin (liver) and lactate (bowels) (14) did not differ significantly between the two groups (P>0.05; Table 1).
Full table
Exclusion criteria included patients with subacute and chronic TAAD, intramural hematoma, penetrating aortic ulcer and traumatic aortic transection and death prior to surgical repair.
Surgical techniques
Our surgical technique has been previously described in detail (15-17). Specifically, right axillary artery cannulation is used for cardiopulmonary bypass (CPB) and unilateral SACP. Upon reaching the goal nasopharyngeal temperature of 18–28 °C, uSACP is initiated at a flow rate of 5–10 mL/kg/min and a mean perfusion pressure of 40–60 mmHg is maintained during CPB. The temperature for circulatory arrest and the temperature for SACP were equal. The procedure involves deployment of an open stent graft, Cronus® (MicroPort, Shanghai, China) into the descending aorta and total arch replacement with a 4-branched vascular graft (Maquet Cardiovascular, Wayne, NJ). The open stent graft was 10 cm in length and 24–28 mm in diameter. The distal landing zone is above T6 in most cases (16). Deployment simply involves gripping the handle in one hand and pulling out the pull wire with the other hand; the stent graft expands automatically, usually within seconds (15). To minimize the time of ischemia, distal reperfusion is initiated once the distal anastomosis is completed; the left carotid artery is reconstructed first, after which the brain is perfused bilaterally and rewarming is started.
Definition of endpoints
In this study, operative mortality refers to all deaths, regardless of cause, occurring during the same hospitalization in which the operation was performed, even if after 30 days and after discharge from the hospital but within 30 postoperative days (18). Operative morbidities include reexploration for bleeding, low cardiac output syndrome, renal failure requiring dialysis, stroke and paralysis.
Statistical analysis
All statistical analyses were performed with SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation (SD) or number and percentages as appropriate. Student’s t-test or Wilcoxon rank sum test and chi-square or Fisher’s exact tests were used for comparisons as appropriate. Risk factors for operative mortality and morbidities were identified with multivariate analysis using a forward stepwise binary logistic regression model. The differences in the variations over time of postoperative serum levels of aspartate aminotransferase, total bilirubin, creatinine and lactate were evaluated using a mixed effect analysis of variance model. All statistical tests were 2-sided and any P value of <0.05 was considered statistically significant.
Results
Operative data
At the start of circulatory arrest, the mean nasopharyngeal temperature was 21.7±2.5 °C for the entire series. In the DHCA group, HCA started at a significantly lower temperature than in the MHCA group (19.2±0.7 vs. 24.0±1.0 °C; P<0.01). The time of CPB was shorter in the MHCA group than in the DHCA group, which almost reached statistical significance (211±54 vs. 238±62 minutes, P=0.053). The time of aortic cross-clamp was significantly shorter in the MHCA group than the DHCA group (118±27 vs. 142±45 minutes, P=0.005). The two groups had similar times of HCA (28±8 vs. 29±9 minutes, P=0.465) and operation (8.5±1.9 vs. 9.2±2.0 hours, P=0.108), respectively (Table 2). Of note, the HCA time was 30 minutes or less in 68.9% of patients (51/74) and >50 minutes in only 1 patient (1.3%) of the entire cohort.

Full table
Composite graft root replacement was required in 41.0% (16/39) vs. 40.0% (14/35) of patients in the MHCA vs. DHCA groups, respectively (P=0.929). Concomitant coronary artery bypass grafting was performed in three patients of the MHCA group (7.7%).
Operative mortality and morbidity
Operative mortality was 12.2% (9/74), including 4 deaths in the MHCA (10.2%) and 5 in the DHCA groups (14.3%), respectively. However, mortality rate did not differ significantly between the two groups (P=0.862). The leading cause of death was multiorgan failure, identified in 5 (6.7%, 5/74) of the 9 expired patients (55.5%), with 2 in the DHCA group and 3 in the MHCA group, respectively. Other causes included cerebral hemorrhage in 2 patients (5.7%, 2/35) of the DHCA group, cerebral infarct in 1 (2.6%, 1/39) and sepsis in 1 (2.6%, 1/39) of the MHCA group, respectively.
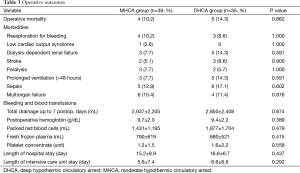
The total amount of postoperative drainage up to 7 postoperative days did not differ between the two groups (2,937 vs. 2,850 mL, P=0.874). Nor were there any difference in the transfusion of blood products (P>0.05) and the incidence of reexploration for bleeding (10.2% in MHCA vs. 8.6% in DHCA, P=1.000) between the two groups. No statistical difference was found in the incidence of renal failure requiring dialysis (7.7% in MHCA vs. 14.3% in DHCA, P=0.591). A similar percentage of patients sustained stroke (5.1% vs. 8.6%, P=0.900) and paralysis (7.7% vs. 5.7%, P=1.000) in each group, respectively. There were no statistical differences between the MHCA and DHCA group in the incidences of low cardiac output, prolonged ventilation, sepsis and multiorgan failure (P>0.05; Table 3).
Full table
Postoperative vital organ functions
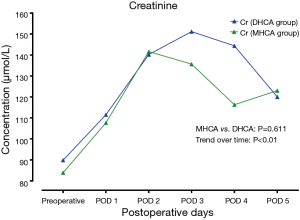
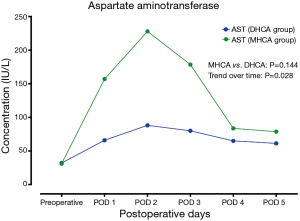
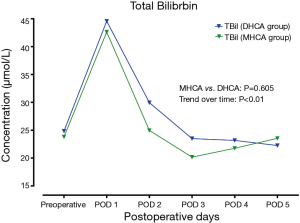
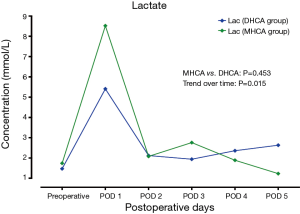
In the MHCA and DHCA groups, a significant temporal effect with increases in the concentrations of creatinine, aspartate aminotransferase, total bilirubin and lactate was observed postoperatively (P<0.05). Creatinine reached its peak concentration (P=0.015) at postoperative day 2 in the MHCA group and at day 3 in the DHCA group. In both groups, the peak level was at postoperative day 2 for aspartate aminotransferase (P=0.028), and at day 1 for total bilirubin (P<0.01) and lactate at (P<0.01), respectively. However, mixed effect analysis of variance modeling revealed that these temporal trends did not differ between the MHCA and DHCA groups in the levels of creatinine (P=0.611), aspartate aminotransferase (P=0.144), total bilirubin (P=0.605) and lactate (P=0.453) as shown in Figures 1-4.
Risk factors for operative mortality and morbidities
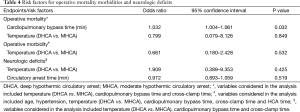
In multivariable analysis, the temperature during HCA (MHCA vs. DHCA) did not affect the operative mortality [odds ratio (OR), 0.799; 95% confidence interval (CI), 0.079–8.126; P=0.849], morbidities (OR, 1.909; 95% CI, 0.389–9.353; P=0.425) and neurologic complications (OR, 0.661; 95% CI, 0.180–2.428; P=0.532). The cardiopulmonary time (in minutes) was the only risk factor for operative mortality (OR, 1.032; 95% CI, 1.004–1.061; P=0.023). No risk factors were identified for operative morbidities and neurologic events (Table 4).
Full table
Discussion
Acute TAAD is a catastrophic event with very high risk of mortality and morbidity (19,20). Although DHCA with or without adjunctive cerebral perfusion has been an important tool for cerebral protection in aortic arch surgery and its safety was proven over the past two decades, continued debates exist regarding the use of DHCA (1-3). Some studies have shown the adverse effects of DHCA, such as prolonged CPB time which leads to increased systemic inflammatory response and subsequent coagulation disorders, postoperative bleeding and higher need for blood transfusion, and organ dysfunction (6,10,11). As a result, the past decade has seen a departure from traditional DHCA techniques to warmer temperatures in HCA. Moreover, several studies (21-24) have compared the techniques of DHCA to MHCA and some have shown that aortic arch surgery could be performed safely under MHCA rather than DHCA (6-8,25). However, it is difficult to compare DHCA and MHCA directly because most centers have achieved good outcomes with a single strategy. Although Chen and associates compared the early outcomes of MHCA vs. DHCA in a large series of 288 patients with acute TAADs, total arch repair was performed only in 9 (11.0%) vs. 23 (11.2%) patients of the DHCA vs. MHCA groups, while hemiarch repair was the procedure in 88.9% (256/288) of their patients (26). In contrast, all patients in this study were managed with total arch replacement, which provides an opportunity to compare directly the impact of moderate and deep HCA on operative outcomes while all other confounding factors (patients, cannulation, cerebral perfusion and surgical technique) are similar.
Although some studies (21-24) have suggested that MHCA may not provide adequate protection for the neurologic system and visceral organs, especially with prolonged duration of circulatory arrest, some high-volume centers have achieved excellent results with the use of warmer temperatures in aortic arch repair (4-10). Experimental animal models (27) also showed that further cooling did not decrease brain oxygen consumption efficiently. Over the past decade, our protocol of HCA has evolved considerably as we accrued more experience, and now we routinely perform arch surgery at 25–28 °C, a temperature that is higher than that used by other institutions (1-3). The results of this study show that the use of MHCA in total arch replacement and frozen elephant trunk under unilateral cerebral perfusion was not associated with increased risks of operative mortality and morbidities, neurological complications and impaired visceral functions (the liver, kidney and bowels), which implies that that aortic arch surgery can be safely performed under MHCA and uSACP in the emergency settings for patients with acute TAAD.
Neurologic complications after arch surgery are believed to be mainly associated with the use of DHCA (23,24,28). SCAP has increasingly been adopted to minimize the incidence of neurologic complications (29,30). In this study, the incidence of neurologic complications did not differ between the DHCA and MHCA groups, nor was the temperature in HCA identified as a risk factor for neurologic complications. Our results suggest that MHCA could provide equivalent cerebral protection as DHCA in emergency aortic arch surgery for patients with acute TAAD. In this study, the time of HCA was not a risk factor for neurologic complications either, which is inconsistent with other reports in which HCA time was identified as a risk factor for stroke or temporary neurologic dysfunction (14,31). A possible explanation for this difference may be that the HCA time was >30 minutes in only 31.1% (23/74) and >50 minutes in only 1.3% (1/74) of patients in this series. Mounting experimental and clinical evidence (28,29) have suggested that the safety limit of HCA might approximate 30–40 minutes. When the HCA time exceeds 45 minutes, the incidence of neurologic deficit will increase drastically (32). Based on the results of this study we speculate that a HCA time of ≤30 minutes may not affect the incidence of neurologic deficits considerably.
Although it is believed that HCA, especially DHCA, can induce coagulopathy (33), results in literature are conflicting. Zierer and colleagues (34) found a marked reduction in the mean chest tube drainage, CPB time and intensive care unit stay in patients undergoing MHCA compared to those with DHCA. However, Harrington and associates (22) reported that DHCA was not associated with increased postoperative hemorrhage. This study found no significant difference in the postoperative drainage and the transfusion requirements between the two groups, which indicates that both MHCA and DHCA may have similar impacts on the blood elements and the coagulation process in this cohort of patients with acute TAAD.
A major concern of using more moderate levels of hypothermia is a compromise in the suppression of visceral organ metabolism as opposed to DHCA (12). Clinically, the kidneys are most sensitive to ischemia among the viscera, followed by the liver and the bowel (5,8). The most frequent indicator of visceral organ injury identified in aortic surgery is acute renal failure. In this study, there was no significant difference in the incidence of dialysis-dependent renal failure and the temporal trend in the changes of creatinine levels between the two groups, nor was any statistical difference identified in the temporal trend of the hepatic and intestinal functions following emergency total arch repair. Given that both groups had similar visceral ischemia (HCA) times, it does imply that moderate hypothermia in the current study provided equivalent visceral protection compared with deep hypothermia.
Although the CPB time did not differ between the MHCA and DHCA groups, CPB time was identified as the only risk factor that significantly affects operative mortality, which is consistent with the finding of our previous study (17) and that of others (35). DHCA is associated with longer times of CPB, aortic cross-clamp and the procedure because it entails longer periods for cooling and rewarming, and longer time for hemostasis, with a potential increase in the risk of ischemic reperfusion injury (21-23). Taken the average CPB times in both groups, the risk of operative mortality in the DHCA group (238 minutes) would be 2.3-fold higher than in the MHCA group (211 minutes). Given the similar outcomes in terms of operative mortality and morbidity, and neurologic and visceral protections in the MHCA and DHCA groups, an important implication is that MHCA may be a better approach in emergency aortic arch repair for patients with acute TAAD by avoidance of posing such patients to the longer CPB times and potential adverse risks of DHCA (12), including multiorgan endothelial dysfunction (23) .
Study limitations
This study has several imitations although it is part of a prospective clinical trial. Most important is its relatively small sample size compared with other large series, which affects the power of statistical analysis, especially in the identification of risk factors. It is also limited by the absence of sensitive and specific indicators of visceral organ injury. The variables chosen in this study measuring visceral organ functions are unspecific and influenced by a lot of other factors. Another concern pertains to the young average age of patients in this study (47 years) although senior age is not a contraindication for aortic arch reconstruction in our center. Therefore, data in this study may not be applicable to older patients who might be more prone to neurologic ischemic events. Furthermore, as no consensus exists on the levels of temperature for HCA (12), both groups in this study would be considered as DHCA by the Johns Hopkins definition and therefore caution should be taken when interpreting the results of this study. In addition, this study represents a single-center experience susceptible to inherent selection biases. Despite these limitations, the homogeneous nature of this study with respect to patients, cerebral perfusion method and surgical procedure does allow for a more intuitive comparison of the impacts of MHCA vs. DHCA.
Conclusions
The results of this study show that the temperature in HCA did not affect operative mortality in aortic arch surgery for patients with acute type A dissection. This study implies the clinical safety and efficacy of moderate HCA in emergency aortic arch repair for such patients, which can provide comparable cerebral and visceral organ protection while decreasing CPB and aortic cross-clamp times without increasing the risk of operative mortality and morbidity.
Acknowledgements
We acknowledge the help from Prof. Jing Liu (Beijing Institute of Heart Lung and Blood Vessel Diseases and Beijing Anzhen Hospital, Capital Medical University, China) with statistical assistance. We also thank Ji Che (Beijing Institute of Heart Lung and Blood Vessel Diseases and Beijing Anzhen Hospital, Capital Medical University, China) for editorial assistance.
Funding: This study was supported by a grant from Beijing Municipal Science & Technology Commission (No.Z141100002114025).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hammon JW, Shore-Lesserson L, Dickinson TA. Temperature Management Guidelines. Ann Thorac Surg 2015;100:385. [Crossref] [PubMed]
- Englum BR, Andersen ND, Husain AM, et al. Degree of hypothermia in aortic arch surgery - optimal temperature for cerebral and spinal protection: deep hypothermia remains the gold standard in the absence of randomized data. Ann Cardiothorac Surg 2013;2:184-93. [PubMed]
- Di Luozzo G, Griepp RB. Cerebral protection for aortic arch surgery: deep hypothermia. Semin Thorac Cardiovasc Surg 2012;24:127-30. [Crossref] [PubMed]
- Ziganshin BA. Which method of cerebral protection do you prefer to use for aortic arch surgery? Aorta (Stamford) 2013;1:69-70. [Crossref] [PubMed]
- Leshnower BG, Myung RJ, Kilgo PD, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg 2010;90:547-54. [Crossref] [PubMed]
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9. [Crossref] [PubMed]
- Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg 2008;135:908-14. [Crossref] [PubMed]
- Zierer A, Detho F, Dzemali O, et al. Antegrade cerebral perfusion with mild hypothermia for aortic arch replacement: single-center experience in 245 consecutive patients. Ann Thorac Surg 2011;91:1868-73. [Crossref] [PubMed]
- Halkos ME, Kerendi F, Myung R, et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg 2009;138:1081-9. [Crossref] [PubMed]
- Leshnower BG, Myung RJ, Thourani VH, et al. Hemiarch replacement at 28°C: an analysis of mild and moderate hypothermia in 500 patients. Ann Thorac Surg 2012;93:1910-5; discussion 1915-6.
- Urbanski PP, Lenos A, Bougioukakis P, et al. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg 2012;41:185-91. [PubMed]
- Luehr M, Bachet J, Mohr FW, et al. Modern temperature management in aortic arch surgery: the dilemma of moderate hypothermia. Eur J Cardiothorac Surg 2014;45:27-39. [Crossref] [PubMed]
- Yan TD, Bannon PG, Bavaria J, et al. Consensus on hypothermia in aortic arch surgery. Ann Cardiothorac Surg 2013;2:163-8. [PubMed]
- Khaladj N, Peterss S, Pichlmaier M, et al. The impact of deep and moderate body temperatures on end-organ function during hypothermic circulatory arrest. Eur J Cardiothorac Surg 2011;40:1492-9; discussion 1499. [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- Ma WG, Zhang W, Wang LF, et al. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Ma WG, Zheng J, Zhang W, et al. Frozen elephant trunk with total arch replacement for type A aortic dissections: Does acuity affect operative mortality? J Thorac Cardiovasc Surg 2014;148:963-70; discussion 970-2. [Crossref] [PubMed]
- Society of Thoracic Surgeons. STS Adult Cardiac Surgery Database Data Specifications version 2.81. Available online: http://www.sts.org/sites/default/files/documents/STSAdultCVDataSpecificationsV2_81.pdf. Accessed May 1, 2015.
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, et al. Selective antegrade cerebral perfusion and mild (28°C-30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg 2012;144:1042-9. [Crossref] [PubMed]
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005;129:112-22. [Crossref] [PubMed]
- Mazzeffi M, Marotta M, Lin HM, et al. Duration of deep hypothermia during aortic surgery and the risk of perioperative blood transfusion. Ann Card Anaesth 2012;15:266-73. [Crossref] [PubMed]
- Harrington DK, Lilley JP, Rooney SJ, et al. Nonneurologic morbidity and profound hypothermia in aortic surgery. Ann Thorac Surg 2004;78:596-601. [Crossref] [PubMed]
- Cooper WA, Duarte IG, Thourani VH, et al. Hypothermic circulatory arrest causes multisystem vascular endothelial dysfunction and apoptosis. Ann Thorac Surg 2000;69:696-702; discussion 703. [Crossref] [PubMed]
- Hagl C, Tatton NA, Khaladj N, et al. Involvement of apoptosis in neurological injury after hypothermic circulatory arrest: a new target for therapeutic intervention? Ann Thorac Surg 2001;72:1457-64. [Crossref] [PubMed]
- Minatoya K, Ogino H, Matsuda H, et al. Evolving selective cerebral perfusion for aortic arch replacement: high flow rate with moderate hypothermic circulatory arrest. Ann Thorac Surg 2008;86:1827-31. [Crossref] [PubMed]
- Leshnower BG, Thourani VH, Halkos ME, et al. Moderate versus deep hypothermia with unilateral selective antegrade cerebral perfusion for acute type A dissection. Ann Thorac Surg 2015;100:1563-8; discussion 1568-9. [Crossref] [PubMed]
- Ehrlich MP, McCullough JN, Zhang N, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 2002;73:191-7. [Crossref] [PubMed]
- Hagl C, Khaladj N, Karck M, et al. Hypothermic circulatory arrest during ascending and aortic arch surgery: the theoretical impact of different cerebral perfusion techniques and other methods of cerebral protection. Eur J Cardiothorac Surg 2003;24:371-8. [Crossref] [PubMed]
- Svensson LG. Antegrade perfusion during suspended animation? J Thorac Cardiovasc Surg 2002;124:1068-70. [Crossref] [PubMed]
- Kazui T, Yamashita K, Washiyama N, et al. Usefulness of antegrade selective cerebral perfusion during aortic arch operations. Ann Thorac Surg 2002;74:S1806-9; discussion S1825-32.
- Girardi LN, Krieger KH, Lee LY, et al. Management strategies for type A dissection complicated by peripheral vascular malperfusion. Ann Thorac Surg 2004;77:1309-14; discussion 1314. [Crossref] [PubMed]
- Ergin MA, Griepp EB, Lansman SL, et al. Hypothermic circulatory arrest and other methods of cerebral protection during operations on the thoracic aorta. J Card Surg 1994;9:525-37. [Crossref] [PubMed]
- Paparella D, Rotunno C, Guida P, et al. Hemostasis alterations in patients with acute aortic dissection. Ann Thorac Surg 2011;91:1364-9. [Crossref] [PubMed]
- Zierer A, Aybek T, Risteski P, et al. Moderate hypothermia (30 degrees C) for surgery of acute type A aortic dissection. Thorac Cardiovasc Surg 2005;53:74-9. [Crossref] [PubMed]
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]


