Perspective and practice patterns of mediastinal staging among thoracic surgeons
Introduction
Lung cancer is the most common cause of cancer-related death worldwide, with an estimated 235,760 new diagnoses and 131,880 deaths from lung cancer in the U.S. in 2021 (1). Advances in detection and diagnosis technology have resulted in opportunities for earlier surgical treatment. Accurate preoperative staging and restaging of mediastinal lymph nodes in patients with potentially resectable non-small cell lung cancer is of paramount importance. Mediastinoscopy and endobronchial ultrasound (EBUS) guided Transbronchial needle aspiration are two of the most commonly used techniques for invasive mediastinal staging of lung cancer.
Introduced during the 1950’s, Mediastinoscopy has been the technique of choice for staging of the mediastinum and regarded as the gold standard for many years. The advancement in videoscopic-assisted surgery, specifically the introduction of video-assisted mediastinoscopic lymphadenectomy (VAMLA) and transcervical-extended mediastinal lymphadenectomy (TEMLA) by the end of the 20th century has added to the diagnostic accuracy and the surgical radicality of mediastinoscopy (2,3). Since the advent of EBUS in early 2000’s, EBUS has been proposed as a safe, less invasive alternative to mediastinoscopy to stage mediastinal lymph nodes in patients with lung cancer. Multiple studies proved EBUS to be cost effective when compared with mediastinoscopy, with similar diagnostic accuracy and lower complication rates (4,5).
With the increased popularity and training in minimally invasive and endoscopic procedures among practitioners, combined endosonographic lymph node biopsy techniques have become a valid alternative to standard mediastinoscopy and regarded as the primary mediastinal staging choice in several institutions and societies worldwide, with surgical mediastinal staging recommended when endosonographic staging does not show malignant nodal involvement in high-risk patients (6-9). In addition to lung cancer staging, EBUS has been shown to be a useful tool for diagnosis of several other mediastinal benign and malignant pathologies such as lymphoma, sarcoidosis, tuberculosis, and mediastinal cysts (10). While guidelines for interventional pulmonary training (including radial and linear EBUS) with recommendations for number of procedures needed for competency exist (11-13), and practice patterns were studied by different societies (14), limited data exist on the experience and training gained by thoracic surgeons (15). With the increased utilization of endosonographic procedures nowadays, we sought to determine what training is being offered and whether there is a paradigm shift in mediastinal staging among practicing thoracic surgeons in United States. We believe that this data might have implications in terms of the design of a training curriculum specifically for thoracic surgeons in the future. We present the following article in accordance with the SURGE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-183/rc).
Methods
Survey
An anonymous 28-questions web-based electronic survey was constructed and asked the responders to provide demographic, training, and practice patterns with mediastinoscopy and EBUS (Appendix 1). The survey was pilot tested among 3 thoracic surgeons and one pulmonologist for clarity, content, and ease of interpretation. Practicing thoracic surgeons in the United States were identified by 2 separate databases (New York general thoracic surgical club and Brigham and women’s thoracic surgery alumni databases) in order to achieve larger capture. E-mail invitations were sent with a link to the online survey. Participation was voluntary, and to preserve the complete anonymity, the survey did not ask identifying information such as training program names, unique demographic information, and other specific participant identifiers. The survey was open between August 2018 to January 2019. Training and practice patterns were compared between two periods: 2003–2010 and 2011–2018. The year 2003 was used as a cut off for graduation as this was the year EBUS was introduced.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Maimonides Medical Center (No. 2019-01-12-MMC) and individual consent for this questionnaire based retrospective analysis was waived.
Statistical analysis was performed by using Descriptive statistics to summarize quantitative data.
Results
A total of 98 thoracic surgeons responded to the survey. There was a 93% completion rate. Sixty-six percent of the respondents completed their training in thoracic surgery after the year 2000.
Training
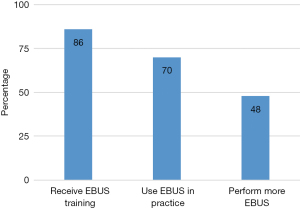
Eighty-seven percent (84/97) received EBUS training of any kind. Forty-three percent (35/82) trained with EBUS during residency or fellowship while 30% (25/82) trained by a fellow surgeon or pulmonologist during practice years. The rest trained by special hands-on courses either during fellowship or while on the job.
Year of graduation
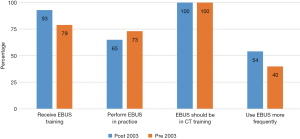
Fifty-one percent (50/98) surgeons completed fellowship after 2003. Of these, 93% reported that they had received EBUS training of any kind. Sixty-five percent reported they perform EBUS in their current practice. Fifty-four percent reported they perform EBUS more frequently than mediastinoscopy. Seventy-five percent believe mediastinoscopy is more accurate than EBUS in diagnosing lymphoma or sarcoidosis. Sixty-nine percent responded that EBUS is safer than mediastinoscopy (Figure 1).
Forty-nine percent (48/98) surgeons completed their training prior to 2003 and of these 79% reported they had received EBUS training of any kind, 44% of which learned in a hands-on course taught by pulmonologists and 42% by courses taught by other surgeons. Seventy-three percent perform EBUS in their current practice. Sixty percent however reported they perform more mediastinoscopy than EBUS. Sixty-five percent believe that EBUS is safer than mediastinoscopy.
EBUS exposure during training
All of the respondents (100%) of the responders believe that EBUS should be formally incorporated into cardiothoracic surgery (CTS) training. Seventy-seven percent believe that less exposure to mediastinoscopy during CTS training could affect the future of CTS trainees negatively while 89% of them answered that less exposure to EBUS during CTS training could affect the future of CTS trainees negatively. Interestingly, 17% disagree, 6% neither agree nor disagree that less exposure to mediastinoscopy during CTS training could affect the future of CTS trainees negatively.
Current practice
While 87% reported they received EBUS training of any kind, 69% (57/82) responded that they preform EBUS in their current practice. Of those who perform EBUS in their current practice, 86% (49/57) prefer to perform EBUS in the operating room and under general anesthesia 89% (51/57), rather than at the bronchoscopy suite and under moderate sedation respectively. Only 22% (13/57) responded that they also perform endoscopic ultrasound. Sixty-seven percent (38/57) of the surgeons that perform EBUS in their current practice perform EBUS for purposes other than cancer staging. Majority of them also reported that they perform less mediastinoscopy since the advent of EBUS 84% (48/57) (Figure 2).
Preference for EBUS
Forty-eight percent responded that they perform EBUS more frequently than mediastinoscopy.
From those who prefer EBUS, 55% (21/38) trained with EBUS during CTS training. Seventy-six percent (29/38) feel that EBUS is safer than mediastinoscopy and allows access to lymph nodes or lesions not accessible by mediastinoscopy 87% (33/38). 82% (31/38) prefer EBUS over mediastinoscopy in order to avoid re-do mediastinoscopy and in irradiated mediastinum 74% (28/38) (Figure 3).
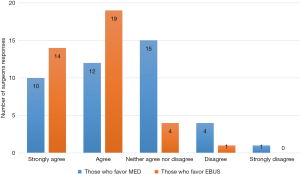
While only 16% (6/38) feel that EBUS is more accurate than mediastinoscopy, 67% believed it was the preferred procedure. From those who prefer EBUS over mediastinoscopy—81% (30/37) practice at an academic setting with the rest practicing in a community setting.
Preference for mediastinoscopy
Fifty two percent responded that they perform mediastinoscopy more frequently than EBUS. Out of them 52% (22/42) also perform EBUS.
For respondents who prefer mediastinoscopy, only 30% (13/42) trained with EBUS during CTS training. Of those who prefer mediastinoscopy, 77% (30/42) report that EBUS is primarily performed by pulmonologist at their institution, 36% (14/42) feel more comfortable performing mediastinoscopy over EBUS although they received some EBUS training.
Majority of those who prefer mediastinoscopy over EBUS reported that they perform more accurate staging compared to EBUS, that mediastinoscopy is more accurate in diagnosing Lymphoma or sarcoidosis and that frozen section can be done at the same interval as resection. 95% (38/42) of the respondents who prefer mediastinoscopy practice at an academic or community with academic affiliation setting with the rest practice at community setting.
NOT performing EBUS at all
Interestingly, 100% of the surgeons that reported that they do not perform EBUS in their practice reported that they received EBUS training of any kind. 40% (8/20) of those who responded that they do not perform EBUS at their practice reported that they feel more comfortable performing mediastinoscopy, 90% (18/20) EBUS is primarily performed by pulmonologists at their institution. Thirty-six percent (9/24) of those who do not perform EBUS at all feel that EBUS is safer than mediastinoscopy and only 20% (4/20) believe that mediastinoscopy is the preferred procedure over EBUS. Ninety-one percent (22/24) practice at an academic or academic affiliated institution.
Practice trends—extent of lymph node biopsy
In order to assess practice trends, we quarried the participants on their lymph node biopsy routine. Ninety-four percent (36/38) of those who prefer using EBUS biopsy 3 or more lymph node stations, 92% (35/38) biopsy nodes under 1 cm and 86% (33/38) biopsy N1 nodes while 8% (3/38) never biopsy N1 nodes (Figure 4).
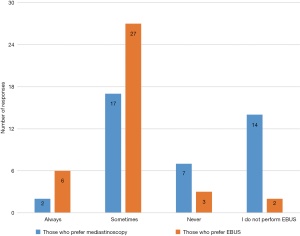
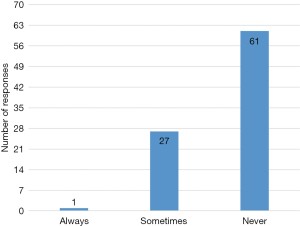
Ninety-seven percent (39/40) of those who prefer using mediastinoscopy biopsy 3 or more lymph node stations, 97% (39/40) biopsy nodes under 1cm and only 27% (11/40) biopsy N1 (tracheobronchial angle) nodes while 70% (28/40) never biopsy N1(tracheobronchial angle) nodes (Figure 5).
Discussion
Accurate mediastinal staging and restaging is mandatory in order to assess prognosis and to select the appropriate treatment protocol for lung cancer. Mediastinoscopy and EBUS-guided transbronchial needle aspiration are the two most commonly used invasive methods for mediastinal staging for lung cancer.
EBUS has been proposed as a safe, less invasive alternative to mediastinoscopy to stage mediastinal lymph nodes in patients with lung cancer (16). Multiple studies demonstrated similar accuracy for mediastinal lymph node sampling (17,18). Others found that EBUS is superior to mediastinoscopy in terms of its diagnostic performance for mediastinal staging of cN1-3 (5).
Since its introduction into practice during the early 2000’s, thoracic surgeons have been getting variable exposure to EBUS either during fellowship training, hands-on courses, or post-graduate practice with the help of fellow surgeon or pulmonology colleague. multiple reports exist on the use of EBUS for mediastinal staging and its efficacy comparing to the gold standard mediastinoscopy (6-9,11-14); however, limited data exist on the exposure and the training that thoracic surgeons are getting over the years with EBUS (15,18,19).
Using an online survey, we sought to investigate the status of mediastinal staging among practicing thoracic surgeons in United States. Specifically, we wanted to identify whether there is a change in training and practice patterns since the advent of EBUS into clinical practice in the early 2000’s.
We found that while majority of practicing thoracic surgeons received some form of training with EBUS, still more than 50% prefer using mediastinoscopy to stage the mediastinum. Despite this, majority of the participants reported that EBUS is safer than mediastinoscopy regardless of if they prefer using EBUS or mediastinoscopy.
Although less surgeons who completed their training prior to 2003 reported that they received training with EBUS compared to those who completed their training after 2003, we found that more surgeons that were trained prior to 2003 were actually performing EBUS (Figure 1). The reasons for this are not entirely clear but could reflect that perhaps the older respondents (largely practicing in academic settings) may have been early EBUS adopters and established practice patterns previously in which thoracic surgeons staged their own patients.
Training
All respondent practicing surgeons believed that EBUS should be formally incorporated into thoracic surgery training with 89% of them actually believe that less EBUS during training could negatively affect the future of CTS trainees. Simultaneously, 73% reported that less mediastinoscopy during training could negatively impact the future of CTS trainees negatively. This highlights the relevance of EBUS in the hands of the thoracic surgeon not only as a diagnostic tool but also as a complementary tool to the good old mediastinoscopy. Knowing best the anatomy of the mediastinum and trained with bronchoscopy—thoracic surgeon proficient with both EBUS and mediastinoscopy has the ability to decide which tool or combination of tools is adequate in a specific patient (19). With the recent guidelines preference for minimally invasive endoscopic mediastinal staging, an experienced thoracic surgeon proficient with both EBUS and mediastinoscopy can increase the effectiveness of multidisciplinary team discussions ideally (6-9,20).
Currently, there is no accepted number of procedures that a thoracic surgeon should perform in order to achieve EBUS competency. The American Board of Thoracic Surgery requires trainees to log 10 EBUS cases and 15 mediastinoscopy cases as part of their training curriculum (21). There are no certification requirements before performing EBUS independently.
Although major societies have recommended at least 30–50 supervised procedures to establish EBUS competency, this requirement may be less for surgeons who routinely perform mediastinal procedures, minimally invasive procedures, and bronchoscopies.
Studying their early learning curve, Groth et al. reported 96% sensitivity with 97.8% accuracy following their first 10 EBUS guided biopsies (15).
As EBUS becomes the standard of care in many centers, we humbly suggest that a Society-based survey and task force can help to initiate and design an ideal training pathway for thoracic surgeon to gain proficiency in endosonographic procedures [EBUS and endoscopic ultrasound (EUS)] (6,22).
With the introduction of innovations and minimally invasive techniques into every surgical discipline, we believe that similar to mediastinoscopy, a thoracic surgeon who utilizes EBUS will have the choice to offer therapeutic intervention at the same time as the diagnostic procedure. With the limited operating room resources, the ability to achieve both diagnosis and treatment at the same setting is crucial in the modern surgeon toolbox.
EBUS has several distinctive advantages as reported in our survey
Reevaluation of the mediastinum following previous mediastinoscopy, neoadjuvant therapy, or previous mediastinal radiation is likely safer with EBUS compared to Mediastinoscopy. Although commonly accepted, we did not find comparative studies comparing the safety between the two methods (20). Out of the respondents, 82% and 74% prefer EBUS over mediastinoscopy in order to avoid redo-Mediastinoscopy and irradiated mediastinum respectively.
Similar to other reports EBUS is regarded as a safe, less invasive alternative to mediastinoscopy with an equal high diagnostic accuracy. Regardless of preference (EBUS or mediastinoscopy) the majority of the participants reported that they feel EBUS is safer than mediastinoscopy. Majority of the participants who reported that they do not perform EBUS in their practice do so due to the fact that pulmonologists are performing EBUS at the same institute.
One of the disadvantages of mediastinoscopy is its inability to biopsy posterior or deep sub-carinal nodes and majority of N1 nodes; additionally, the risk of recurrent laryngeal nerve injury during biopsy—particularly the left paratracheal nodes during mediastinoscopy is well established. This was echoed in our survey as well—87% of surgeons performing EBUS reported that they can access lesions not accessible by mediastinoscopy, 94% reported that they biopsy 3 or more stations, 92% biopsy nodes smaller than 1 cm and 86% reported that they biopsy N1 nodes as a routine. Only 8% reported that they never biopsy N1 nodes during EBUS. On the contrary, during Mediastinoscopy 27% of surgeons reported that they routinely biopsy nodes under 1 cm and 70% reported that they never biopsy N1 nodes. This highlights the ability of EBUS to access more lymph node stations over mediastinoscopy and is being regarded as one of its advantages. In an era where neoadjuvant treatment for stage II lung cancers is currently being evaluated in the setting of clinical trials, pathologic staging of N1 nodes may be more crucial. Additionally, in considering sub-lobar resection for smaller tumors, preoperative knowledge and pathologic staging of N1 nodes by EBUS is paramount and has the potential to change surgical approach.
Majority of surgeons reported that they perform EBUS in the operating room and under general anesthesia. To our practice, performing mediastinal staging under general anesthesia is well-tolerated and allows a thorough mediastinal staging without the need for skin incision. Another advantage of endosonographic proficiency by surgeons is the addition of endoscopic ultrasound fine needle aspiration (EUS-FNA) to EBUS as reported by several authors (15,19,22-25). Based on these reports and others, it is generally accepted that combined endosonography (i.e., EBUS and EUS done at the same procedure) is a powerful and safe diagnostic tool for variety of diseases affecting the mediastinum. Surprisingly, in-spite of this body of evidence, only 22% of the responders in our cohort reported that they perform also EUS in their practice. The thoracic surgeon, with proficiency in upper endoscopy as well as bronchoscopy, is uniquely positioned to perform complete mediastinal staging including paraesophageal and inferior ligament nodes.
Importance of mediastinoscopy
Although mediastinoscopy is still regarded by some as the gold-standard approach for mediastinal staging, it has limited ability to assess the posterior subcarinal, lower mediastinal and hilar lymph nodes. With the advent of EBUS and the proficiency gained by bronchoscopists world-wide, many studies confirm that both mediastinoscopy and EBUS have similar sensitivity for detection of mediastinal metastases. Recent recommendations by the American College of Chest Physicians (ACCP) and the European Society of Thoracic surgeons (ESTS) led to the preference of endoscopical mediastinal staging with EBUS-TBNA or EUS-FNA (or both) as the tests of first choice, with the recommendation to be followed by mediastinoscopy in patients with high pretest probability of mediastinal metastatic disease (7-9,26).
For non-lung cancer disease of the mediastinum and complex mediastinal lymphadenopathies such as sarcoidosis, tuberculous lymphadenitis and lymphoma-mediastinoscopy provides high diagnostic benefit specifically if the target lesion is located in the paratracheal region (10,27,28). In our study, most respondents prefer mediastinoscopy over EBUS in diagnosing lymphoma or sarcoidosis and in situations that required frozen section at the same time.
Mediastinoscopy is an invasive procedure that requires general anesthesia and is performed in an operating room. A study comparing the cost between mediastinoscopy and EBUS showed that endoscopic needle-based staging is less expensive than mediastinoscopy if the staging is performed in the endoscopy suite. However, if being performed in the operating room, EBUS is more expensive than mediastinoscopy but generates 3.6 times less the amount of mediastinoscopy waste (29). Others found that EBUS remains cost effective compared to mediastinoscopy regardless of placement of the procedure (30-32). Designated center cost analysis and staging protocols should be considered in light of the shortage of operating room resources, and the fact that majority of the surgeons prefer using EBUS in the operating room and under general anesthesia.
Despite the shift to minimally invasive endoscopic staging in many centers, there is still lack for EBUS-TBNA expertise in other centers, and mediastinoscopy is the preferred choice in many thoracic surgery training programs for mediastinal staging, re-staging, and diagnosis of recurrent mediastinal disease. With the improved quality of imaging systems and enhanced visibility in video-assisted mediastinoscopy, it has become easier to teach and perform accurate and safe mediastinal staging nowadays. Therefore, it is important that thoracic surgeons will acquire adequate training to obtain proficiency in both approaches (i.e., endosonographic staging and mediastinoscopy), as mediastinoscopy should still be the first choice for staging by thoracic surgeons when needed (2,21,22,24,33).
Our survey has several limitations. First, although we used two separate large databases, we recognize that there might be selection bias in our study as it was not designed to capture all practicing thoracic surgeons in the US. Further, we could not confirm some overlapping between some of the members of these 2 databases, hence we could not precisely record accurate response rate but only the number of responders. Although this limits the statistical validity of the study, we feel that the relatively high number of responders may provide a preliminary estimate of the practice patterns among the thoracic surgeons in the US and the information could be useful for thoracic surgery program directors and society stakeholders for future design of an ideal study of training and practice patterns, and invoke consideration of future dedicated endosonographic training curriculum among thoracic surgery trainees. Incorporating consistent, dedicated endosonographic training into thoracic surgery curriculums will allow surgeons to have a role in advanced endoscopic procedures as the technology evolves and expands in the near future. Our survey was not designed to provide comparison between groups, as of such we were able only to provide patterns based on descriptive quantitative statistics only.
Conclusions
Emerging minimally invasive diagnostic modalities will continue to evolve and challenge established approaches. To remain at the forefront of diagnosis and treatment of mediastinal and thoracic pathologies in timely fashion, thoracic surgeons should be trained and invest the necessary effort to utilize all tools and new technologies available firsthand. We call for thoracic surgery residency program directors to consider incorporating endosonographic proficiency into their curricula, and thoracic surgical societies to become involved in certifying credentials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-183/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-183/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-183/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute. SEER Cancer Stat Facts: Lung and Bronchus Cancer [Internet]. 2021 [cited 2021 Dec 27]. Available online: https://seer.cancer.gov/statfacts/html/lungb.html
- Huertgen M, Tripsky J, Hartert M. Video-Assisted Mediastinoscopic Lymphadenectomy (VAMLA): Recipe and Cooking Secrets (a Tutorial). Oper Tech Thorac Cardiovasc Surg 2020;25:140-70. [Crossref]
- Hartert M, Tripsky J, Huergen M. Video-assisted mediastinocopic lymphadenectomy (VAMLA) for staging & treatment of non-small cell lung cancer (NCLSC). Mediastinum 2020;4:3. [Crossref] [PubMed]
- Figueiredo VR, Cardoso PFG, Jacomelli M, et al. EBUS-TBNA versus surgical mediastinoscopy for mediastinal lymph node staging in potentially operable non-small cell lung cancer: a systematic review and meta-analysis. J Bras Pneumol 2020;46:e20190221. Erratum in: J Bras Pneumol 2021;47:e20190221errata. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Hegde PV, Liberman M. Mediastinal Staging: Endosonographic Ultrasound Lymph Node Biopsy or Mediastinoscopy. Thorac Surg Clin 2016;26:243-9. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. Erratum in: Endoscopy 2015;47:c1. [Crossref] [PubMed]
- Yazgan S, Ucvet A, Gursoy S, et al. Surgical Experience of Video-Assisted Mediastinoscopy for Nonlung Cancer Diseases. Thorac Cardiovasc Surg 2021;69:189-93. [Crossref] [PubMed]
- Tanner NT, Pastis NJ, Silvestri GA. Training for linear endobronchial ultrasound among US pulmonary/critical care fellowships: a survey of fellowship directors. Chest 2013;143:423-8. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Training and proficiency in endobronchial ultrasound-guided transbronchial needle aspiration: A systematic review. Respirology 2017;22:1547-57. [Crossref] [PubMed]
- Naur TMH, Konge L, Nayahangan LJ, et al. Training and certification in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis 2017;9:2118-23. [Crossref] [PubMed]
- Turner SR, Seyednejad N, Nasir BS. Patterns of Practice in Mediastinal Lymph Node Staging for Non-Small Cell Lung Cancer in Canada. Ann Thorac Surg 2018;106:428-34. [Crossref] [PubMed]
- Groth SS, Whitson BA, D'Cunha J, et al. Endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes: a single institution's early learning curve. Ann Thorac Surg 2008;86:1104-9; discussion 1109-10. [Crossref] [PubMed]
- Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 2017;9:S83-97. [Crossref] [PubMed]
- Ge X, Guan W, Han F, et al. Comparison of Endobronchial Ultrasound-Guided Fine Needle Aspiration and Video-Assisted Mediastinoscopy for Mediastinal Staging of Lung Cancer. Lung 2015;193:757-66. [Crossref] [PubMed]
- Andrade RS. Relevance of endobronchial ultrasonography to thoracic surgeons. Semin Thorac Cardiovasc Surg 2010;22:150-4. [Crossref] [PubMed]
- Bonta PI, Crombag L, Annema JT. Linear endobronchial and endoesophageal ultrasound: a practice change in thoracic medicine. Curr Opin Pulm Med 2016;22:281-8. [Crossref] [PubMed]
- American Board of Thoracic Surgery. Index case requirements [Internet]. [Cited 2021 Dec 27]. Available online: https://www.abts.org/ABTS/CertificationWebPages/Operative_Requirements/Index%20Case%20Requirements-2017.aspx
- Oliveira RL, Liberman M. Endosonographic Mediastinal Lymph Node Staging in Non-Small Cell Lung Cancer: How I Teach It. Ann Thorac Surg 2017;104:18-21. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Waddell T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg 2013;96:1756-60. [Crossref] [PubMed]
- Yasufuku K. Relevance of endoscopic ultrasonography and endobronchial ultrasonography to thoracic surgeons. Thorac Surg Clin 2013;23:199-210. [Crossref] [PubMed]
- Gilbert S, Wilson DO, Christie NA, et al. Should endobronchial ultrasonography be part of the thoracic surgeon's armamentarium? J Thorac Cardiovasc Surg 2009;137:413-8. [Crossref] [PubMed]
- Marcoux M, Ost DE. What's new in endobronchial ultrasound for mediastinal staging?. Curr Opin Pulm Med 2020;26:346-58. [Crossref] [PubMed]
- Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804-9. [Crossref] [PubMed]
- Garwood S, Judson MA, Silvestri G, et al. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest 2007;132:1298-304. [Crossref] [PubMed]
- Andrade RS, Podgaetz E, Rueth NM, et al. Endobronchial ultrasonography versus mediastinoscopy: a single-institution cost analysis and waste comparison. Ann Thorac Surg 2014;98:1003-7. [Crossref] [PubMed]
- Steinfort DP, Liew D, Conron M, et al. Cost-benefit of minimally invasive staging of non-small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol 2010;5:1564-70. [Crossref] [PubMed]
- Chouaid C, Salaün M, Gounant V, et al. Clinical efficacy and cost-effectiveness of endobronchial ultrasound-guided transbronchial needle aspiration for preoperative staging of non-small-cell lung cancer: Results of a French prospective multicenter trial (EVIEPEB). PLoS One 2019;14:e0208992. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Rochau U, Siebert U, et al. Cost-effectiveness of mediastinal lymph node staging in non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:1567-78. [Crossref] [PubMed]
- Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012;186:255-60. [Crossref] [PubMed]