
Patch augmentation vs. valve replacement for patients with atrial functional mitral regurgitation and long-standing atrial fibrillation
Introduction
The mechanism of atrial functional mitral regurgitation (AFMR) and atrial fibrillation, including mitral annular dilatation and posterior leaflet tethering associated with left atrial enlargement, has gradually been elucidated (1). Recently, surgical interventions for AFMR due to atrial fibrillation have become an area of interest because different techniques are required at each stage of AFMR according to the pathology (2,3). Atrial fibrillation often causes mitral annular and left atrial dilatation, which lead to insufficient coaptation of the mitral leaflets. Some patients who have long-standing atrial fibrillation develop AFMR and posterior leaflet tethering due to deviation of the posterior leaflet. This deviation is caused by considerable dilation of the mitral annulus and the left atrium (atriogenic tethering or atrial hamstringing) (1). Sakaguchi et al. reported that ring annuloplasty for AFMR with excessive leaflet tethering may not be sufficient to achieve long-term correction of mitral regurgitation (MR) (2). Therefore, AFMR with long-standing atrial fibrillation, which is associated with severe shortening of the posterior leaflet with tethering, requires intervention for the shortened or tethered posterior leaflet. Ring repair alone cannot control AFMR because of insufficient leaflet coaptation, which is associated with recurrent MR.
Recently, PA repair for a shortened or tethered posterior leaflet in patients with AFMR and long-standing atrial fibrillation has been used (3,4). However, few studies have examined the outcomes of PA repair for AFMR (3,4), and the outcomes of PA repair have not been compared with those of mitral VR. Therefore, in this retrospective study, we compared the outcomes of PA repair with a tethered posterior leaflet with those of mitral VR for AFMR with long-standing atrial fibrillation. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-828/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Osaka Metropolitan Medical School Hospital and Osaka City General Hospital (approval No. 3817; approval date, 31 July 2017). Written informed consent for this retrospective study was obtained from all the patients at the time of cardiovascular surgery.
Patients
From April 2008 to November 2021, we performed mitral valve repair with PA in 16 patients who had AFMR with a tethered posterior leaflet and severe left atrial enlargement due to long-standing atrial fibrillation at Osaka Metropolitan University Hospital and Osaka City General Hospital. Additionally, we performed mitral VR for AFMR with a tethered posterior leaflet and severe left atrial enlargement due to long-standing atrial fibrillation in 23 patients between April 2012 and November 2021 at Osaka Metropolitan University Hospital. The patients who had congestive heart failure caused by AFMR and long-standing atrial fibrillation even after medical management, including rhythm control, were eligible for inclusion in the study. We excluded patients with a reduced left ventricular (LV) ejection fraction (LVEF) (<50%) because decreased LV function may be associated with other cardiac diseases. Finally, we compared the outcomes of PA (n=16; PA group) with those of VR (n=15; VR group) for AFMR with a tethered posterior leaflet and severe atrial enlargement due to long-standing atrial fibrillation. We defined AFMR with a tethered posterior leaflet as severe shortening and tethering of the posterior leaflet and mitral annular dilatation with or without pseudo-prolapse of the anterior leaflets in accordance with the guidelines of the Japanese Circulation Society (5). Preoperative comorbidities and perioperative complications were defined by referring to the Japan Cardiovascular Surgery Database (http://www.jacvsd.umin.jp). Major adverse cardiac events after hospital discharge included cardiac death, readmission for congestive heart failure, cardiac thromboembolic events, and cardiac reoperation.
Surgical techniques
We performed mitral valve surgery for AFMR using the conventional approach, which consisted of cardiopulmonary bypass with ascending aortic cannulation and bicaval venous cannulation to the superior and inferior vena cava through a median sternotomy. We approached the mitral valve through the inferior approach or the transseptal approach. We performed aortic VR or coronary artery bypass grafting in the conventional fashion during cardiac arrest if necessary.
In the PA group, we performed PA with fresh autologous pericardium without glutaraldehyde fixation for AFMR to obtain an adequate coaptation length when the posterior leaflet was shorter than 10 mm (3,4). PA repair consisted of transversely cutting the center of the shortened posterior leaflet and sewing the pericardial patch using continuous suture with 5-0 monofilament polypropylene (Figure 1). The incision of the P2 posterior leaflet was extended to P1 or P3, where coaptation loss was identified using preoperative echocardiography or intraoperative findings. The harvested autologous pericardial patch was trimmed to a square shape to expand the height of the posterior leaflet over 20 mm during sewing. If the patients demonstrated pseudo-prolapse of the anterior leaflets, neochordal repair was performed with the loop technique using a CV4 expanded polytetrafluoroethylene suture (Gore-Tex®; W. L. Gore & Associates, Flagstaff, AZ, USA) and a felt pledget. The loop technique has been described previously (6). We selected a mitral ring that was one size smaller than the intercommissural distance.
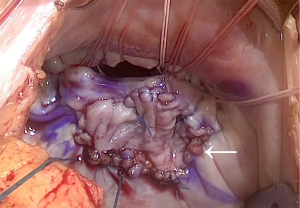
In the VR group, we placed a bioprosthetic valve or mechanical valve with non-everting or everting mattress spaghetti-pledged 2-0 polyester suture. We removed the mitral valve leaflets and tendons without preserving subvalvular tissue because residual subvalvular tissue might have pannus formation, which may be associated with limiting prosthetic valve leaflet motion in the future.
Left atrial plication using horizontal mattress and continuous 4-0 Prolene suture with a felt pledget was performed from the left atrial appendage to the caudal atrial septum close to the posterior leaflet annulus, and from the left atrial appendage to the cranial atrial septum through the left atrial roof, with or without the middle posterior leaflet annulus to the left atrial roof through the posterior wall of the left atrium between the left and right pulmonary veins (Figure S1). Left atrial appendage was closed by intra-atrial horizontal mattress and continuous 4-0 Prolene suture without devices. Right atrial plication was also performed to plicate the right atriotomy with removal of the redundant right atrium. We did not perform atrial plication and left atrial appendage closure if the patients had a weak atrial wall that led to severe bleeding.
Anticoagulant therapy
After surgery, the patients underwent oral anticoagulant therapy with warfarin or non-vitamin K antagonist direct oral anticoagulants. Warfarin was controlled within 1.8–2.2 of the international normalized ratio as the standard, and within 2.0–2.5 of the international normalized ratio in patients who underwent mechanical VR. If the patients had a bleeding tendency, we controlled warfarin at a lower level of the international normalized ratio than the standard level.
Echocardiography
All patients underwent transthoracic echocardiography before and after surgery. The left atrial volume index (LAVI) was calculated by dividing the left atrial volume by the body surface area. MR severity was defined using a multiparametric approach, including assessments of the color Doppler-derived jet area, the effective regurgitant orifice area, the MR volume and fraction, and the pulmonary vein flow velocity pattern (7). The mean mitral valve pressure gradient (PG) was obtained by tracing the continuous wave Doppler signal for integration of instantaneous gradients over the diastolic filling period. The severity of tricuspid regurgitation (TR) was defined using a multiparametric approach, including assessments of the color Doppler-derived jet area, the continuous wave Doppler-derived jet density and contour, and the hepatic vein flow velocity pattern (7). Continuous wave Doppler was used to measure tricuspid valve peak velocity (v) (in m/s) and the TR PG (in mmHg), which was calculated as 4 × v2. We measured the P2 posterior leaflet lengths and the posterior leaflet tethering angle in mid-systole. The posterior leaflet tethering angle was defined as the angle comprising the annular line, and the line drawn between the posterior annulus and the tip of the posterior leaflet (3). The patients who had coaptation loss and a short length (<12 mm) of the posterior leaflet with a posterior leaflet tethering angle >30 degrees were candidates for this study.
Follow-up
Excluding the patients who died and underwent reoperation with VR for recurrent MR during hospitalization, 29 patients were followed up as outpatients every 6–12 months. Follow-up patients were censored on the last known date of echocardiography for recurrent MR and on the last known date that they visited the hospital. The median follow-up duration was 1.7 years [interquartile range (IQR), 1.2–3.9 years]. The median follow-up index was 0.93 [IQR, 0.56–0.98].
Statistical analysis
Data were analyzed using EZR, version 1.52 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Numerical variables are expressed as median [IQR] and were analyzed using the non-parametric Mann-Whitney U test. Categorical variables are expressed as number (percentage) and were compared using the χ2 test or Fisher’s exact test, as appropriate. The overall survival rate, rate of freedom from major adverse cardiac events after hospital discharge, rate of readmission for congestive heart failure, thromboembolic event rate, and cardiac reoperation rate were expressed using Kaplan-Meier estimates, and differences between the two groups were evaluated using the log-rank test. The univariate Cox regression analysis was used to identify the factors associated with thromboembolic events. Receiver operating characteristic (ROC) curves were designed to identify cut-off values to predict the risk of thromboembolic events. The specificity and sensitivity were calculated, as well as the positive and negative predictive values. The best possible cut-off point was defined as the highest Youden index [(specificity + sensitivity) − 1]. A P value of <0.05 was considered statistically significant.
Results
Patients’ preoperative and intraoperative characteristics
Table 1 summarizes the preoperative and intraoperative characteristics in the PA and VR groups. The median age of patients was 72.5 [67.8–78.3] and 76.0 [74.5–80.0] years in the PA and VR groups, respectively (P=0.160). The VR group had a significantly higher rate of chronic renal disease than the PA group (P=0.009). There was no significant difference in LAVI. The median preoperative TR PG was 30.5 and 39.0 mmHg in the PA and VR groups, respectively (P=0.072). The PA group had a lower EuroScore II than the VR group (PA: 2.81 vs. VR: 4.82, P=0.027).
Table 1
| Variables | PA group (n=16) | VR group (n=15) | P value |
|---|---|---|---|
| Age, years | 72.5 [67.8–78.3] | 76.0 [74.5–80.0] | 0.160 |
| Sex, female/male | 4 (25.0)/12 (75.0) | 7 (46.7)/8 (53.3) | 0.273 |
| BSA, m2 | 1.67 [1.57–1.73] | 1.51 [1.41–1.69] | 0.206 |
| Hypertension | 9 (56.3) | 12 (80.0) | 0.252 |
| Dyslipidemia | 2 (12.5) | 2 (13.3) | 1.000 |
| Diabetes mellitus | 1 (6.3) | 1 (6.7) | 1.000 |
| Smoking | 8 (50.0) | 6 (40.0) | 0.722 |
| Chronic renal disease | 2 (12.5) | 9 (60.0) | 0.009 |
| Hemodialysis | 0 (0.0) | 1 (6.7) | 1.000 |
| Cerebrovascular disease | 0 (0.0) | 4 (26.7) | 0.043 |
| Respiratory disease | 2 (12.5) | 3 (20.0) | 0.654 |
| Previous cardiac surgery | 1 (6.3) | 2 (13.3) | 0.600 |
| NYHA class ≥III | 9 (56.3) | 10 (66.7) | 0.716 |
| Preoperative LVEF, % | 60.0 [59.0–67.5] | 63.0 [58.5–66.0] | 0.984 |
| Preoperative LV diastolic dimension, mm | 59.5 [53.8–61.5] | 54.0 [51.3–56.0] | 0.205 |
| Preoperative LAVI, mL/m2 | 131.4 [77.0–202.6] | 161.0 [85.5–202.5] | 0.711 |
| Preoperative MR grade | 1.000 | ||
| Moderate to severe | 2 (12.5) | 1 (6.7) | |
| Severe | 14 (87.5) | 14 (93.3) | |
| Preoperative TR PG, mmHg | 30.5 [28.0–38.0] | 39.0 [31.1–52.5] | 0.072 |
| Preoperative TR grade | 0.639 | ||
| Mild | 2 (12.5) | 1 (6.7) | |
| Moderate | 8 (50.0) | 6 (40.0) | |
| Moderate to severe | 0 (0.0) | 2 (13.3) | |
| Severe | 6 (37.5) | 6 (40.0) | |
| EuroScore II | 2.81 [2.33–5.31] | 4.82 [3.13–9.20] | 0.027 |
| Operation time, min | 345 [309–404] | 288 [264–344] | 0.086 |
| Cardiopulmonary bypass time, min | 206 [183–236] | 172 [153–192] | 0.012 |
| Aortic clamp time, min | 164 [149–178] | 137 [126–161] | 0.058 |
| Concomitant operation | |||
| Aortic VR | 0 (0.0) | 1 (6.7) | 0.484 |
| Coronary artery bypass grafting | 0 (0.0) | 2 (13.3) | 0.226 |
| Atrial plication | 7 (43.8) | 9 (60.0) | 0.479 |
| Left atrial appendage closure | 11 (68.8) | 14 (93.3) | 0.172 |
Data are presented as n (%) or median [interquartile range]. PA, patch augmentation; VR, valve replacement; BSA, body surface area; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LV, left ventricular; LAVI, left atrial volume index; MR, mitral regurgitation; TR, tricuspid regurgitation; PG, pressure gradient.
Fifteen patients in the PA group underwent mitral valve repair with the Carpentier-Edwards Physio II annuloplasty ring (Edwards Lifesciences, Irvine, CA, USA) (28 mm in two patients, 30 mm in five patients, 32 mm in five patients, 34 mm in two patients, 36 mm in one patient), while the SJM Rigid Saddle Ring (Abbott Laboratories, Chicago, IL, USA) was used in one patient (30 mm). Six patients concomitantly underwent the loop technique for anterior leaflet prolapse. Four patients in the VR group underwent VR with the Mosaic bioprosthesis (Medtronic Inc., Minneapolis, MN, USA), while the Epic valve (Abbott Laboratories) was used in eight patients, the Carpentier-Edwards PERIMOUNT valve (Edwards Lifesciences) was used in one patient, the SJM mechanical valve (Abbott Laboratories) was used in one patient, and the ATS mechanical valve (Medtronic) was used in one patient. All patients underwent concomitant tricuspid valve repair. Only one patient underwent the DeVega procedure for severe tricuspid valve regurgitation in the VR group, whereas 30 patients underwent ring annuloplasty. In the PA group, two patients underwent PA of the tricuspid valve anterior leaflet due to anterior leaflet shortening. Atrial plication was performed in 16 patients, and left atrial appendage closure was performed in 25 patients. The PA group had a longer operation time (PA: 345 vs. VR: 288 min, P=0.086), cardiopulmonary bypass time (PA: 206 vs. VR: 172 min, P=0.012), and aortic clamp time (164 vs. 137 min, P=0.058) than the VR group.
Postoperative outcomes during hospitalization
Table 2 shows the postoperative outcomes of patients during hospitalization. After surgery, one patient in the PA group who did not undergo atrial plication and left appendage closure died of cerebral infarction caused by cardiac thrombosis, whereas no patients died in the VR group (P=1.000). Six patients in each group had postoperative morbidities (P=1.000). One patient required mitral VR for perforation of the patched posterior leaflet (Figure S2). Five patients required re-exploration for bleeding (PA: 2 patients vs. VR: 3 patients). Postoperative LAVI, mean mitral valve PG, and TR PG were not significantly different between the PA and VR groups. In the PA group, postoperative MR grade was less than mild in fifteen patients, and mild to moderate in one patient. The postoperative TR grade was not significantly different between the PA and VR groups.
Table 2
| Variables | PA group (n=16) | VR group (n=15) | P value |
|---|---|---|---|
| Postoperative LVEF, % | 57.5 [53.0–62.3] | 55.0 [49.5–59.0] | 0.177 |
| Postoperative LV diastolic dimension, mm | 52.0 [47.0–55.3] | 45.0 [43.5–54.0] | 0.227 |
| Postoperative LAVI, mL/m2 | 86.8 [58.9–118.3] | 67.0 [45.0–92.0] | 0.173 |
| Postoperative mean mitral valve PG, mmHg | 4.0 [3.0–5.0] | 4.0 [4.0–7.0] | 0.144 |
| Postoperative MR grade | 0.673 | ||
| None or trivial | 12 (75.0) | 14 (93.3) | |
| Mild | 3 (18.8) | 1 (6.7) | |
| Mild to moderate | 1 (6.3) | 0 (0.0) | |
| Postoperative TR PG, mmHg | 25.0 [22.0–27.0] | 25.2 [24.0–40.0] | 0.355 |
| Postoperative TR grade | 0.722 | ||
| Trivial | 10 (62.5) | 7 (46.7) | |
| Mild | 5 (31.3) | 7 (46.7) | |
| Moderate | 1 (6.3) | 1 (6.7) | |
| Mortality | 1 (6.3) | 0 (0.0) | 1.000 |
| Morbidities | 6 (37.5) | 6 (40.0) | 1.000 |
| Reoperation for recurrent MR | 1 (6.3) | 0 (0.0) | |
| Re-exploration for bleeding | 2 (12.5) | 3 (20.0) | |
| Cerebral infarction | 1 (6.3) | 0 (0.0) | |
| Low output syndrome | 1 (6.3) | 0 (0.0) | |
| LV rupture | 0 (0.0) | 1 (6.7) | |
| Need for continuous hemodialysis | 2 (12.5) | 1 (6.7) | |
| Pneumonia | 1 (6.3) | 2 (13.3) | |
| Tracheotomy | 0 (0.0) | 2 (13.3) | |
| Pacemaker implantation | 0 (0.0) | 2 (13.3) |
Data are presented as n (%) or median [interquartile range]. PA, patch augmentation; VR, valve replacement; LVEF, left ventricular ejection fraction; LV, left ventricular; LAVI, left atrial volume index; PG, pressure gradient; MR, mitral regurgitation; TR, tricuspid regurgitation.
Mid-term outcomes: survival rate and major adverse cardiovascular event rate
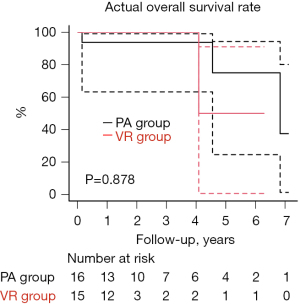
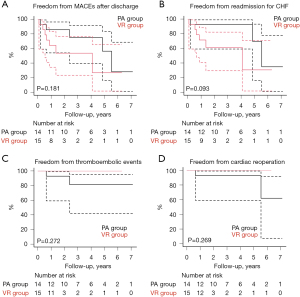
The overall survival rate was 93.8% and 100% at 3 years and 75.0% and 50.0% at 5 years in the PA and VR groups, respectively (P=0.878) (Figure 2, Table S1). The cause of late death was cancer in two patients in the PA group and congestive heart failure in one patient in the VR group. The rate of freedom from major adverse cardiac events after hospital discharge was 75.0% and 53.6% at 3 years and 56.2% and 26.8% at 5 years in the PA and VR groups, respectively (P=0.181) (Figure 3A, Table S1). In the PA group, two patients had recurrent severe MR, and two patients required reoperation owing to recurrent MR and left atrial thrombosis. Two patient who did not undergo atrial plication and left appendage had cerebral infarction and left atrial thrombosis. Three patients were readmitted to hospital owing to congestive heart failure. In the VR group, six patients were readmitted to hospital owing to congestive heart failure associated with chronic atrial fibrillation, including one patient with a permanent pacemaker for sick sinus syndrome. One patient who underwent mechanical VR experienced cerebral bleeding. No patients in the VR group required reoperation or demonstrated structural valve deterioration. The rate of freedom from readmission for congestive heart failure, thromboembolic events, and cardiac reoperation after hospital discharge have no significant difference, respectively (P=0.093, 0.272, and 0.269) (Figure 3B-3D, Table S1).
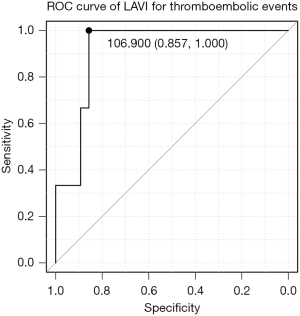
Factors associated with thromboembolic events
We assessed the factors associated with thromboembolic events over the entire period. The univariate Cox regression analysis showed that postoperative LAVI (hazard ratio 1.02; 95% CI: 1.004–1.034; P=0.016) was associated with thromboembolic events (Table 3). The ROC curve showed that the postoperative LAVI cut-off value for thromboembolic events was 106.9 mL/m2 (specificity, 0.857; sensitivity, 1.000; area under the curve, 0.917; 95% CI: 0.801–1.000) (Figure 4).
Table 3
| Variables | Thromboembolic events | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| Age, years | 0.95 | 0.820–1.100 | 0.490 |
| Sex, male | 0.99 | 0.089–10.96 | 0.993 |
| Hypertension | <0.01 | 0–inf | 0.999 |
| Chronic renal disease | <0.01 | 0–inf | 0.999 |
| Cerebral disease | <0.01 | 0–inf | 0.999 |
| NYHA ≥3 | 1.19 | 0.105–13.48 | 0.888 |
| Preoperative LV diastolic dimension, mm | 0.97 | 0.849–1.116 | 0.702 |
| Preoperative TR PG, mmHg | 0.97 | 0.864–1.093 | 0.636 |
| VR | <0.01 | 0–inf | 0.999 |
| Atrial plication | <0.01 | 0–inf | 0.999 |
| Left atrial appendage closure | <0.01 | 0–inf | 0.999 |
| Postoperative LAVI, mL/m2 | 1.02 | 1.004–1.034 | 0.016 |
| Postoperative TR PG, mmHg | 1.04 | 0.915–1.175 | 0.570 |
For numerical variables, the hazard ratio refers to an increase of 1. CI, confidence interval; inf, infinity; NYHA, New York Heart Association; LV, left ventricular; TR, tricuspid regurgitation; PG, pressure gradient; VR, valve replacement; LAVI, left atrial volume index.
Discussion
Long-standing atrial fibrillation is associated with significant functional MR and TR, which is in turn associated with a very poor prognosis despite preserved LVEF (8). In this situation, surgical interventions are recommended to improve prognosis (3,9). Mitral valve repair with ring annuloplasty only for AFMR with excessive posterior leaflet shortening and tethering does not effectively control MR (2). Additional repair techniques, such as PA repair, are required to obtain good mitral valve leaflet coaptation. Mitral VR may be useful because patients with long-standing atrial fibrillation are usually older with severe degenerative changes in the mitral leaflets. However, few studies have reported the outcomes of PA repair compared with those of mitral VR for AFMR with a tethered posterior leaflet. Our study showed that PA repair for AFMR with a tethered posterior leaflet and severe atrial enlargement achieves comparable outcomes to those of mitral VR.
PA repair provides good mitral valve leaflet coaptation, which leads to good control of MR. Recent studies have shown good early outcomes with low rates of recurrent MR and reoperation after PA for ischemic MR or rheumatic disease by deep leaflet coaptation (10,11). Additionally, Rahmani et al. revealed that posterior leaflet PA significantly reduces the forces on the chordae tendinae from the posterior papillary muscle with good hemodynamics in vitro using a functional ischemic MR valve simulation (12). However, PA repair had relatively higher recurrent MR and reoperation rates than the other mitral valve repair techniques. Fukunaga et al. reported a lower freedom from reoperation rate after PA than with non-PA repair (93.4% vs. 96.9% and 68.8% vs. 89.7% at 2 and 5 years, respectively) (13). Additionally, PA repair carries a risk of patch detachment or rupture after surgery, as well as calcification of glutaraldehyde-fixed pericardium (13,14). We also experienced three patients with recurrent MR, including one patient with patch perforation and two patients who required reoperation in the relatively early-term. No patients demonstrated calcification of the pericardial patch, which may be because we did not use glutaraldehyde-fixed pericardium. A recent report showed that in mitral valve repair, fresh autologous pericardium can be used with the expectation of durable long-term valve function without evidence of late patch calcification, stiffness, or aneurysmal degeneration (15). In contrast, Ikeda et al. reported that extended PA was not associated with recurrent MR or reoperation 3 years after surgery, which emphasizes the importance of large and wide augmentation to prevent recurrent MR (11). In the present study, PA repair required longer cardiopulmonary bypass and cardiac arrest times than mitral VR, even though the patients who underwent VR usually underwent concomitant procedures. A recent meta-analysis showed a good long-term outcome with a low reoperation rate after mitral bioprosthetic VR for MR (16). In high-risk patients, VR may be a useful procedure to obtain good outcomes with a low reoperation rate. PA or VR should be chosen based on the patient’s background.
Long-standing atrial fibrillation is also associated with severe right and left atrial enlargement, usually with MR and TR, which requires additional atrial reduction surgery (17). Severe atrial enlargement leads to smoke-like flow in the atrium with a possibility of thrombosis and compression of the bronchus and lung, followed by decreased respiratory function (18). Therefore, atrial reduction surgery is required to improve atrial function and lung compression. Sawazaki et al. showed that aggressive atrial volume reduction of bilateral enlarged atria improved respiratory function (19). Moreover, recent studies have shown that left atrial volume reduction concomitant with atrial fibrillation surgery helped to restore both left atrial contraction and compliance with a high rate of restoration to sinus rhythm (20,21). Matsumori et al. also suggested that left atrial plication improved the horizontal mitral valve angle, which affected the durability of mitral valve repair (22). Additionally, our results show that LAVI is associated with thromboembolic events after surgery for AFMR, and the patients who did not undergo atrial plication and left atrial appendage closure had thromboembolic events. Therefore, atrial reduction surgery with appropriate volume reduction is needed to prevent or improve the events associated with atrial enlargement. However, atrial reduction surgery poses a risk of bleeding because of inherent weakening of the atrial wall, although several atrial reduction techniques, including plication and resection, have been reported with good outcomes (18). Thus, the technique should be chosen with consideration of the bleeding risk.
The mortality and morbidity rates of surgery for atrial enlargement are high. Long-standing atrial fibrillation is associated with huge atrial enlargement with MR and TR, followed by left and right congestive heart failure (8). Zheng et al. reported an operative mortality rate of 13%, a low cardiac output syndrome rate of 13%, and a respiratory failure rate of 10% after surgery for left atrial enlargement (23). Our results also showed that 6.5% of patients experienced respiratory complications requiring tracheotomy. In particular, the VR group had a high tracheotomy rate, which may have been derived from the higher EuroScore II compared with the PA group. Furthermore, patients who had long-standing atrial fibrillation with atrial enlargement were usually older and had chronic heart failure, which is associated with weak tissues and cardiac cachexia (24). Our results also showed a relatively high morbidity rate, including reoperation for postoperative bleeding and LV rupture, although few patients with low output syndrome required intra-aortic balloon pumping. Coagulopathy caused by heart failure and weak tissue injury may lead to a bleeding tendency and LV rupture. Additionally, some patients had congestive heart failure or required pacemaker implantation after surgery. Long-standing atrial fibrillation that cannot be corrected carries a risk of sinus node dysfunction and persistent pulmonary hypertension, which induces congestive heart failure and requires permanent pacemaker implantation (8,25). Therefore, because patients with long-standing atrial fibrillation may develop congestive heart failure even after surgery, medical treatments should be carefully considered in cooperation with a cardiologist.
This study has some inherent limitations that should be noted. First, the study was retrospective in nature and was not a randomized controlled study. Moreover, inherited risk factors that we could not detect or exclude may have led to selection bias. Heterogeneity of repair for AFMR and concomitant operations may also affect the outcomes. In addition, the PA group had a lower EuroScore II and a higher rate of renal and cerebral diseases than the VR group, which may have affected the outcomes. Second, the number of included patients was relatively small, which may have influenced the results. However, it may be difficult to obtain a large number of patients with AFMR and to exclude selection bias because the number of patients with AFMR is relatively small (1,8). Finally, the follow-up duration may have been too short to declare robust long-term results after surgery for AFMR. Therefore, further follow-up is required to examine the outcomes after surgery for AFMR.
Conclusions
PA repair for AFMR caused by severe posterior leaflet shortening with atrial enlargement may achieve good outcomes that are comparable with those of VR. However, PA repair required a longer cardiopulmonary bypass time and had a higher reoperation rate than VR. Therefore, in high-risk patients, VR may be a good choice because of its good mid-term outcomes without reoperation, especially for non-expert surgeons. Surgical procedures should be chosen while considering the patient’s background. Atrial reduction surgery with appropriate volume reduction could be considered to prevent thromboembolic events because the postoperative LAVI is associated with thromboembolic events after surgery.
Acknowledgments
We thank Emily Woodhouse, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-828/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-828/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-828/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-828/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Osaka Metropolitan Medical School Hospital and Osaka City General Hospital (approval No. 3817), and written informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kagiyama N, Mondillo S, Yoshida K, et al. Subtypes of Atrial Functional Mitral Regurgitation: Imaging Insights Into Their Mechanisms and Therapeutic Implications. JACC Cardiovasc Imaging 2020;13:820-35. [Crossref] [PubMed]
- Sakaguchi T, Totsugawa T, Orihashi K, et al. Mitral annuloplasty for atrial functional mitral regurgitation in patients with chronic atrial fibrillation. J Card Surg 2019;34:767-73. [Crossref] [PubMed]
- Takahashi Y, Abe Y, Takashi M, et al. Mid-term results of valve repairs for atrial functional mitral and tricuspid regurgitations. Gen Thorac Cardiovasc Surg 2020;68:467-76. [Crossref] [PubMed]
- Takahashi Y, Shibata T, Hattori K, et al. Extended posterior leaflet extension for mitral regurgitation in giant left atrium. J Heart Valve Dis 2014;23:88-90. [PubMed]
- Izumi C, Eishi K, Ashihara K, et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ J 2020;84:2037-119. [Crossref] [PubMed]
- Shibata T, Kato Y, Motoki M, et al. Mitral valve repair with loop technique via median sternotomy in 180 patients. Eur J Cardiothorac Surg 2015;47:491-6. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Abe Y, Akamatsu K, Ito K, et al. Prevalence and Prognostic Significance of Functional Mitral and Tricuspid Regurgitation Despite Preserved Left Ventricular Ejection Fraction in Atrial Fibrillation Patients. Circ J 2018;82:1451-8. [Crossref] [PubMed]
- Balogh Z, Mizukami T, Bartunek J, et al. Mitral Valve Repair of Atrial Functional Mitral Regurgitation in Heart Failure with Preserved Ejection Fraction. J Clin Med 2020;9:3432. [Crossref] [PubMed]
- Malhotra A, Majmudar S, Siddiqui S, et al. Midterm Results of Mitral Valve Repair With Pericardial Leaflet Augmentation: A Single-Center Experience. Semin Thorac Cardiovasc Surg 2020;32:433-40. [Crossref] [PubMed]
- Ikeda N, Yamaguchi H, Takagaki M, et al. Extended Posterior Leaflet Augmentation for Ischemic Mitral Regurgitation – Augmented Posterior Leaflet Snuggling up to Anterior Leaflet. Circ J 2019;83:567-75. [Crossref] [PubMed]
- Rahmani A, Rasmussen AQ, Honge JL, et al. Mitral valve mechanics following posterior leaflet patch augmentation. J Heart Valve Dis 2013;22:28-35. [PubMed]
- Fukunaga N, Sakata R, Koyama T. Reoperative analysis after mitral valve repair with glutaraldehyde-treated autologous pericardium. Interact Cardiovasc Thorac Surg 2017;25:912-7. [Crossref] [PubMed]
- Gomibuchi T, Takano T, Wada Y, et al. Patch detachment after mitral valve repair with posterior leaflet augmentation: a case report. J Cardiothorac Surg 2015;10:118. [Crossref] [PubMed]
- Quinn RW, Wang L, Foster N, et al. Long-term Performance of Fresh Autologous Pericardium for Mitral Valve Leaflet Repair. Ann Thorac Surg 2020;109:36-41. [Crossref] [PubMed]
- Malvindi PG, Mastro F, Kowalewski M, et al. Durability of Mitral Valve Bioprostheses: A Meta-Analysis of Long-Term Follow-up Studies. Ann Thorac Surg 2020;109:603-11. [Crossref] [PubMed]
- Shibata T, Takahashi Y, Fujii H, et al. Surgical considerations for atrial functional regurgitation of the mitral and tricuspid valves based on the etiological mechanism. Gen Thorac Cardiovasc Surg 2021;69:1041-9. [Crossref] [PubMed]
- Apostolakis E, Shuhaiber JH. The surgical management of giant left atrium. Eur J Cardiothorac Surg 2008;33:182-90. [Crossref] [PubMed]
- Sawazaki M, Tomari S, Tsunekawa T, et al. Aggressive atrial volume reduction for bilateral giant atria improves respiratory function. Ann Thorac Surg 2013;95:1464-6. [Crossref] [PubMed]
- Marui A, Saji Y, Nishina T, et al. Impact of left atrial volume reduction concomitant with atrial fibrillation surgery on left atrial geometry and mechanical function. J Thorac Cardiovasc Surg 2008;135:1297-305. [Crossref] [PubMed]
- Scherer M, Therapidis P, Miskovic A, et al. Left atrial size reduction improves the sinus rhythm conversion rate after radiofrequency ablation for continuous atrial fibrillation in patients undergoing concomitant cardiac surgery. Thorac Cardiovasc Surg 2006;54:34-8. [Crossref] [PubMed]
- Matsumori M, Kawashima M, Aihara T, et al. Efficacy of left atrial plication for atrial functional mitral regurgitation. Gen Thorac Cardiovasc Surg 2021;69:458-65. [Crossref] [PubMed]
- Zheng SH, Sun YQ, Meng X, et al. Left atrial plication for left atrium associated with mitral valve disease. Zhonghua Wai Ke Za Zhi 2005;43:918-20. [PubMed]
- Arámbula-Garza E, Castillo-Martínez L, González-Islas D, et al. Association of cardiac cachexia and atrial fibrillation in heart failure patients. Int J Cardiol 2016;223:863-6. [Crossref] [PubMed]
- Sairaku A, Nakano Y, Oda N, et al. Prediction of sinus node dysfunction in patients with long-standing persistent atrial fibrillation using the atrial fibrillatory cycle length. J Electrocardiol 2012;45:141-7. [Crossref] [PubMed]


