
Robotic approach for pediatric pulmonary resection: preliminary investigation and comparative study with thoracoscopic approach
Introduction
Congenital pulmonary airway malformation (CPAM) and intralobar pulmonary sequestration (IPS) with an estimated incidence of 1:10,000 are the most common lung anomalies observed in pediatric thoracic surgery (1,2). Lobectomy is the standard treatment for these conditions (3,4). With the progress of minimally invasive technology, thoracoscopic lobectomy has gained wider acceptance from both surgeons and parents/guardians of pediatric patients because of its cosmetical advantages, painlessness, and fast recovery. However, to overcome the limitations of conventional thoracoscopy, such as a 2D view field and rigid instruments, it is often necessary to employ adaptive port distribution, because, otherwise, the operation may become relatively difficult and it may even become difficult to complete it.
Currently, the robotic surgery system with an articulation instrument affording a 3D vision is widely used in the pediatric urologic and general surgery (5). Robotic lobectomy has been gaining increasing acceptance since Morgan and Ashton in 2003 independently carried out their landmark robotic lobectomy for general thoracic surgery in adult patients in the United States and Europe, respectively (6,7). By 2015, more than 8,600 cases of robotic lobectomy have been reported worldwide (8). However, robot-assisted thoracic lobectomy or segmentectomy have been performed in very few pediatric cases. In 2008, Meehan was the first to use a robot in pediatric lobectomy. He reported four cases of robotic lobectomy and two of segmentectomy, wherein a robotic seal device was used to dissect the lung parenchyma and seal vessels (9). In 2013, Cundy reviewed the first decade of robotic surgery in children and reported 2,393 operations on 1,840 pediatric patients, including 18 cases of lobectomy and 3 cases of segmentectomy; however, they did not illustrate technical details regarding the surgery (8). Comparative studies between robotic and conventional thoracoscopic surgeries for diaphragmatic hernia, esophageal atresia, and mediastinal cysts in children (10) and for lung resection in adults (11-13) have been reported. However, there is still a lack of sufficient evidence to indicate whether robot-assisted pulmonary resection is superior to the conventional thoracoscopic approach in pediatric patients.
The da Vinci robotic surgical system was introduced in our hospital in 2015. With the extensive experience in thoracoscopic lobectomy, along with robotic manipulation skills acquired through conducting numerous pediatric general surgeries and mediastinal tumors (14,15), we launched the first robotic pediatric lobectomy in China mainland in 2018 (16). This study aims to share our initial experience with this procedure. It also conducts a comparative study with the thoracoscopic approach. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-526/rc).
Methods
Study design
This is a retrospective study conducted on consecutive patients who underwent lobectomy or segmentectomy at our institution from January 2017 to January 2021 using either a robotic surgery system or a conventional thoracoscope. Surgery was indicated for CPAM and IPS. The decision to use the robotic surgery system or the conventional thoracoscope was made by the guardian/parent(s) of the patients after the doctor informed them the main characteristics of the two approaches in terms of the incisions made, instruments used, procedure, operation time, and cost (Table 1). Patients with a history of empyema, lung abscess, or more than three episodes of pneumonia were excluded. All the operations were performed by the same surgeon and the same assistant. The data of the patients who successfully underwent the complete procedure without conversion were retrieved and analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. [2022]LSZ(0589)). Written informed consent was obtained from all patients.
Table 1
| Items | Robotic | Thoracoscopic |
|---|---|---|
| Incisions | One 12-mm, two 8-mm and one 5-mm | One 10-mm and two 5-mm, or three 5-mm incisions |
| Instruments | Robotic surgery has greater magnification, clearer images and more flexible and stable instruments | |
| Procedure | Almost the same | |
| Operation time | Robotic surgery had slightly longer total operation time, but the actual operative time was about the same | |
| Costs | Robotic surgery costs about $2,500 more than the thoracoscopic | |
All the cases were divided into two groups based on the surgical approach used: robotic pulmonary resection (RPR) group and thoracoscopic pulmonary resection (TPR) group. Data on general information (age, sex, and weight), surgical features (total operative time, pure operative time, and conversion), body temperature (>38 ℃), and postoperative complications (pneumothorax, hemothorax, air leak, atelectasis, or residual lesions) of the two groups were collected and compared (Table 2). The postoperative complications are listed in Table 3 (17-23). The total operative time refers to the time from the opening of the incision to its closure, which includes pure operative time, docking time, undocking time, and instrument-replacement time in the RPR group and pure operative time and instrument-replacement time in the TPR group.
Table 2
| Variable | RPR group (n=29) | TPR group (n=42) | P value |
|---|---|---|---|
| Sexa | NA | ||
| Male | 16 | 24 | |
| Female | 13 | 18 | |
| Age (month)b | 68.1±47.2 [6–156; 81] | 64.6±45.1 [2–149; 72] | 0.896 |
| Weight (kg)b | 23.0±14.6 [8–81; 25] | 21.9±12.1 [6–56; 23] | 0.831 |
| Proceduresa | NA | ||
| RUL | 3 | 5 | |
| LUL | 2 | 1 | |
| RLL | 10 | 16 | |
| LLL | 8 | 13 | |
| RML | 1 | 1 | |
| RS5 | 1 | 0 | |
| RS6 | 1 | 1 | |
| LS6 | 1 | 1 | |
| RS7-10 | 2 | 2 | |
| RS9-10 | 0 | 2 | |
| Pathology resultsa | |||
| CPAMs | 21 | 29 | |
| IPS | 8 | 13 | |
| Operative results | |||
| Operative time (min)c | 148.3±36.8 [80–227] | 118.3±22.5 [90–160] | <0.001 |
| Pure operative time (min)c | 103.9±28.5 [42–197] | 111.4±18.3 [80–155] | 0.045 |
| Docking time (min)c | 18.9±3.0 [10–20] | – | NA |
| Conversiona | 1 (1/29) | 2 (2/42) | 0.505 |
| Postoperative results | |||
| Postoperative fever (>38 ℃)a | 2/29 | 11/42 | 0.039 |
| Air leaka | 0 | 1 | |
| Drainage time (day)c | 1.9±0.9 [1–5] | 1.8±0.7 [1–7] | 0.842 |
| Hospital stay (day)c | 3.5±1.2 [2–6] | 3.8±1.5 [2–9] | 0.603 |
| Further results | |||
| Scard | 1.6±1.1 | 1.4±0.6 | 0.090 |
RPR, robotic pulmonary resection; TPR, thoracoscopic pulmonary resection; a, number; b, mean ± standard deviation [range; median]; c, mean ± standard deviation [range]; d, mean ± standard deviation. NA, not applicable; RUL, right upper lobectomy; LUL, left upper lobectomy; RLL, right lower lobectomy; LLL, left lower lobectomy; RML, right middle lobectomy; LS6, left dorsal segmentectomy; RS6, right dorsal segmentectomy; RS5, right lateral segmentectomy; RS7-10, right basilar segmentectomy; RS9-10, right lateral and posterior basilar segmentectomy; CPAM, congenital pulmonary airway malformation; IPS, intralobar pulmonary sequestration.
Table 3
| Complications | Definition |
|---|---|
| Pneumothorax | Defined as air present in the pleural space. Diagnosed by chest radiograph following chest tube removal. The thorax space is compressed by more than 20% |
| Hemothorax | Bloody fluid drained through chest tube for more than 3 days |
| Air leak | Different amount of air bubbles present on coughs or spontaneous respiration. The air leak lasting beyond postoperative day 5 is defined as persistent air leak |
| Atelectasis | Diagnosed by chest radiograph following chest tube removal |
| Residual lesions | Diagnosed by CT scan at 3 months’ follow-up in clinics |
Pain control
During the operation, 1 mL of 0.3% ropivacaine was infused at each incision site. One dose of tramadol was administered on the operative night. Then, ibuprofen was provided orally as needed.
NASA-TLX questionnaire
The original National Aeronautics and Space Administration Task Load Index (NASA-TLX) (24), including the workload items comprising mental demand (MD), physical demand (PD), temporal demand (TD), frustration (Fr), effort (Ef), and performance (Pe), was employed in this study. Each item was marked on a 20-point scale (low =1, high =20). One NASA-TLX questionnaire was completed by the chief surgeon within 24 h of each procedure. The NASA-TLX score was defined as the raw sum of six subscale ratings (5= low, 100= high).
Surgical techniques
The patients were placed in the lateral decubitus position with the affected side up. CO2 insufflation was used when single-lung ventilation could not be achieved with a pressure of 4–6 mmHg at a flow rate of 1–2 L/min. The specimen was brought out through the enlarged camera port incision. A chest tube, placed through one of the middle axillary port incisions, was left in all cases. For wound closure, fascia closure was performed first, after which a tension-reduction suture was used in the subcutaneous layer. Then, the skin was bonded with skin glue instead of using intermittent sutures.
TPR
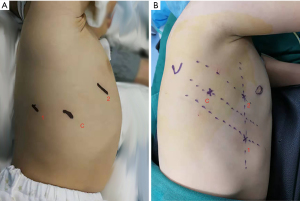
Three or four 5- or 3-mm ports were used for the procedure. For the upper or middle lobe lesions, the 5th–8th–9th intercostal spaces from front to back port distribution were used (Figure 1A), while for the lower lobe, the 5th intercostal space on the middle axillary line and the 4th and 6th or 7th intercostal spaces on the anterior axillary line port distribution (Figure 1B) were used. Lung parenchyma was treated by Ensure (Ethicon, Cincinnati, OH, USA). The vessels and adjacent tissues were mobilized by a cautery hook. The vessels and bronchus were sealed by Hem-o-Lock (Teleflex Medical, Research Triangle Park, NC, USA) or ECHELON FLEX™ Powered Plus Articulating Endoscopic Linear Cutters (Ethicon Endo-Surgery, Inc., Chihuahua, Mexico). One 5-mm trocar might be changed to a 12-mm one if a linear stapler was applied.
RPR
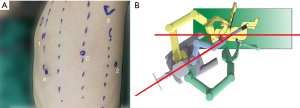
A standard da Vinci Surgical Si System (Intuitive Surgical, California, USA) was used with one camera arm and two instrument arms for all procedures. The ports were distributed at the 5th, 8th, and 9th intercostal spaces from the front to the back (Figure 2A). After positioning the ports, the robot cart was entered from over the patient’s back 30° off the longitudinal access of the patient (Figure 2B) and docked with the ports. Then, a 5-mm accessary port was used at the 8th intercostal space between the anterior and middle axillary line (this port was enlarged to a 12-mm one if a stapler was applied). The lung parenchyma was treated using Harmonic Scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) and the vessels and adjacent tissues were mobilized using a cautery hook by the surgeon at the cart. The vessels and bronchus were sealed (Figure 3A-3C) by Hem-o-Lock (Teleflex Medical, Research Triangle Park, NC) or ECHELON FLEX™ Powered Plus Articulating Endoscopic Linear Cutters (Ethicon Endo-Surgery, Inc., Chihuahua, Mexico) by an assistant surgeon at the operating table.
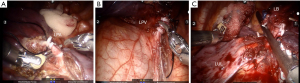
Follow-up
All the patients were followed-up in the outpatient department at 1, 3, 6, 12, and 24 months after discharge. Their scar was assessed using the Scar Cosmesis Assessment and Rating (SCAR) Scale (25) at the three-month postoperative interview by the same two nurses. The mean value was used to eliminate variability from the results. Chest X-ray was performed at every follow-up routine test and a CT scan was conducted six months after the operation.
Statistical analysis
The data were analyzed using the IBM SPSS Statistics 22.0 software (SPSS Inc., Illinois, USA). Age, weight, operative time, length of drainage, and length of hospital stay were normally distributed by the Shapiro Wilk test (26). All quantitative data were expressed by the mean ± standard deviation (range). Comparisons between the two groups were performed using Pearson’s Chi-square test for categorical variables, while Student’s t-test or Mann-Whitney U test was employed for continuous variables. Correlation between the total workload and sub-items was tested using Pearson’s correlation test. A P value of <0.05 was considered statistically significant.
Results
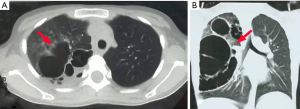
The cohort used in this study comprised 74 patients. One case in the RPR group and two in the TPR group were converted to thoracotomy, while the rest were successfully completed. The RPR group had 29 cases (lobectomy =24 cases; segmental resection =5) and the TPR group had 42 cases (lobectomy =36; segmental resection =6). Patients’ demographics and procedures are listed in Table 2. The groups had patients with similar age and weight. The RPR group had the youngest patient of age 6 months and weight 8 kg. The causes for conversion were hemostatic clip falling off the distal stump of the inferior pulmonary vein (IPV) in the RPR group and dissection difficulty owing to heavy inflammatory adhesion in the TPR group. The total operative time for RPR was 148.3±36.8 min, while it was 118.3±22.5 min for TPR (P<0.001). After removing the instrument-replacement time and docking time, the pure operative time became 103.9±28.5 min for RPR and 111.4±18.3 min for TPR (P=0.045). Fewer patients reported fever postoperatively (2/29 in the RPR group vs. 11/42 in the TPR group; P=0.039). The time for chest-tube drainage was 1.9±0.9 d in the RPR group and 1.8±0.7 d in the TPR group (P=0.842). The duration of hospital stay was 3.5±1.2 d in the RPR group and 3.8±1.5 d in the TPR group (P=0.603). Note that postoperative air leak was observed in an 11-year-old male patient of the TPR group. This patient had type I CPAM in his right upper lobe (Figure 4). His upper lobe bronchus was fragile and closed with PSEE45A-loaded GST45B (Ethicon Endo-Surgery, Inc., Chihuahua, Mexico). Air leakage occurred on the third day of operation after a sudden burst of severe cough. A continuous formation of bubbles was observed in a water-sealed bottle when the boy took deep breaths. However, only occasional bubbles formed with normal breathing. Air leakage stopped after a week of prolonged drainage and antibiotic therapy.
The surgeon-workload scores (NASA-TLX) were 55.2±4.7 in the RPR group and 62.9±6.0 in the TPR group (P<0.01). Pearson’s correlation test showed that the scores for the physical demands (r=0.904), frustration (r=0.898), and performance (r=0.855) correlated well with the total workload (Table 4).
Table 4
| Group | Mental demand 1–20 | Physical demand 1–20 | Temporal demand 1–20 | Frustration 1–20 | Effort 1–20 | Performance 1–20 | Total 5–100 |
|---|---|---|---|---|---|---|---|
| Proceduresa | |||||||
| RPR (N=29) | 13.0±1.7 | 10.7±1.3 | 12.5±1.4 | 10.1±2.2 | 13.9±0.6 | 6.0±1.1 | 55.2±4.7 |
| TPR (N=44) | 14.0±2.9 | 14.1±1.6 | 12.8±1.3 | 11.8±1.7 | 13.9±1.7 | 8.9±1.6 | 62.9±6.0 |
| t | 4.092 | 8.610 | 0.458 | 3.028 | -0.292 | 7.774 | 5.348 |
| P | <0.01 | <0.01 | 0.649 | 0.004 | 0.772 | <0.01 | <0.01 |
| Pearson’s r | 0.718 | 0.904 | 0.738 | 0.898 | 0.560 | 0.855 | 1 |
a, mean ± standard deviation. NASA-TLX, National Aeronautics and Space Administration Task Load Index; RPR, robotic pulmonary resection; TPR, thoracoscopic pulmonary resection.
The follow-up time of the two groups ranged from 6 to 54 months. No pneumothorax, hemothorax atelectasis, pneumonia, and residual lesions were reported by any of the patients after discharge. The scar scores were 1.6±1.1 and 1.4±0.6 for the RPR and TPR groups, respectively (P=0.090).
Discussion
Anatomic pulmonary resection in children by minimal invasive approaches is challenging because of the narrow thoracic space and small anatomical structure of children (27). Therefore, very few reports have discussed the technical details of anatomic pulmonary resection. In addition, few studies have compared the robotic and thoracoscopic approaches. Moreover, no consensus has yet been reached on the indication for segmental resection and lobectomy (3,28). In this study, we performed segmental resection if the lesion was confined to the lung segment without any severe infection and lobectomy if the lesion involved more than half the volume of the corresponding lobe. The study results revealed that robotic surgery, like the thoracoscopic approach, could safely and effectively complete the lobectomy and segmentectomy in children weighing >8 kg. Though the total operative time prolonged because of the complexity of the robotic system, the doctor’s sense of fatigue significantly reduced. Additionally, the pure operative time used for anatomical dissection shortened and the blood loss during operation was also less, indicating improved operative efficiency and accuracy of the robotic approach.
Robot thoracic surgery involves three steps: docking, operation, and undocking. Therefore, this procedure is more complex than conventional thoracoscopic procedures, and the total operative time is also relatively longer. The operation time that we observed in our study was similar to that of Meehan’s report (9) (66–280 min, average 160 min). However, when compared to thoracoscopic procedures from larger series, such as Rothenberg (27) (35–240 min, mean 115 min) and Lieber (29) (85–237 min, mean 146 min), our operative time was not very long. A comparison of the results obtained for the TPR group showed that the robotic procedure was more time-consuming than the thoracoscopic procedure. However, if we remove the time taken for replacing the instruments and scrubbing the camera from both groups, the pure operation time becomes shorter in the RPR group than that in the TPR group. Given the use of advantageous techniques in the robotic approach, such as an upgraded visual system and hand tremor filtration and articulating instruments, the robotic surgery system could facilitate the dissection process and afford an improved operative efficiency. In terms of the operation time alone, no learning curve was needed for robotic pediatric pulmonary resection (30), possibly because we have sufficient experience regarding robotic pulmonary resection, both through thoracoscopic and robotic general surgeries.
In addition, the filtration of action tremor diminishing the impact caused by the beating of the heart and large blood vessels made the dissection and anatomic mobilization more stable and accurate (31), which may explain why fewer patients in the RPR group reported fever postoperatively. Moreover, the articulating instruments of the robotic system allowed anatomic mobilization of the structures in situ with low tension, rather than retracting the parenchyma to a certain angle and relatively higher tension to facilitate the dissection in a conventional thoracoscopic procedure, in which the parenchyma gets sometimes torn.
However, trocar placement is of considerable importance while articulating instruments for the robotic system, especially for younger children. Two aspects should be considered here: how to distribute the four trocars on the small chest of younger children to avoid instrument collision and how to enter the 12-mm trocar through their tiny intercostal space. To overcome the first difficulty, we shifted the trocar for the left hand to as back as the scapula line and as inferior as the edge of the diaphragm, making full use of the limited thoracic area. This distribution method allowed us to complete the robotic lobectomy for patients as young as 6 months old (weighing 8 kg) using the da Vinci Si surgery system without any instrument collision. For the second aspect, we applied the STEP procedure described previously (32).
The main procedures of the robotic anatomic lobectomy were consistent with that of the classical thoracoscopic surgery (4). For lobectomy, in general, we mobilized the pulmonary vein before mobilizing the pulmonary artery, and then sealed the artery and the vein successively. We finally mobilized and sealed the bronchus. In one case of the RPR group, the IPV was ligated and cut-off before treating the artery. Unfortunately, the hemostatic clip fell off from the distal stump of the IPV when the lower lobe was rotated, resulting in uncontrollable bleeding. Consequently, the operation had to be converted. In retrospect, if the artery had been treated, even if the hemostatic clip fell off, it could be handled calmly and would not cause an inevitable consequence.
Advanced surgical instruments and techniques are being continuously developed to achieve better patient care. However, little attention is being given to the mental and physical health of surgeons that arise from the use of new technologies (33,34). In conventional minimal invasive surgery for children, the operating area is usually crowded, especially in case of single-site surgeries. As a result, surgeons tend to adopt a poor posture to free up some operating space, which makes them prone to fatigue (35). In the current study, the NASA-TLX index (36), which is widely used for rating the work burden of medical staff, was used (24,37). The total workload score was significantly higher in the TPR group than that in the RPR group, mainly owing to grueling physical demands and the need to maintain the top performance in every case. In contrast, the ergonomic design of the robotic surgery system with advanced techniques helps maintain the surgeon their posture, which significantly reduces the physical strain and improves their performance (34,38,39).
Nevertheless, the robotic surgery system still needs improvements. For example, the haptic feedback of the robotic surgical system is even less than that in conventional thoracoscopy. In addition, the surgical instruments are too large to be used in smaller children. Consequently, the incisions were larger in the RPR group; however, the scar score showed no significant difference when assessed three months after the operation. We will not pursue robotic lobectomy and segmental resection in patients younger than 6 months or lighter than 8 kg (9). Moreover, at present, in many countries, there are additional charges for robotic surgery, as it involves the use of machines and instruments, which limits the popularity of robotic surgery to certain extent.
The study has several limitations as well. First, it was a retrospective study. The sample volume was relatively small because of the low incidence of the condition investigated. The minimum weight of the patients we successfully operated on was 8 kg; however, 8 kg is not a statistical weight cut-off value for robotic surgery. Second, the cases were not selected following the principle of randomness. In addition, the comparison parameters such as the pain score and total fee were not considered.
However, this is the first comparative study on robotic and thoracoscopic approaches in pediatric pulmonary resection. The study results show that robotic pulmonary resection is a safe and feasible treatment protocol for pediatric patients older than 6 months or heavier than 8 kg. Although the process is complex, the advanced techniques of the robotic surgery system, such as ergonomic design, 3D vision technique, and the articulating instrument, significantly reduce the workload of surgeons and achieve improved operative efficiency, which at least in theory, make surgeons more calm and comfortable while performing complex operations, which, in turn, benefits both surgeons and patients.
Acknowledgments
The abstract of this work was accepted for oral presentation at annual meeting of Chinese Society of Pediatric Surgery in 2021 in Changsha, which was delayed to 2022.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-526/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-526/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-526/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-526/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. [2022]LSZ(0589)). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Juan AT. Congenital Cystic Adenomatoid Malformations. In: Lima M. editor. Pediatric Thoracic Surgery. Springer, 2013:241-51.
- Lau CT, Kan A, Shek N, et al. Is congenital pulmonary airway malformation really a rare disease? Result of a prospective registry with universal antenatal screening program. Pediatr Surg Int 2017;33:105-8. [Crossref] [PubMed]
- Wong KKY, Flake AW, Tibboel D, et al. Congenital pulmonary airway malformation: advances and controversies. Lancet Child Adolesc Health 2018;2:290-7. [Crossref] [PubMed]
- Koga H, Nakamura H, Murakami H, et al. Thoracoscopic Pulmonary Lobectomy for Densely Fused Pulmonary Lobes in Children with Congenital Pulmonary Airway Malformation: Technical Tips. J Laparoendosc Adv Surg Tech A 2019;29:415-9. [Crossref] [PubMed]
- Cundy TP, Shetty K, Clark J, et al. The first decade of robotic surgery in children. J Pediatr Surg 2013;48:858-65. [Crossref] [PubMed]
- Ashton RC Jr, Connery CP, Swistel DG, et al. Robot-assisted lobectomy. J Thorac Cardiovasc Surg 2003;126:292-3. [Crossref] [PubMed]
- Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883-7; discussion 887. [Crossref] [PubMed]
- Linsky P, Wei B. Robotic lobectomy. J Vis Surg 2017;3:132. [Crossref] [PubMed]
- Meehan JJ, Phearman L, Sandler A. Robotic pulmonary resections in children: series report and introduction of a new robotic instrument. J Laparoendosc Adv Surg Tech A 2008;18:293-5. [Crossref] [PubMed]
- Ballouhey Q, Villemagne T, Cros J, et al. Assessment of paediatric thoracic robotic surgery. Interact Cardiovasc Thorac Surg 2015;20:300-3. [Crossref] [PubMed]
- Merritt RE, Kneuertz PJ, D'Souza DM. Successful Transition to Robotic-Assisted Lobectomy With Previous Proficiency in Thoracoscopic Lobectomy. Innovations (Phila) 2019;14:263-71. [Crossref] [PubMed]
- Sarkaria IS, Gorrepati ML, Mehendale S, et al. Lobectomy in octogenarians: real world outcomes for robotic-assisted, video-assisted thoracoscopic, and open approaches. J Thorac Dis 2019;11:2420-30. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Zhang X, Tang S, Cao G, et al. Robotic-assisted Soave Pull-through for Hirschsprung's Disease in Infants. Chin J Min Inv Surg 2016;16:165-7, 84.
- Chi SQ, Cao GQ, Li S, et al. Outcomes in robotic versus laparoscopic-assisted choledochal cyst excision and hepaticojejunostomy in children. Surg Endosc 2021;35:5009-14. [Crossref] [PubMed]
- Li S, Tang S, Cao G, et al. Preliminary experience of thoracoscopic pulmonary lobectomy using da Vinci robotic-system in children. Journal of Clinical Pediatric 2020;19:619-21, 47.
- Hallifax R, Janssen JP. Pneumothorax-Time for New Guidelines? Semin Respir Crit Care Med 2019;40:314-22. [Crossref] [PubMed]
- LaGrasta C, McLellan M, Connor J. Clinical descriptors of pneumothorax following chest tube removal in paediatric cardiac surgery. Cardiol Young 2021;31:121-4. [Crossref] [PubMed]
- Miyahara S, Iwasaki A. Diagnosis and Treatment of Hemothorax. Kyobu Geka 2015;68:650-3. [PubMed]
- Bronstein ME, Koo DC, Weigel TL. Management of air leaks post-surgical lung resection. Ann Transl Med 2019;7:361. [Crossref] [PubMed]
- Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg 2010;89:1327-35. [Crossref] [PubMed]
- Lagier D, Zeng C, Fernandez-Bustamante A, et al. Perioperative Pulmonary Atelectasis: Part II. Clinical Implications. Anesthesiology 2022;136:206-36. [Crossref] [PubMed]
- Liu J, Chen SW, Liu F, et al. The diagnosis of neonatal pulmonary atelectasis using lung ultrasonography. Chest 2015;147:1013-9. [Crossref] [PubMed]
- Tubbs-Cooley HL, Mara CA, Carle AC, et al. The NASA Task Load Index as a measure of overall workload among neonatal, paediatric and adult intensive care nurses. Intensive Crit Care Nurs 2018;46:64-9. [Crossref] [PubMed]
- Kantor J. The SCAR (Scar Cosmesis Assessment and Rating) scale: development and validation of a new outcome measure for postoperative scar assessment. Br J Dermatol 2016;175:1394-6. [Crossref] [PubMed]
- Wei J. The adoption of repeated measurement of variance analysis and Shapiro-Wilk test. Front Med 2022;16:659-60. [Crossref] [PubMed]
- Rothenberg SS, Middlesworth W, Kadennhe-Chiweshe A, et al. Two decades of experience with thoracoscopic lobectomy in infants and children: standardizing techniques for advanced thoracoscopic surgery. J Laparoendosc Adv Surg Tech A 2015;25:423-8. [Crossref] [PubMed]
- Lee S, Kim DH, Lee SK. Efficacy of segmental resection in patients with prenatally diagnosed congenital lung malformations. Interact Cardiovasc Thorac Surg 2017;24:425-9. [PubMed]
- Lieber J, Urla CI, Baden W, et al. Experiences and challenges of thorcoscopic lung surgery in the pediatric age group. Int J Surg 2015;23:169-75. [Crossref] [PubMed]
- Song G, Sun X, Miao S, et al. Learning curve for robot-assisted lobectomy of lung cancer. J Thorac Dis 2019;11:2431-7. [Crossref] [PubMed]
- Durand M, Musleh L, Vatta F, et al. Robotic lobectomy in children with severe bronchiectasis: A worthwhile new technology. J Pediatr Surg 2021;56:1606-10. [Crossref] [PubMed]
- Li S, Cao G, Zhou R, et al. Feasible techniques in robotic thoracoscopic repair of congenital esophageal atresia: case report and literature review. Surg Case Rep 2021;7:142. [Crossref] [PubMed]
- Gofrit ON, Mikahail AA, Zorn KC, et al. Surgeons' perceptions and injuries during and after urologic laparoscopic surgery. Urology 2008;71:404-7. [Crossref] [PubMed]
- Park A, Lee G, Seagull FJ, et al. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg 2010;210:306-13. [Crossref] [PubMed]
- Rasool S, Singh M, Jain S, et al. Comparison of open, laparoscopic and robot-assisted pyeloplasty for pelviureteric junction obstruction in adult patients. J Robot Surg 2020;14:325-9. [Crossref] [PubMed]
- Hart S. Nasa-Task Load Index (Nasa-TLX): 20 Years Later. Human Factors and Ergonomics Society Annual Meeting Proceedings 2006:904-8.
- Lowndes BR, Forsyth KL, Blocker RC, et al. NASA-TLX Assessment of Surgeon Workload Variation Across Specialties. Ann Surg 2020;271:686-92. [Crossref] [PubMed]
- Butler KA, Kapetanakis VE, Smith BE, et al. Surgeon fatigue and postural stability: is robotic better than laparoscopic surgery? J Laparoendosc Adv Surg Tech A 2013;23:343-6. [Crossref] [PubMed]
- Stefanidis D, Wang F, Korndorffer JR Jr, et al. Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 2010;24:377-82. [Crossref] [PubMed]


