
Clinical impact of SUVmax of interstitial lesions in lung cancer patients with interstitial lung disease who underwent pulmonary resection
Introduction
Lung cancer (LC) is a common complication in patients with interstitial lung disease (ILD), with an incidence ranging from 13% to 20.4% (1-3). Early-stage LC patients with existing ILD may undergo pulmonary resection in case of preserved pulmonary function. Acute exacerbation of ILD (AE-ILD) can often lead to acute respiratory failure, resulting in a life-threatening complication in LC patients with ILD who underwent surgery. In previous studies, the incidence and mortality rate of postoperative AE-ILD (pAE-ILD) are reported as 7.4–22.9% and 33.3–100%, respectively (4-6). Thus, the prediction of pAE-ILD is important in LC patients with ILD undergoing pulmonary resection to guide the surgical management and avoid this complication.
Numerous studies have reported the predictive factors for pAE-ILD, such as elevated lactate dehydrogenase (LDH) level, elevated Krebs von den Lungen-6 (KL-6), decreased % vital capacity (VC), and fibrosis grade on high-resolution computed tomography in LC patients with ILD (5,7,8). Recently, Sato et al. reported a risk scoring system for the prediction of pAE-ILD in LC patients with ILD who underwent surgery, which consists of seven clinical factors: AE history, surgical procedure, usual interstitial pneumonia (UIP) pattern on CT, male sex, use of corticosteroid prior to surgery, serum KL-6 level, and %VC (4,9). Moreover, in a previous study, we found that the maximum standardized uptake value (SUVmax) of contralateral interstitial lesions on fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) images was a significant predictive factor for the development of AE-ILD in LC patients with ILD who received chemotherapy (10). However, it is unclear whether the SUVmax of contralateral interstitial lesions predicts pAE-ILD in LC patients with ILD who underwent pulmonary resection, as research is limited in this area.
Herein, we examined the correlation between SUVmax of the contralateral interstitial lesions and ILD parameters to clarify whether SUVmax was a predictive factor for pAE-ILD in LC patients with ILD who underwent pulmonary resection. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-604/rc).
Methods
Patients
We recruited 123 LC consecutive patients with ILD who underwent 18F-FDG PET/CT prior to surgery and surgical pulmonary resection at our institute between August 2010 and April 2019. We excluded one patient who underwent salvage surgery and five patients in whom we were unable to estimate the SUVmax since the 18F-FDG PET/CT examination was performed at another institution. The remaining 117 patients were enrolled in this retrospective study which was designed by investigator-initiation (Figure S1). Their baseline demographic and clinical parameters before lung resection were obtained from medical records, and the following parameters being included: age, sex, Eastern Cooperative Oncology Group (ECOG) performance Status (PS) (11), smoking status, medical history of ILD, histology, pathological stage, laboratory test results, pulmonary function, and surgical procedure type. The ILD-Gender-Age-Physiology (GAP) score was calculated based on the following five variables: ILD pattern, sex, age, forced vital capacity (FVC), and diffusing capacity for carbon monoxide (DLco) (12). The pathological stage was classified using the eighth edition of the TNM classification. The risk score for predicting the occurrence of AE-ILD after pulmonary resection was calculated based on seven variables: AE history, surgical procedures, UIP pattern on CT finding, male sex, use of corticosteroid prior to surgery, serum KL-6 level, and %VC (9). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Kumamoto University Hospital (IRB number: 2399) approved this research. The requirement of written informed consent from all patients was waived due to the retrospective nature of the study.
Diagnosis of ILD and AE-ILD
ILD was diagnosed based on evidence of diffuse parenchymal and interstitial lung abnormalities on chest CT and classified into two groups based on the UIP pattern using the international consensus criteria of the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association. UIP was identified as the presence of the following CT findings: opacities with honeycombing with/without traction bronchiectasis with subpleural and basal predominance as well as in a heterogeneous distribution (13). AE-ILD was diagnosed, using the consensus statement of the same organizations above when the following clinical criteria were identified: subjective development of dyspnoea within 30 days, progressive hypoxemia due to impaired pulmonary gas exchange, new bilateral alveolar infiltrates regardless of the extent of the segment on CT findings, and the absence of the following conditions: infection, pneumothorax, pulmonary embolism, congestive heart failure, or worsening of lung tumours (14). pAE-ILD was diagnosed when the development of AE-ILD was identified within 30 days postoperatively. ILD and AE-ILD on all CT scans was evaluated by two independent experienced pulmonologists (KA and KS). CT findings were considered concordant when both pulmonologists reached the same result. When they reached different results, CT scans were re-examined, and the final findings were agreed upon by consensus between the two pulmonologists.
Acquisition and data analysis of 18F-FDG PET/CT imaging
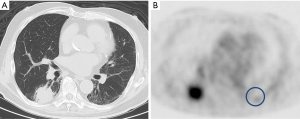
The SUVmax of interstitial lesions was measured as explained in our previous study protocol (10). In brief, after fasting for at least 5 h, a whole-body 18F-FDG PET/CT scan was performed in the patients, 60 min after receiving injections of approximately 185–296 MBq of 18F-FDG, using PET/CT scanners (Gemini-GXL or Gemini-TF, Philips Healthcare, Cleveland, OH, USA; Discovery-IQ, GE Healthcare, Milwaukee, WI, USA). The highest 18F-FDG uptake was measured by a circular region-of-interest with a fixed diameter of approximately 30 mm on PET images corresponding to the interstitial lesion region on CT and defined as SUVmax (Figure 1).
Statistical analysis
The clinical values were compared using Chi-square or Fisher’s exact tests and Mann-Whitney U tests. Quantitative variables were described as medians. Pearson’s correlation test was used to evaluate the correlation between the two values. The cumulative incidences of pAE-ILD and AE-ILD after 30 postoperative days were calculated using Gray’s test to consider the competing risks which prevented the occurrence of death in cases of AE-ILD by other causes, such as cancer-specific death. Two-tailed P value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 27.0; IBM, Armonk, NY, USA) and R statistical software (R Foundation for Statistical Computing, version. 4.0.2).
Results
Patient characteristics
The characteristics of the 117 patients who underwent 18F-FDG PET/CT scans prior to the surgery and lung resection are shown in Table 1. The median age of the patients was 71 (range, 39–86) years, and 103 (88.0%) patients were males. The ECOG PS was 0–1 in 114 patients (97.4%) and 112 (95.7%) patients were smokers. In the histological type, adenocarcinoma, squamous cell carcinoma, and small cell carcinoma accounted for 49 (41.9%), 47 (40.2%), and 9 (7.7%) patients, respectively. Most patients (115, 98.3%) were diagnosed with stage 0–III LC. UIP patterns in CT findings were observed in 27 (23.1%), while non-UIP patterns were seen in 90 (76.9%) patients. The median serum LDH and KL-6 levels, %FVC, %DLco, ILD-GAP score, and risk score for predicting AE after pulmonary resection were 202.5 U/L, 487.0 U/mL, 95.1%, 64.8%, 1.0, and 7.0, respectively. Lobectomy type of surgical resection was performed in 96 (82.1%) patients. Among patients with a preoperative history of ILD, by 9 (7.7%) and 21 (17.9%) patients used steroids and pirfenidone, respectively. The median SUVmax of the contralateral interstitial lesions was 1.61 (range, 0.82–3.70). The median follow-up time since the lung surgery was 786 days (range, 9–3,315 days).
Table 1
| Characteristics | Value (N=117) |
|---|---|
| Median age, years, (range) | 71 (39–86) |
| Sex (male/female), N (%) | 103/14 (88.0/12.0) |
| ECOG PS (0–1/2), N (%) | 114/3 (97.4/2.6) |
| Smoking status (yes/no), N (%) | 112/5 (95.7/4.3) |
| Histology (Ad/Sq/Sm/other), N (%) | 49/47/9/12 (41.9/40.2/7.7/10.3) |
| PStage (0/I/II/III/IV), N (%) | 4/73/17/21/2 (3.4/62.4/14.5/17.9/1.7) |
| ILD pattern (UIP/non-UIP), N (%) | 27/90 (23.1/76.9) |
| Median LDH, U/L (range) | 202.5 (132–350) |
| Median KL-6†, U/mL (range) | 487.0 (149–717) |
| Median %FVC, % (range) | 95.1 (55.2–136.3) |
| Median %DLco‡, % (range) | 64.8 (40.6–98.6) |
| Median ILD-GAP, score‡ (range) | 1.0 (−2–5) |
| Surgical procedure (lobectomy/segmentectomy/partial resection), N (%) | 96/2/19 (82.1/1.7/16.2) |
| Preoperative steroid use (yes/no), N (%) | 9 /108 (7.7/92.3) |
| Preoperative pirfenidone use (yes/no), N (%) | 21/96 (17.9/82.1) |
| History of AE (yes/no), N (%) | 0/117 (0.0/100.0) |
| Median RS for predicting AE after pulmonary resection† (range) | 7.0 (0–14) |
| Median SUVmax of contralateral interstitial lesion (range) | 1.61 (0.82–3.70) |
†, six patients were not evaluated for KL-6 and RS for predicting AE after pulmonary resection were not evaluated in six patients; ‡, nine patients were not evaluated for %DLco and ILD-GAP score. ECOG PS, Eastern Cooperative Oncology Group Performance Status; Ad, adenocarcinoma; Sq, squamous cell carcinoma; Sm, small cell carcinoma; pStage, pathological stage; ILD, interstitial lung disease; UIP, usual interstitial pneumonia; LDH, lactate dehydrogenase; KL-6, Krebs von den Lungen-6; %FVC, percent predicted forced vital capacity; %DLco, percent predicted diffusing capacity for carbon monoxide; GAP, Gender-Age-Physiology; AE, acute exacerbation; RS, risk score; SUVmax, maximum standard uptake value.
Correlation between the SUVmax of contralateral interstitial lesions and pAE-ILD
The pAE-ILD occurred in eight patients, among whom four patients died. The incidence and mortality rate of pAE-ILD were 6.8% and 50%, respectively.
Table 2 shows the absence of a significant association between SUVmax of contralateral interstitial lesions and pAE-ILD, while the proportion of UIP pattern, high level of KL-6, and high-risk score for predicting AE-ILD after pulmonary resection in the AE-ILD group were significantly higher than those in the non-AE-ILD group. Additionally, there was no significant association between the SUVmax of ipsilateral interstitial lesions and pAE-ILD (Figure S2).
Table 2
| Characteristics | Non-pAE-ILD (N=109) | pAE-ILD (N=8) | P value |
|---|---|---|---|
| Median age, years, (range) | 71.0 (39–86) | 77.5 (66–84) | 0.014 |
| Sex (male/female), N (%) | 95/14 (87.2/12.8) | 8/0 (100.0/0.0) | 0.349 |
| ECOG PS (0–1/2), N (%) | 106/3 (97.2/2.8) | 8/0 (100.0/0.0) | 0.807 |
| Smoking status (yes/no), N (%) | 104/5 (95.4/4.6) | 8/0 (100.0/0.0) | 0.697 |
| Histology (NSCLC/SCLC), N (%) | 100/9 (91.7/8.3) | 8/0 (100.0/0.0) | 0.516 |
| pStage (0–II/III–IV), N (%) | 89/20 (81.7/18.3) | 5/3 (62.5/37.5) | 0.189 |
| ILD (UIP/non-UIP), N (%) | 22/87 (20.2/79.8) | 5/3 (62.5/37.5) | 0.016 |
| Median LDH, U/L (range) | 203.0 (132–350) | 151.0 (149–228) | 0.059 |
| Median KL-6†, U/mL (range) | 461.0 (149–1,855) | 680.5 (409–2,019) | 0.080 |
| Median %FVC, % (range) | 96.2 (55.2–136.3) | 90.1 (72.9–125.2) | 0.315 |
| Median %DLco‡, % (range) | 64.8 (40.6–98.6) | 75.8 (48.0–104.1) | 0.897 |
| ILD-GAP score‡ (<1/≥1), N (%) | 19/81 (19.0/81.0) | 0/8 (0.0/100.0) | 0.201 |
| Surgical producer (lobectomy/limited surgery), N (%) | 88/21 (80.7/19.3) | 8/0 (100.0/0.0) | 0.194 |
| Preoperative steroid use (yes/no), N (%) | 9/100 (8.3/91.7) | 0/8 (0.0/100.0) | 0.516 |
| Preoperative pirfenidone use (yes/no), N (%) | 19/90 (17.4/82.6) | 2/6 (25.0/75.0) | 0.438 |
| RS for predicting AE after pulmonary resection† (0–10/11–14), N (%) | 85/18 (82.5/17.5) | 3/5 (37.5/62.5) | 0.009 |
| Median SUVmax of contralateral interstitial lesion (range) | 1.61 (0.82–3.70) | 1.62 (1.40–2.05) | 0.944 |
‡, nine patients were not evaluated for %DLco and ILD-GAP score; †, six patients were not evaluated for KL-6 and RS for predicting AE after pulmonary resection. AE, acute exacerbation; ILD, interstitial lung disease; LDH, lactate dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group Performance Status; pStage, pathological stage; UIP, usual interstitial pneumonia; LDH, lactate dehydrogenase; KL-6, Krebs von den Lungen-6; %FVC, percent predicted forced vital capacity; %DLco, percent predicted diffusing capacity for carbon monoxide; GAP, Gender-Age-Physiology; RS, risk score; SUVmax, maximum standard uptake value.
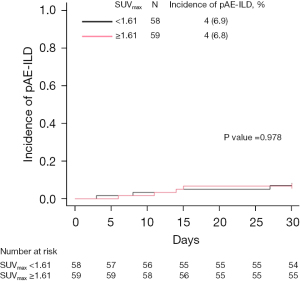
We defined the median SUVmax of contralateral interstitial lesions (1.61) as the cut-off value and divided the patients into high SUVmax (≥1.61) and low SUVmax (<1.61) groups. No significant differences in the incidence of pAE-ILD were observed between those two groups (6.8 vs. 6.9%; P=0.978, Gray’s test; Figure 2).
Correlation between SUVmax of contralateral interstitial lesions and severity and activity of ILD
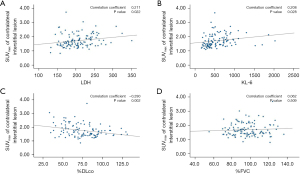
There were significant positive correlations between SUVmax of contralateral interstitial lesions and LDH (r=0.211, P=0.022), KL-6 (r=0.208, P=0.028), and negative correlation between SUVmax and %DLco (r=−0.290, P=0.002), except for %FVC (Figure 3). The median SUVmax in the UIP pattern group was significantly higher than that in the non-UIP pattern group (1.740 vs. 1.520, P=0.018; Figure S3).
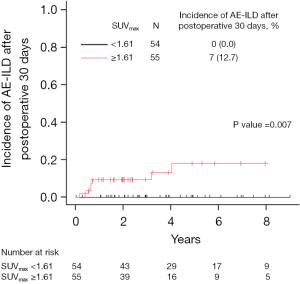
Moreover, we followed up with the patients without pAE-ILD within 30 days postoperatively and identified seven additional patients who developed AE-ILD. There was a significantly higher incidence of AE-ILD after 30 days postoperatively in the high SUVmax group than that in the low SUVmax group (incidence rate; 12.7% vs. 0.0%; P=0.007, Figure 4). Additionally, the median SUVmax of contralateral interstitial lesions in the AE-ILD group after 30 days postoperatively was significantly higher than that in the non-AE-ILD group (2.210 vs. 1.575, P=0.006; Figure S4).
Discussion
This study aimed to examine whether SUVmax (calculated from the 18F-FDG PET/CT images) of contralateral interstitial lesions was a predictive factor for pAE-ILD in LC patients with ILD who underwent pulmonary resection. Our results revealed that the SUVmax of contralateral interstitial lesions was not associated with the occurrence of pAE-ILD in these patients. Further analysis of the results for disease indicators of ILD revealed that SUVmax was significantly correlated with the UIP pattern, elevation of LDH and KL-6, decrease in %DLco, and a higher incidence of AE-ILD after 30 days postoperatively in LC patients with ILD. This result suggests that SUVmax of contralateral interstitial lesions may be a marker of the severity and activity of ILD, and supports the decision to perform surgery for LC in those patients, considering the risk of developing AE-ILD in the future.
The current study showed that the incidence and 90-day mortality of pAE-ILD in LC patients with ILD who underwent lung surgery was 6.8% and 50%, respectively, which is consistent with previous reports (4-6). pAE-ILD is a life-threatening complication; therefore, the prevention and management of pAE-ILD is crucial in LC patients with ILD undergoing pulmonary resection. Recently, the perioperative administration of an antifibrotic agent, pirfenidone, for LC surgery was reported to reduce the frequency of pAE-ILD in LC patients with idiopathic pulmonary fibrosis (IPF) (15-17), however, this study did not demonstrate the efficacy of pirfenidone in preventing pAE-ILD. The preventive effect of pirfenidone is currently being evaluated in a randomized phase III trial of pirfenidone vs. placebo (PIII-PEOPLE study, UMIN000029411) in LC patients with IPF. Although our study did not determine a significant difference, Sato et al. reported that limited surgery was associated with a lower frequency of pAE-ILD (4). In LC patients with ILD, limited surgery could be a useful method of preventing pAE-ILD.
There is limited evidence on the clinical significance of 18F-FDG uptake in interstitial lesions in PET/CT images in patients with ILD. Several previous reports demonstrated that the accumulation of 18F-FDG uptake on interstitial pneumonia lesions in ILD patients was negatively correlated with DLco and positively correlated with KL-6 (18,19). These factors are indicators of disease progression for interstitial pneumonia in ILD patients (20-22). Our results also showed a correlation between SUVmax of contralateral interstitial lesions and disease progression markers of ILD. Moreover, patients with ILDs, especially IPF, experience AE-ILD in the natural course of disease progression, and its incidence is approximately 4–20% of cases annually (14,23). Notably, the high accumulation of 18F-FDG uptake in interstitial lesions was significantly correlated with the incidence of AE-ILD after 30 days postoperatively in LC patients with ILD who received pulmonary surgery. Hence, the SUVmax of contralateral interstitial lesions might demonstrate disease severity and activity of ILD in LC patients with ILD.
The current study displayed a significant association between pAE-ILD and the UIP pattern, elevation of KL-6, and a high score in the risk score system for predicting AE after surgery proposed by Sato et al, which is consistent with previous studies (4,6,7,9). However, there were no significant differences in the correlation between the SUVmax of contralateral interstitial lesions and pAE-ILD, in contrast to previous similar reports (24,25), and our previous study on LC patients with ILD treated with chemotherapy (10). The reason for the discrepancy between our result and other similar previous reports is believed to be the higher 18F-FDG accumulation in interstitial lesions of ILD patients with the UIP pattern with honeycomb cysts than in ILD patients with the non-UIP pattern, as reported by Umeda et al. (26). Compared to previous reports, our study may have included more mild cases with fewer UIP patterns on chest CT and fewer instances of low pulmonary function such as higher %DLco. Additionally, two possible reasons may explain the discrepancy between our current and previous results: the different types of triggers for AE-ILD and the duration of observation. First, lung damage due to hyperoxia, single-lung ventilation, and hyperextension of the contralateral lung have been considered as contributors to the development of surgical procedure-related AE-ILD. Conversely, it has been proposed that chemotherapy-related AE-ILD is caused by direct cytotoxic lung injury and an immune-mediated mechanism (27,28). Additionally, the effect of these AE-ILD triggers occurs only once in case of surgery, but repetitively in case of chemotherapy. Thus, the various causes of AE-ILD triggers and the number of lung attacks by these triggers might have caused the differences in our two reports. Second, pAE-ILD in this study was detected during a short duration of observation (within 30 days after surgery), while in our previous study on LC patients with ILD treated with chemotherapy, AE-ILD was detected in long-follow-up periods (the occurrence of AE-ILD from the first administration of chemotherapy to the time of death regardless of the cause or the last follow-up). As mentioned above, patients with a high SUVmax of contralateral interstitial lesions more frequently experienced AE-ILD after 30 days postoperatively. Thus, the SUVmax of contralateral interstitial lesions might be useful for the prediction of AE-ILD in the natural disease course in patients with ILD.
The mechanism of 18F-FDG accumulation in interstitial lesions may involve fibroblast cells stimulated by transforming growth factor-β (TGF-β). Previous reports had demonstrated that the TGF-β-stimulated fibroblast cells have increased glucose transporter-1 at the cell membrane and active metabolism (29), which leads to increased 18F-FDG uptake in interstitial lesions on PET image (30,31). Additionally, TGF-β-stimulated fibroblast cells not only produce excessive collagen type-1, which contributes to the progression of pulmonary fibrosis (32), but also release cytokines, such as IL-6, and produce inflammatory changes in the lung microenvironment (33). Thus, the 18F-FDG accumulation in interstitial lesions might represent an increase in TGF-β-stimulated fibroblast cells, which might reflect a pulmonary microenvironment with increased fibrosis and inflammation.
This study had several limitations. First, this study was retrospectively conducted at a single institution among the Japanese population only. Moreover, there was a risk of bias because of the nature of the retrospective study. Second, the surgical procedure of lobectomy or limited surgery was decided by each surgeon while considering the patient’s pulmonary function and comorbidity, which may have affected our results. Third, several clinical variables such as LDH and KL-6 were elevated because of the activity of interstitial pneumonia and cancer progression (34-36). Therefore, the study results should be interpreted with caution, and large-scale, multi-centre, prospective studies are required to analyse these findings.
In conclusion, SUVmax of contralateral interstitial lesions was not a predictive factor for pAE-ILD. However, it was an indicator of disease severity and activity of ILD in LC patients with ILD who underwent pulmonary resection. Further studies are needed to evaluate the relationship between 18F-FDG accumulation in interstitial lesions and the pathophysiology of ILD.
Acknowledgments
The authors thank Ms. Tamura and Ms. Kasai, secretaries at the Department of Respiratory Medicine at Kumamoto University Hospital, for their assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-604/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-604/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-604/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-604/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Kumamoto University Hospital (IRB number: 2399) and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14:723-8. [Crossref] [PubMed]
- Kreuter M, Ehlers-Tenenbaum S, Schaaf M, et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis 2015;31:266-74. [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-1611.e3. [Crossref] [PubMed]
- Watanabe A, Miyajima M, Mishina T, et al. Surgical treatment for primary lung cancer combined with idiopathic pulmonary fibrosis. Gen Thorac Cardiovasc Surg 2013;61:254-61. [Crossref] [PubMed]
- Sugiura H, Takeda A, Hoshi T, et al. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann Thorac Surg 2012;93:937-43. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg 2012;14:146-50. [Crossref] [PubMed]
- Shintani Y, Ohta M, Iwasaki T, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg 2010;58:182-5. [Crossref] [PubMed]
- Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. [Crossref] [PubMed]
- Akaike K, Saruwatari K, Oda S, et al. Predictive value of 18F-FDG PET/CT for acute exacerbation of interstitial lung disease in patients with lung cancer and interstitial lung disease treated with chemotherapy. Int J Clin Oncol 2020;25:681-90. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723-8. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180071. [Crossref] [PubMed]
- Iwata T, Yoshida S, Fujiwara T, et al. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients With Idiopathic Pulmonary Fibrosis. Ann Thorac Surg 2016;102:1905-10. [Crossref] [PubMed]
- Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. [Crossref] [PubMed]
- Justet A, Laurent-Bellue A, Thabut G, et al. [18F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir Res 2017;18:74. [Crossref] [PubMed]
- Nobashi T, Kubo T, Nakamoto Y, et al. 18F-FDG Uptake in Less Affected Lung Field Provides Prognostic Stratification in Patients with Interstitial Lung Disease. J Nucl Med 2016;57:1899-904. [Crossref] [PubMed]
- Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773-9. [Crossref] [PubMed]
- Qiu M, Chen Y, Ye Q. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Clin Respir J 2018;12:1084-92. [Crossref] [PubMed]
- Yokoyama A, Kondo K, Nakajima M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006;11:164-8. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Yamamichi T, Shimada Y, Masuno R, et al. Association between F-18 fluorodeoxyglucose uptake of noncancerous lung area and acute exacerbation of interstitial pneumonia in patients with lung cancer after resection. J Thorac Cardiovasc Surg 2020;159:1111-1118.e2. [Crossref] [PubMed]
- Kagimoto A, Tsutani Y, Handa Y, et al. Prediction of Acute Exacerbation of Interstitial Pneumonia Using Visual Evaluation of PET. Ann Thorac Surg 2021;112:264-70. [Crossref] [PubMed]
- Umeda Y, Demura Y, Ishizaki T, et al. Dual-time-point 18F-FDG PET imaging for diagnosis of disease type and disease activity in patients with idiopathic interstitial pneumonia. Eur J Nucl Med Mol Imaging 2009;36:1121-30. [Crossref] [PubMed]
- Amundson WH, Racila E, Allen T, et al. Acute exacerbation of interstitial lung disease after procedures. Respir Med 2019;150:30-7. [Crossref] [PubMed]
- Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 2012;13:39. [Crossref] [PubMed]
- Xie N, Tan Z, Banerjee S, et al. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am J Respir Crit Care Med 2015;192:1462-74. [Crossref] [PubMed]
- Cho SJ, Moon JS, Nikahira K, et al. GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax 2020;75:227-36. [Crossref] [PubMed]
- Win T, Screaton NJ, Porter JC, et al. Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging 2018;45:806-15. [Crossref] [PubMed]
- Tsukui T, Ueha S, Abe J, et al. Qualitative rather than quantitative changes are hallmarks of fibroblasts in bleomycin-induced pulmonary fibrosis. Am J Pathol 2013;183:758-73. [Crossref] [PubMed]
- Epstein Shochet G, Brook E, Bardenstein-Wald B, et al. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res 2020;21:56. [Crossref] [PubMed]
- Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark 2016;16:415-23. [Crossref] [PubMed]
- Yuh YJ, Kim SR. actate Dehydrogenase (LDH) as a Tumor Marker for Non-small Cell Lung Cancer. Cancer Res Treat 2002;34:339-44. [Crossref] [PubMed]
- Miyazaki K, Kurishima K, Kagohashi K, et al. Serum KL-6 levels in lung cancer patients with or without interstitial lung disease. J Clin Lab Anal 2010;24:295-9. [Crossref] [PubMed]


