
Effects of colloid-based (hydroxyethylstarch 6% 130/0.42, gelafundin 4%) and crystalloid-based volume regimes in cardiac surgery: a retrospective analysis
Introduction
Crystalloids and colloids are given to maintain blood pressure and also to avoid blood product transfusions, in which even moderate bleeding is seen to be associated with an increased morbidity and mortality especially after cardiac surgery (1). Colloids have been used during cardiac surgery because of longer perseverance intravascularly compared to crystalloids (2,3). Especially hydroxyethylstarch (HES) had been used during cardiac surgery for decades, but in 2013, the European Medical Association (EMA) restricted its application which mandated changes in common used volume regimes (4). The restriction was based on higher mortality rates and acute kidney injury (AKI) in septic and critically ill patients receiving HES (5,6). But the use of last generation HES (6% 130/0.42 tetrastarch) has been discussed to be safe in comparison to other colloids or crystalloids especially for cardiac surgery (7). After the restriction for HES, the overall administration of colloids decreased of which gelatin (GELA) was increasingly used (8). But GELA is also critically discussed to be associated with need of higher noradrenalin dosages compared to tetrastarches (9), bleeding and the need for transfusion (3,10,11). The effectiveness and safety of GELA is currently being investigated in an ongoing multi-center study in critically ill patients (12).
In this retrospective study, we aimed to evaluate the effects of changed volume replacement management after the restriction of HES to GELA or to CRYS mono regimen. Because the EMA restricted HES due to death and kidney injury, we focused primarily on mortality and AKI and secondarily on blood loss, transfusions, hemodynamic support, and intensive care unit length of stay (ICU LOS). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-450/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of the University of Ulm (approval ID 50-17) and individual consent for this retrospective analysis was waived. Relying entirely upon existing data, the committee waived the need for retroactive study-specific patient consent. All consecutive patients who were treated at least by coronary artery bypass grafting (CABG) between January 2012 and December 2014 were assessed for eligibility to cover similar pre- and post-restriction periods in clinical practice. Patients with insufficient data coverage or various colloid administration were excluded. Study data were extracted from routine patient records and anonymized.
The observation period ended on postoperative day (POD) 3, except mortality which was additionally calculated at the end of intensive care and at discharge from hospital, as well as the length of ICU stay. Primary outcomes were mortality and AKI [according to the Acute Kidney Injury Network (AKIN) criteria] (13). Secondary outcomes were blood loss, transfusions, hemodynamic support (vasopressor administration per time normalized to body weight), mechanical ventilation, and the ICU LOS.
The determinants were type and volume of the administered volume replacement solutions. Three subgroups were selected for the present analysis based on the exposure:
- Patients who received just crystalloid but no colloid (CRYS);
- Patients who received gelafundin 4% (B.Braun, Gelafundin 4%, Melsungen), but no other colloid in addition to crystalloid (GELA); and
- Patients who received tetraspan 6% (B.Braun, Tetraspan® 6 % Infusionslösung, Melsungen, Germany), but no other colloid in addition to crystalloid (HES).
The standard of blood product administration was carried out according to the currently applicable cross-sectional guidelines of the German Medical Association for therapy with blood components and plasma derivatives with a focus on patient-specific threshold values based especially on a combined diagnosis of laboratory parameters, echocardiography and course of operation. The standard surgical procedure was also the administration of tranexamic acid in all operations with at least one coronary artery bypass.
Statistical analysis
Data were analyzed with the software R (R Foundation for Statistical Computing, Vienna, Austria) and add-on packages as specified below. Statistical tests and effects of factors were considered significant if P was less than 0.05.
Univariate analyses
Normalized data such as doses per body weight, cumulative data as the sum of administered substances or of excreted/drained volumes within the observation period, and other derived data were computed from the recorded data. Normality of continuous numeric data was assessed with the Shapiro-Wilk test and visualized by Q-Q plots. Non-normally distributed data are reported as median (first quartile to third quartile). Groups were compared with the Conover-Iman test using a Holm correction for post-hoc tests (conover.test package). Count data were evaluated with χ2 tests followed by pairwise two-proportion z-tests with Bonferroni correction for multiple testing. Ordinal data were assessed with cumulative link models and Tukey post-tests (ordinal and emmeans packages).
Longitudinal data
Time courses of continuous numeric data were fitted with linear mixed-effects models (nlme package) using time and group with an interaction term as fixed effects and patients as random intercepts. If there were significant effects of group or of the time: group interaction, data were further investigated for each level of time using the Conover-Iman test to identify pairwise differences between the groups. Colloid volumes were compared with the Wilcoxon rank sum test at each level of time as the CRYS patients did not receive colloids per definition. Longitudinal data of vasopressor and volume administration were characterized by increasing numbers of patients over time who did not receive further doses owing to improving hemodynamic stability or ICU discharge. Count data were derived from the dose data indicating the number of patients receiving a particular compound at each level of time. These data were evaluated as binary longitudinal data using generalized estimating equations (geepack package) to assess effects of group and time. Differences between groups at individual time points were identified by χ2 tests. The doses of patients still receiving them were then analyzed with mixed-effects models as described above.
Multiple regression analysis
The primary outcome parameters hospital survival, renal replacement therapy (RRT), and AKI were analyzed by calculating multiple logistic regression models (generalized linear regression using the logit link function). Selection of factors for the initial models was guided by clinical experience and by univariate correlation analyses. Correlations between numerical factors and the dichotomous outcome variables were assessed with point-biserial correlation coefficients. Correlations between dichotomous or categorical factors and the outcome variables were analyzed by χ2 tests. Potential confounders that were considered in regression analyses included the baseline data age, gender, acute coronary syndrome, chronic obstructive pulmonary disease (COPD), dialysis, ejection fraction, emergency surgery, hyperlipidemia, insulin-dependent and non-insulin-dependent diabetes mellitus, myocardial infarction within the last two weeks, previous myocardial infarction, heart failure according to the New York Heart Association (NYHA) classification, RRT, reoperation, stroke, serum creatinine, platelet count, antiplatelet therapy, and vasopressors. Perioperative factors included concomitant valve surgery, the number of anastomosed vessels during CABG, hematocrit, cumulative blood losses and vasopressor doses, as well as administration of blood products including packed red blood cells (PRBCs), platelet concentrates (PCs), prothrombin complex concentrate (PCC), and fresh frozen plasma (FFP). The initial models were simplified step-wise until a minimum of Akaike’s information criterion was reached. Regression results are reported as exponentiated estimates (adjusted odds ratios) calculated from the raw data and from scaled/centered continuous numeric data. Confounding was assessed by removing potential confounding factors from the minimal adequate model one at a time. Confounding was assumed to be present if the scaled/centered estimate β of the exposure variable changed by 10% or more when removing a factor.
Results
Patient population
A total of 1,540 patient datasets were included covering >98.8% of all CABG patients in the predefined time range (Table 1). Groups were comparable in all preoperative parameters except antiplatelet therapy (P=0.021).
Table 1
| Parameters | CRYS (n=205) | GELA (n=397) | HES (n=938) | P |
|---|---|---|---|---|
| Age (years) | 69 (61 to 76) | 70 (63 to 75) | 71 (62 to 76) | 0.75 |
| Weight (kg) | 84 (75 to 93) | 83 (71 to 92) | 81 (72 to 92) | 0.27 |
| BMI (kg/m2) | 28.0 (25.0 to 31.2) | 27.8 (24.9 to 30.5) | 27.4 (24.9 to 30.5) | 0.30 |
| Gender | 0.74 | |||
| Male | 160 (78%) | 319 (80%) | 738 (79%) | |
| Female | 45 (22%) | 78 (20%) | 200 (21%) | |
| EuroScore II | 1.82 (1.08 to 3.46) | 2.13 (1.17 to 3.97) | 2.06 (1.17 to 3.83) | 0.21 |
| SAPS II | 28.0 (24.0 to 32.5) | 29.0 (25.0 to 33.0) | 29.0 (24.0 to 33.0) | 0.85 |
| NYHA | 0.17 | |||
| I | 18 (9%) | 21 (5%) | 44 (5%) | |
| II | 51 (25%) | 114 (29%) | 246 (26%) | |
| III | 117 (57%) | 237 (60%) | 551 (59%) | |
| IV | 18 (9%) | 23 (6%) | 90 (10%) | |
| EF | 0.53 | |||
| >50% | 145 (71%) | 262 (66%) | 651 (68%) | |
| 31% to 50% | 52 (25%) | 89 (22%) | 207 (22%) | |
| 21% to 30% | 5 (2%) | 23 (6%) | 54 (6%) | |
| 20% and less | 1 (0%) | 8 (2%) | 24 (3%) | |
| Emergency | 64 (32%) | 128 (32%) | 273 (29%) | 0.09 |
| Dialysis prior | 6 (3%) | 3 (1%) | 15 (2%) | 0.12 |
| Antiplatelet therapy prior | 180 (88%) | 346 (87%) | 771 (82%) | 0.021 |
| Marcumar prior | 10 (5%) | 21 (5%) | 56 (6%) | 0.36 |
| Vasopressors prior | 1 (0%) | 7 (2%) | 14 (1%) | 0.19 |
| Hypertension | 191 (93%) | 365 (92%) | 848 (90%) | 0.46 |
| Diabetes mellitus | 67 (33%) | 137 (35%) | 281 (30%) | 0.24 |
| Hemoglobin (g∙dL−1) | 14.0 (12.7 to 15.0) | 13.7 (12.5 to 14.9) | 14.1 (12.7 to 15.0) | 0.16 |
| Hematocrit (%) | 41 (38 to 44) | 41 (38 to 44) | 42 (38 to 44) | 0.30 |
| Thrombocytes (109∙L−1) | 195 (166 to 236) | 202 (167 to 244) | 201 (168 to 242) | 0.61 |
| Glomerular filtration rate (mL∙min−1 per 1.73 m2) | 50 (31 to 57) | 50 (40 to 55) | 51 (40 to 56) | 0.58 |
| Creatinine (µmol∙L−1) | 88 (77 to 102) | 86 (75 to 104) | 85 (74 to 99) | 0.07 |
| ALT (U∙L−1) | 27.5 (21.0 to 38.0) | 26.0 (19.0 to 35.0) | 26.0 (19.0 to 37.0) | 0.78 |
| AST (U∙L−1) | 24.0 (21.0 to 30.0) | 26.0 (21.0 to 33.0) | 25.0 (21.0 to 32.0) | 0.41 |
| γ-GT (U∙L−1) | 37.0 (25.0 to 57.0) | 38.5 (25.0 to 68.8) | 36.0 (25.0 to 56.0) | 0.25 |
| AP (IU∙L−1) | 68.5 (57.8 to 83.0) | 67.0 (57.0 to 82.0) | 66.5 (56.0 to 80.0) | 0.26 |
Data indicate frequencies as number of patients (% within group) unless specified otherwise. Continuous data are presented as median (1st quartile to 3rd quartile). P values <0.05 indicate significant differences between groups. CRYS, crystalloids; GELA, gelafundin; HES, hydroxyethyl starch; BMI, body mass index; SAPS, Simplified Acute Physiology Score; NYHA, New York Heart Association; EF, ejection fraction; ALT, alanine-aminotransferase; AST, aspartate-amino-transferase; γ-GT, gamma-glutamyl-transferase; AP, alkaline phosphatase.
Mortality
Mortality was without differences between groups (Table 2, Figure 1). We calculated a multiple logistic regression based on demographic and perioperative factors from sufficiently complete datasets of n=1,432 patients to assess the role of colloids in hospital survival. The minimal adequate model {χ2[14]=161.075, P<0.001, McFadden Pseudo-R2=0.620} is summarized in Tables S1,S2. Each liter of colloid solution decreased the adjusted odds of survival by a factor of 0.501 (95% CI : 0.354 to 0.710, P<0.001). The type of colloid was not part of the minimal adequate model, indicating similar effects of both tetraspan and gelafundin. Total infusion volume exerted a protective effect. Each liter of total volume increased the adjusted odds of survival by a factor of 1.216 (95% CI: 1.007 to 1.468, P=0.042). Cumulative blood losses at 18 h postoperative, intraoperative PRBC transfusions, cumulative norepinephrine administration on the operative day, and PCC administration were tested as potential confounders which are known to affect mortality, but may also have influenced colloid administration. The scaled and centered estimate of the effect of total colloid volume were altered by factors of 1.0, 0.92, 1.06, and 1.09, respectively, indicating that none of these factors acted as a confounder.
Table 2
| Parameters | CRYS (n=205) | GELA (n=397) | HES (n=938) | P |
|---|---|---|---|---|
| Mortality | ||||
| 72 h | 2 (1%) | 4 (1%) | 8 (1%) | 0.958 |
| ICU | 2 (1%) | 7 (2%) | 24 (3%) | 0.305 |
| hospital | 2 (1%) | 7 (2%) | 24 (3%) | 0.305 |
| ICU stay (d) | a | b | c | <0.0001 |
| 0 to 2 | 47 (23%) | 70 (18%) | 109 (12%) | |
| 3 to 5 | 129 (63%) | 232 (58%) | 520 (55%) | |
| 6 to 10 | 26 (13%) | 82 (21%) | 247 (26%) | |
| >10 | 2 (1%) | 11 (3%) | 59 (6%) | |
| Mechanical ventilation (h) | 11.0 (9.0 to 13.3)a | 11.0 (9.0 to 16.0)b | 12.0 (10.0 to 17.3)c | <0.0001 |
| Acute kidney injury | 0.061 | |||
| none | 143 (70%) | 260 (65%) | 574 (61%) | |
| AKIN stage 1 | 43 (21%) | 90 (23%) | 230 (25%) | |
| AKIN stage 2 | 6 (3%) | 14 (4%) | 45 (5%) | |
| AKIN stage 3 | 10 (5%) | 26 (7%) | 64 (7%) | |
| Renal replacement therapy | 8 (4%) | 24 (6%) | 62 (7%) | 0.341 |
| PRBC units | a | b | c | <0.0001 |
| 0 | 150 (73%) | 230 (58%) | 422 (45%) | |
| 1 to 5 | 51 (25%) | 149 (38%) | 414 (44%) | |
| 6 to 10 | 4 (2%) | 13 (3%) | 84 (9%) | |
| >10 | 0 (0%) | 5 (1%) | 18 (2%) | |
| PC units | a | a | b | <0.0001 |
| 0 | 196 (96%) | 375 (94%) | 760 (81%) | |
| 1 to 3 | 7 (3%) | 20 (5%) | 156 (17%) | |
| 4 to 10 | 2 (1%) | 2 (1%) | 20 (2%) | |
| >10 | 0 (0%) | 0 (0%) | 2 (0%) | |
| Fibrinogen units | a | a | b | <0.0001 |
| 0 | 199 (97%) | 380 (96%) | 825 (88%) | |
| 1 to 5 | 6 (3%) | 15 (4%) | 943(10%) | |
| 6 to 10 | 0 (0%) | 2 (1%) | 18 (2%) | |
| >10 | 0 (0%) | 0 (0%) | 2 (0%) | |
| FFP units | ab | a | b | 0.0023 |
| 0 | 201 (98%) | 388 (98%) | 881 (94%) | |
| 1 to 3 | 3 (1%) | 5 (1%) | 14 (1%) | |
| 4 to 10 | 1 (1%) | 4 (1%) | 35 (4%) | |
| >10 | 0 (0%) | 0 (0%) | 8 (1%) | |
| PCC | 3 (1%)a | 11 (3%)a | 69 (7%)b | <0.0001 |
| PCC (IU) | 3,500 (3,250 to 4,750) | 3,000 (2,000 to 4,000) | 2,000 (2,000 to 4,000) | 0.45 |
| Hematocrit (%) | 0.0001 | |||
| Baseline | 41 (38 to 44) | 41 (38 to 44) | 42 (38 to 44) | 0.30 |
| Postoperative | 31 (28 to 34)a | 29 (27 to 31)b | 28 (26 to 31)c | <0.0001 |
| 6 h postoperative | 33 (30 to 35)a | 31 (29 to 33)b | 31 (28 to 33)c | <0.0001 |
| Postoperative day 1 | 32 (30 to 35)a | 30 (28 to 33)b | 31 (28 to 33)b | <0.0001 |
| Postoperative day 2 | 29 (26 to 32)ab | 28 (26 to 31)a | 29 (27 to 32)b | <0.0001 |
| Postoperative day 3 | 29 (26 to 31)ab | 28 (26 to 31)a | 29 (26 to 31)b | 0.01 |
| Hemoglobin (g∙dL−1) | <0.0001 | |||
| Baseline | 14.0 (12.7 to 15.0) | 13.7 (12.5 to 14.9) | 14.1 (12.7 to 15.0) | 0.16 |
| Postoperative | 10.5 (9.6 to 11.3)a | 9.7 (9.0 to 10.6)b | 9.6 (8.9 to 10.4)b | <0.0001 |
| 6 h postoperative | 11.2 (10.1 to 12.0)a | 10.5 (9.8 to 11.3)b | 10.3 (9.5 to 11.2)c | <0.0001 |
| Postoperative day 1 | 10.9 (10.0 to 11.9)a | 10.3 (9.5 to 11.1)b | 10.5 (9.6 to 11.2)b | <0.0001 |
| Postoperative day 2 | 9.7 (8.9 to 11.0)a | 9.5 (8.7 to 10.3)b | 9.8 (9.1 to 10.6)a | <0.0001 |
| Postoperative day 3 | 9.6 (8.9 to 10.7)a | 9.3 (8.6 to 10.3)b | 9.6 (8.9 to 10.3)a | 0.01 |
| Platelets (109∙L−1) | 0.136 | |||
| Baseline | 195 (166 to 236) | 202 (167 to 244) | 201 (168 to 242) | 0.61 |
| Postoperative | 148 (123 to 175)a | 133 (106 to 162)b | 126 (101 to 160)c | <0.0001 |
| 6 h postoperative | 161 (128 to 192)a | 151 (122 to 186)b | 144 (115 to 180)b | <0.0001 |
| Postoperative day 1 | 156 (128 to 190) | 150 (121 to 183) | 148 (124 to 183) | 0.20 |
| Postoperative day 2 | 138 (115 to 167) | 138 (113 to 169) | 137 (112 to 171) | 0.99 |
| Postoperative day 3 | 145 (116 to 178) | 143 (115 to 176) | 140 (112 to 173) | 0.22 |
| Creatinine (µmol∙L−1) | 0.462 | |||
| Baseline | 88 (77 to 102) | 86 (75 to 104) | 85 (74 to 99) | 0.07 |
| Postoperative | 91 (77 to 108) | 91 (77 to 107) | 89 (76 to 107) | 0.78 |
| 6 h postoperative | 88 (76 to 107) | 89 (75 to 106) | 89 (76 to 108) | 0.96 |
| Postoperative day 1 | 86 (74 to 108) | 90 (74 to 113) | 90 (74 to 112) | 0.58 |
| Postoperative day 2 | 92 (79 to 118) | 94 (76 to 124) | 95 (77 to 125) | 0.86 |
| Postoperative day 3 | 94 (78 to 125) | 93 (75 to 137) | 98 (78 to 137) | 0.35 |
| Urea (mmol∙L−1) | 0.406 | |||
| Baseline | 5.60 (4.70 to 7.15) | 5.80 (4.70 to 7.40) | 5.70 (4.60 to 7.40) | 0.56 |
| Postoperative | 5.30 (4.30 to 6.10) | 5.30 (4.40 to 6.60) | 5.40 (4.40 to 6.80) | 0.30 |
| 6 h postoperative | 5.50 (4.40 to 6.60) | 5.60 (4.60 to 7.20) | 5.70 (4.60 to 7.40) | 0.06 |
| Postoperative day 1 | 5.50 (4.50 to 7.23) | 5.90 (4.60 to 7.98) | 5.90 (4.60 to 8.10) | 0.17 |
| Postoperative day 2 | 6.50 (5.30 to 9.20) | 6.80 (5.10 to 9.50) | 6.95 (5.00 to 9.50) | 0.86 |
| Postoperative day 3 | 7.30 (5.40 to 10.88) | 7.20 (5.10 to 10.90) | 8.10 (5.60 to 11.70) | 0.08 |
| GFR (mL∙min−1 per 1.73 m2) | 0.011 | |||
| Baseline | 50 (31 to 57) | 50 (40 to 55) | 51 (40 to 56) | 0.58 |
| Postoperative | 54 (43 to 70) | 51 (42 to 55) | 52 (42 to 57) | 0.11 |
| 6 h postoperative | 53 (42 to 71)a | 48 (37 to 54)b | 49 (40 to 56)ab | 0.01 |
| Postoperative day 1 | 47 (32 to 57)ab | 42 (31 to 51)a | 47 (38 to 54)b | 0.02 |
| Postoperative day 2 | 39 (28 to 51) | 41 (29 to 51) | 43 (32 to 51) | 0.15 |
| Postoperative day 3 | 40 (28 to 51) | 39 (29 to 46) | 40 (31 to 49) | 0.50 |
| MAP (mmHg) | 0.0002 | |||
| Preoperative | 98 (90 to 107) | 95 (87 to 106) | 97 (88 to 106) | 0.26 |
| Intraoperative | 76 (72 to 79)a | 73 (68 to 77)b | 74 (70 to 78)a | <0.0001 |
| Postoperative | 77 (73 to 83) | 78 (73 to 86) | 77 (70 to 85) | 0.09 |
| 6 h postoperative | 78 (73 to 83)a | 77 (72 to 84)ab | 77 (72 to 83)b | 0.01 |
| 12 h postoperative | 80 (77 to 87)a | 78 (73 to 83)b | 78 (73 to 83)b | <0.0001 |
| 18 h postoperative | 82 (77 to 87)a | 80 (73 to 87)b | 80 (75 to 86)b | <0.0001 |
| Postoperative day 1 | 81 (77 to 87)a | 78 (73 to 83)b | 78 (73 to 83)b | <0.0001 |
| Postoperative day 2 | 81 (76 to 88) | 80 (73 to 87) | 80 (75 to 86) | 0.34 |
| Postoperative day 3 | 83 (78 to 90) | 82 (73 to 88) | 81 (75 to 90) | 0.07 |
Data indicate frequencies as number of patients (% within group) unless specified otherwise. Continuous data are presented as median (1st quartile to 3rd quartile). P values <0.05 indicate significant differences between groups. Pairwise comparison post test results are indicated as letters (a, b, c); values of groups not sharing a letter are significantly different. CRYS, crystalloids; GELA, gelafundin; HES, hydroxyethyl starch; ICU, intensive care unit; AKIN, acute kidney injury network; PRBC, packed red blood cells; PC, platelet concentrate; FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; GFR, glomerular filtration rate; MAP, mean arterial pressure.
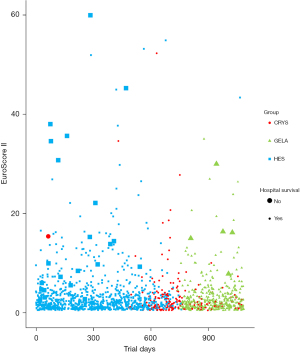
AKI
GFR differed between groups (P=0.011), but AKI (P=0.061) and RRT (P=0.341) did not differ between groups (Table 2, Figure 2). A multiple logistic regression model of AKI as a function of demographic and perioperative factors was calculated to estimate the influence of volume therapy. Sufficiently complete datasets of 996 patients were available for this analysis. Tables S3,S4 summarize the minimal adequate model {χ2[19]=274.594, P<0.001, McFadden Pseudo-R2=0.210}. Total volume and total colloid volume were retained in the model, but their respective adjusted odds ratio estimates of 1.037 (95% CI: 0.994 to 1.082, P=0.089) and 1.097 (95% CI: 0.987 to 1.221, P=0.087) for each liter administered were not considered significant.
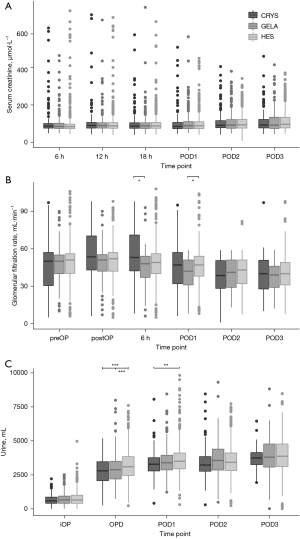
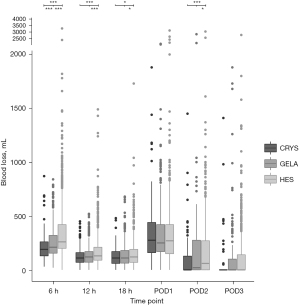
Blood loss
The type of volume replacement was associated with different volumes of blood loss over time (P<0.0001) (Table 3, Figure 3). HES showed the highest fraction of patients losing blood at 18h postoperative and later (P<0.0001) (Table 3). Platelet counts were lowest in HES and highest in CRYS postoperatively (P<0.0001) (Figure 4).
Table 3
| Parameters | CRYS (n=205) | GELA (n=397) | HES (n=938) | P |
|---|---|---|---|---|
| Patients receiving crystalloids | <0.0001 | |||
| Intraoperative | 204 (100%) | 396 (100%) | 934 (100%) | 0.9 |
| OP day | 204 (100%)ab | 394 (99%)a | 938 (100%)b | 0.04 |
| Postoperative day 1 | 157 (77%)a | 328 (83%)a | 889 (95%)b | <0.0001 |
| Postoperative day 2 | 92 (45%)a | 198 (50%)a | 688 (73%)b | <0.0001 |
| Postoperative day 3 | 53 (26%)a | 129 (32%)a | 500 (53%)b | <0.0001 |
| Crystalloid administration (mL) | <0.0001 | |||
| Intraoperative | 2,000 (2,000 to 2,512)a | 2,000 (2,000 to 2,000)a | 2,000 (1,000 to 2,000)b | <0.0001 |
| OP day | 3,850 (3,125 to 4,625)a | 3,750 (3,000 to 4,625)a | 2,900 (2,125 to 3,750)b | <0.0001 |
| Postoperative day 1 | 3,465 (2,721 to 4,000)a | 3,392 (2,738 to 4,143)a | 3,000 (2,400 to 3,874)b | <0.0001 |
| Postoperative day 2 | 2,016 (1,504 to 2,802)a | 2,418 (1,750 to 3,016)b | 2,313 (1,721 to 3,000)ab | 0.04 |
| Postoperative day 3 | 1,624 (1,224 to 2,460) | 2,016 (1,320 to 3,000) | 2,050 (1,472 to 2,701) | 0.05 |
| Total crystalloid administration (mL) | 9,829 (7,510 to 11,862) | 10,055 (7,641 to 13,460) | 10,543 (8,234 to 12,756) | 0.05 |
| Patients receiving colloids | <0.0001 | |||
| Intraoperative | n/a | 339 (85%) | 795 (85%) | 0.83 |
| OP day | n/a | 157 (40%) | 754 (80%) | <0.0001 |
| Postoperative day 1 | n/a | 71 (18%) | 573 (61%) | <0.0001 |
| Postoperative day 2 | n/a | 18 (5%) | 307 (33%) | <0.0001 |
| Postoperative day 3 | n/a | 7 (2%) | 148 (16%) | <0.0001 |
| Colloid administration (mL) | <0.0001 | |||
| Intraoperative | n/a | 500 (500 to 1,000) | 500 (500 to 1,000) | 0.05 |
| OP day | n/a | 500 (500 to 1,000) | 1,000 (500 to 1,500) | <0.0001 |
| Postoperative day 1 | n/a | 500 (500 to 500) | 1,000 (500 to 1,000) | <0.0001 |
| Postoperative day 2 | n/a | 500 (500 to 500) | 500 (500 to 1,000) | 0.38 |
| Postoperative day 3 | n/a | 500 (500 to 500) | 500 (500 to 1,000) | 0.48 |
| Total colloid administration (mL) | n/a | 1,000 (500 to 1,500) | 2,000 (1,000 to 3,038) | <0.0001 |
| Overall volume replacement (mL) | <0.0001 | |||
| Intraoperative | 2,000 (2,000 to 2,500)a | 2,500 (2,000 to 3,000)b | 2,500 (1,500 to 3,000)c | <0.0001 |
| OP day | 3,825 (3,125 to 4,625) | 4,000 (3,100 to 4,922) | 3,750 (3,000 to 4,625) | 0.05 |
| Postoperative day 1 | 3,000 (1,225 to 3,960)a | 3,170 (2,016 to 4,057)b | 3,500 (2,814 to 4,500)c | <0.0001 |
| Postoperative day 2 | 0 (0 to 2,016)a | 0 (0 to 2,482)b | 2,130 (0 to 3,016)c | <0.0001 |
| Postoperative day 3 | 0 (0 to 480)a | 0 (0 to 1,298)b | 809 (0 to 2,343)c | <0.0001 |
| Total overall volume replacement (mL) | 9,829 (7,510 to 11,862)a | 11,125 (8,500 to 14,468)b | 12,746 (10,326 to 15,576)c | <0.0001 |
| Patients with blood loss | <0.0001 | |||
| 6 h postoperative | 203 (99%) | 395 (99%) | 933 (99%) | 0.70 |
| 12 h postoperative | 202 (99%) | 391 (98%) | 925 (99%) | 1.0 |
| 18 h postoperative | 195 (95%)a | 372 (94%)b | 920 (98%)c | 0.0002 |
| Postoperative day 1 | 154 (75%)a | 325 (82%)a | 863 (92%)b | <0.0001 |
| Postoperative day 2 | 41 (20%)a | 110 (28%)a | 454 (48%)b | <0.0001 |
| Postoperative day 3 | 11 (5%)a | 41 (10%)a | 201 (21%)b | <0.0001 |
| Blood loss (mL) | 0.0001 | |||
| 6 h postoperative | 190 (130 to 260)a | 210 (155 to 320)b | 260 (200 to 420)c | <0.0001 |
| 12 h postoperative | 110 (70 to 160)a | 120 (72 to 170)a | 130 (90 to 210)b | <0.0001 |
| 18 h postoperative | 110 (60 to 170)a | 110 (63 to 180)a | 120 (70 to 190)b | 0.01 |
| Postoperative day 1 | 275 (160 to 440) | 250 (170 to 420) | 270 (150 to 420) | 0.87 |
| Postoperative day 2 | 0 (0 to 128)a | 20 (0 to 275)a | 60 (0 to 270)b | <0.0001 |
| Postoperative day 3 | 0 (0 to 0) | 0 (0 to 100) | 0 (0 to 140) | 0.06 |
| Total blood loss (mL) | 660 (410 to 940)a | 750 (500 to 1,090)b | 940 (670 to 1,400)c | <0.0001 |
| Urine (mL) | <0.0001 | |||
| Intraoperative | 580 (400 to 850) | 650 (400 to 925) | 640 (400 to 995) | 0.09 |
| OP day | 2,790 (2,065 to 3,455)a | 2,870 (2,190 to 3,485)a | 3,090 (2,440 to 3,830)b | <0.0001 |
| Postoperative day 1 | 3,270 (2,780 to 3,750)a | 3,380 (2,855 to 3,930)ab | 3,490 (2,930 to 4,100)b | <0.0001 |
| Postoperative day 2 | 3,215 (2,758 to 3,830) | 3,550 (2,845 to 4,383) | 3,410 (2,810 to 4,100) | 0.14 |
| Postoperative day 3 | 3,730 (3,248 to 4,125) | 3,790 (3,060 to 4,760) | 3,855 (3,095 to 4,760) | 0.54 |
| Total urine (mL) | 7,610 (5,450 to 11,670)a | 8,540 (6,110 to 13,420)b | 12,250 (8,386 to 15,098)c | <0.0001 |
| Cumulative volume balance (mL) | <0.0001 | |||
| Intraoperative | 1,350 (840 to 2,010)a | 1,910 (1,342 to 2,620)b | 1,550 (851 to 2,260)c | <0.0001 |
| OP day | 2,235 (1,335 to 3,090)a | 2,860 (1,850 to 3,945)b | 2,126 (1,176 to 3,148)a | <0.0001 |
| Postoperative day 1 | 2,110 (1,080 to 3,050)a | 2,669 (1,525 to 3,920)b | 2,024 (858 to 3,335)a | <0.0001 |
| Postoperative day 2 | 1,630 (405 to 2,630)a | 2,313 (866 to 3,590)b | 1,442 (31 to 2,899)a | <0.0001 |
| Postoperative day 3 | 1,393 (−70 to 2,460)a | 1,896 (245 to 3,267)b | 708 (−873 to 2,343)c | <0.0001 |
Data indicate frequencies as number of patients (% within group) unless specified otherwise. Continuous data are presented as median (1st quartile to 3rd quartile). P values <0.05 indicate significant differences between groups. Pairwise comparison post test results are indicated as letters (a, b, c); values of groups not sharing a letter are significantly different. CRYS, crystalloids; GELA, gelafundin; HES, hydroxyethyl starch; OP, operative.
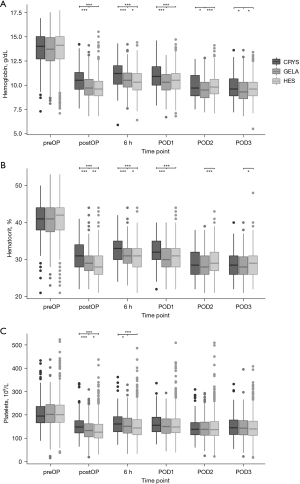
HES patients had the lowest hematocrit, and GELA was lower than CRYS postoperative and 6h postoperative (P<0.0001, Figure 4). Hemoglobin concentrations were lower in colloids compared to CRYS postoperatively until POD1 (P<0.0001) and were lowest in GELA on POD2 and POD3 (P<0.0001).
Blood products
Patients receiving PRBC transfusions were highest in the HES group (55%) followed by GELA (42%) and CRYS (27%) groups (P<0.0001, Table 2). PCs were administered by 19% of the HES patients, 6% of the GELA patients, and 4% of the CRYS patients (P<0.0001). Fibrinogen was administered to a higher fraction of HES patients (12%, P<0.0001) compared to GELA (4%) and CRYS (3%). PCC was administered more frequently in HES than in GELA or CRYS patients (P<0.0001) (Table 2).
Hemodynamic therapy
Mean arterial pressure (MAP) was lowest intraoperatively, with GELA patients slightly below other groups (P<0.0001) (Table 2).
Total replacement volumes were higher in colloid groups compared to CRYS in POD1 and later (P<0.0001) (Table 3). HES received more often noradrenaline than both groups on POD1 through POD3 (P<0.0001), requiring the highest doses from operation to POD2 (P≤0.01) (Figure 5). Cumulative noradrenaline doses were higher in both colloid groups compared to CRYS (P<0.0001). Application of colloids increased the cumulative noradrenaline dose by 0.0158 and 0.0320 µg∙kg−1∙min−1, respectively, controlling for all other factors of the model (Tables S1-S4). Adrenaline was administered more often in colloid groups compared to CRYS (P=0.0003, Table 4). Cumulative adrenaline doses were higher in HES compared to both groups (P=0.03).
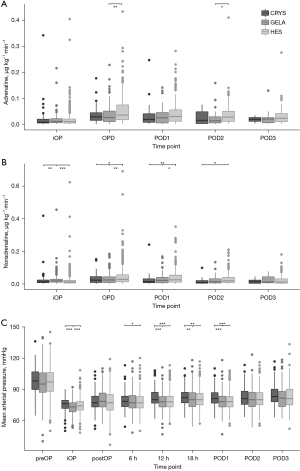
Table 4
| Parameters | CRYS (n=205) | GELA (n=397) | HES (n=938) | P |
|---|---|---|---|---|
| Patients receiving adrenaline at any time | 72 (35%)a | 215 (54%)b | 455 (49%)c | 0.0003 |
| Patients receiving adrenaline | <0.0001 | |||
| Intraoperative | 71 (35%)a | 201 (51%)b | 430 (46%)b | 0.0009 |
| OP day | 43 (21%)a | 125 (32%)b | 293 (31%)b | 0.01 |
| Postoperative day 1 | 27 (13%)a | 76 (19%)ab | 212 (23%)b | 0.008 |
| Postoperative day 2 | 13 (6%)a | 41 (10%)ab | 131 (14%)b | 0.005 |
| Postoperative day 3 | 4 (2%)a | 19 (5%)ab | 79 (8%)b | 0.0007 |
| Adrenaline administration (µg∙kg−1∙min−1) | 0.008 | |||
| Intraoperative | 0.0080 (0.0047 to 0.0195) | 0.0108 (0.0057 to 0.0213) | 0.0100 (0.0045 to 0.0200) | 0.35 |
| OP day | 0.0284 (0.0150 to 0.0441)ab | 0.0255 (0.0100 to 0.0506)a | 0.0351 (0.0174 to 0.0741)b | <0.0001 |
| Postoperative day 1 | 0.0184 (0.0074 to 0.0401) | 0.0249 (0.0049 to 0.0450) | 0.0297 (0.0117 to 0.0554) | 0.04 |
| Postoperative day 2 | 0.0148 (0.0028 to 0.0478)ab | 0.0134 (0.0052 to 0.0287)a | 0.0273 (0.0109 to 0.0500)b | 0.02 |
| Postoperative day 3 | 0.0186 (0.0114 to 0.0258) | 0.0199 (0.0055 to 0.0254) | 0.0227 (0.0099 to 0.0410) | 0.24 |
| Total adrenaline administration (µg∙kg−1∙min−1) | 0.0238 (0.0054 to 0.0609) | 0.0241 (0.0082 to 0.0695) | 0.0337 (0.0076 to 0.1134) | 0.03 |
| Patients receiving dobutamine at any time | 12 (6%)a | 23 (6%)a | 124 (13%)b | 0.0001 |
| Dobutamine administration | <0.0001 | |||
| Intraoperative | 8 (4%)a | 22 (6%)a | 98 (10%)b | 0.0006 |
| OP day | 3 (1%) | 6 (2%) | 37 (4%) | 0.02 |
| Postoperative day 1 | 1 (0%)ab | 1 (0%)a | 23 (2%)b | 0.006 |
| Postoperative day 2 | 0 (0%)ab | 0 (0%)a | 13 (1%)b | 0.01 |
| Postoperative day 3 | 1 (0%) | 0 (0%) | 7 (1%) | 0.2 |
| Dobutamine administration (µg∙kg−1∙min−1) | n/a | |||
| Intraoperative | 1.263 (0.922 to 1.534) | 0.975 (0.601 to 2.233) | 0.771 (0.377 to 1.311) | 0.04 |
| OP day | 0.650 (0.563 to 1.466) | 0.735 (0.357 to 3.693) | 1.494 (0.567 to 2.206) | 0.88 |
| Postoperative day 1 | 0.384 | 2.688 | 1.190 (0.770 to 1.913) | 0.3 |
| Postoperative day 2 | n/a | n/a | 2.06 (0.977 to 3.586) | n/a |
| Postoperative day 3 | 2.773 | n/a | 0.662 (0.422 to 2.550) | 0.28 |
| Total dobutamine administration (µg∙kg−1∙min−1) | 1.315 (0.729 to 2.051) | 1.086 (0.612 to 3.112) | 1.083 (0.489 to 2.043) | 0.62 |
| Patients receiving milrinone at any time | 7 (3%) | 22 (6%) | 57 (6%) | 0.52 |
| Milrinone administration | 0.125 | |||
| Intraoperative | 2 (1%) | 10 (3%) | 21 (2%) | 0.44 |
| OP day | 4 (2%) | 17 (4%) | 28 (3%) | 0.26 |
| Postoperative day 1 | 4 (2%) | 11 (3%) | 28 (3%) | 0.72 |
| Postoperative day 2 | 2 (1%) | 8 (2%) | 21 (2%) | 0.51 |
| Postoperative day 3 | 1 (0%) | 5 (1%) | 17 (2%) | 0.33 |
| Milrinone administration (µg∙kg−1∙min−1) | 0.197 | |||
| Intraoperative | 0.0375 (0.0335 to 0.0414) | 0.0484 (0.0340 to 0.0984) | 0.0370 (0.0312 to 0.0495) | 0.51 |
| OP day | 0.0914 (0.0612 to 0.1854) | 0.0543 (0.0472 to 0.0874) | 0.0684 (0.0413 to 0.1146) | 0.59 |
| Postoperative day 1 | 0.0771 (0.0672 to 0.0834) | 0.0399 (0.0232 to 0.0705) | 0.0522 (0.0292 to 0.1017) | 0.40 |
| Postoperative day 2 | 0.0869 (0.0519 to 0.1219) | 0.0611 (0.0426 to 0.1070) | 0.0246 (0.0118 to 0.0462) | 0.22 |
| Postoperative day 3 | 0.0914 | 0.0351 (0.0219 to 0.0707) | 0.0472 (0.0232 to 0.0910) | 0.66 |
| Total milrinone administration (µg∙kg−1∙min−1) | 0.191 (0.049 to 0.270) | 0.112 (0.068 to 0.233) | 0.105 (0.045 to 0.174) | 0.39 |
| Patients receiving noradrenaline at any time | 192 (94%) | 379 (95%) | 900 (96%) | 0.63 |
| Noradrenaline administration | <0.0001 | |||
| Intraoperative | 175 (85%) | 362 (91%) | 846 (90%) | 0.07 |
| OP day | 134 (65%)a | 313 (79%)b | 704 (75%)b | 0.001 |
| Postoperative day 1 | 60 (29%)a | 168 (42%)b | 487 (52%)c | <0.0001 |
| Postoperative day 2 | 23 (11%)a | 64 (16%)a | 315 (34%)b | <0.0001 |
| Postoperative day 3 | 8 (4%)a | 20 (5%)a | 162 (17%)b | <0.0001 |
| Noradrenaline administration (µg∙kg−1∙min−1) | 0.037 | |||
| Intraoperative | 0.0133 (0.0077 to 0.0238)a | 0.0183 (0.0105 to 0.0290)b | 0.0117 (0.0059 to 0.0221)a | <0.0001 |
| OP day | 0.0229 (0.0087 to 0.0442)a | 0.0239 (0.0079 to 0.0436)a | 0.0279 (0.0126 to 0.0561)b | <0.0001 |
| Postoperative day 1 | 0.0138 (0.0074 to 0.0309)a | 0.0213 (0.0070 to 0.0404)a | 0.0252 (0.0105 to 0.0538)b | <0.0001 |
| Postoperative day 2 | 0.0091 (0.0044 to 0.0202)a | 0.0132 (0.0054 to 0.0312)ab | 0.0192 (0.0077 to 0.0401)b | 0.01 |
| Postoperative day 3 | 0.0127 (0.0036 to 0.0231) | 0.0116 (0.0068 to 0.0433) | 0.0119 (0.0042 to 0.0310) | 0.9 |
| Total noradrenaline administration (µg∙kg−1∙min−1) | 0.032 (0.014 to 0.061)a | 0.043 (0.021 to 0.087)b | 0.049 (0.019 to 0.107)b | <0.0001 |
| Patients receiving furosemide at any time | 160 (78%)a | 323 (81%)a | 852 (91%)b | <0.0001 |
| Furosemide administration | <0.0001 | |||
| Intraoperative | 20 (10%)a | 77 (19%)b | 143 (15%)ab | 0.007 |
| OP day | 104 (51%) | 178 (45%) | 456 (49%) | 0.3 |
| Postoperative day 1 | 122 (60%)a | 269 (68%)a | 745 (79%)b | <0.0001 |
| Postoperative day 2 | 60 (29%)a | 123 (31%)a | 458 (49%)b | <0.0001 |
| Postoperative day 3 | 28 (14%)a | 66 (17%)a | 259 (28%)b | <0.0001 |
| Furosemide administration (mg) | 0.45 | |||
| Intraoperative | 10 (5 to 13)a | 10 (10 to 20)a | 20 (10 to 20)b | 0.01 |
| OP day | 20 (10 to 30) | 20 (10 to 40) | 20 (10 to 40) | 0.05 |
| Postoperative day 1 | 40 (20 to 68) | 40 (20 to 80) | 40 (20 to 60) | 0.71 |
| Postoperative day 2 | 40 (20 to 178) | 40 (20 to 160) | 30 (20 to 90) | 0.11 |
| Postoperative day 3 | 60 (38 to 123) | 40 (20 to 178) | 40 (20 to 162) | 0.37 |
| Total furosemide administration (mg) | 50 (25 to 115)a | 50 (30 to 140)ab | 70 (30 to 150)b | 0.01 |
Data indicate frequencies as number of patients (% within group) unless specified otherwise. Continuous data are presented as median (1st quartile to 3rd quartile). P values <0.05 indicate significant differences between groups. Pairwise comparison post test results are indicated as letters (a, b, c); values of groups not sharing a letter are significantly different. CRYS, crystalloids; GELA, gelafundin; HES, hydroxyethyl starch; OP, operative.
Mechanical ventilation and ICU LOS
Mechanical ventilation differed significantly between all groups (P<0.0001) with shortest duration in the CRYS and longest duration in the HES group. CRYS had the shortest ICU LOS, HES the longest (P<0.0001, Table 2).
Discussion
This retrospective study focused on the effects of volume management changes on GELA or CRYS in cardiac surgery at a university hospital. Main results are: (I) no difference in mortality was observed; (II) no differences were detected for AKI and RRT; (III) HES treated patients showed the highest blood loss, CRYS patients the lowest; (IV) HES had the highest transfusion of PRBCs and PCs and CRYS the lowest; (V) FFP, fibrinogen, and PCC were highest in HES; (VI) both colloids showed higher cumulative noradrenaline doses compared to CRYS; (VII) adrenaline was administered more often in colloid groups; (VIII) CRYS patients showed the shortest time of mechanical ventilation and ICU LOS, while HES patients had the longest ventilation and ICU LOS.
Due to the small number of deaths in our study and in the literature, no firm conclusion can be drawn for cardiac surgery. Large randomized studies in critically ill patients showed increased risk of AKI and need of RRT for the HES (5,6). GELA was rarely used, but some data describe no effects (14), other deleterious effects on kidney function in cardiac surgery (15). However, our data are consistent to a meta-analysis in cardiac surgery which did not find evidence for increased AKI or RRT in tetrastarch (7).
Controversial data are available investigating blood loss in terms of different volumes in cardiac surgery: some data comparing tetrastarch and crystalloids did not observe any differences (16-20), while others reported higher blood loss for tetrastarch (21,22); data comparing tetrastarch and GELA also range from a higher blood loss observed initially in one study in the tetrastarch group (23) to no difference reported in most studies (20,24-26). Results of blood product administration are in line with other data: patients with crystalloids received fewer transfusions compared to tetrastarch (17,21), and GELA received less transfusion than tetrastarch treated (23,24,26).
Despite differences of a few mmHg of MAP in our study, HES and GELA showed the highest cumulative doses of noradrenaline. These findings are in line with other data comparing HES and CRYS (27-29). Higher noradrenaline requirements were described in GELA treated patients compared to tetrastarch (9). Few data are available for catecholamine requirements focused on GELA and crystalloids in cardiac surgery: no difference was observed for noradrenaline and dobutamine requirement in a study of forty patients comparing GELA and 0.9% saline (30).
Despite reports of allergic or anaphylactic reactions after GELA exposure (31), no such reaction was observed in this study, but must be kept in mind as a potential risk.
Keeping in mind that just few data in cardiac surgery are available, our results match existing data that neither differences in mechanical ventilation (18,20,21) nor in ICU LOS (17,18,21) were observed.
Limitations of our study are the retrospective design, the almost linear sequence of volume regimens, and the fact that the decision for a volume regimen was at the discretion of the physician which could thus be prone to confounders and/or misinterpretations.
Overall, this retrospective investigation of three volume replacement managements showed no difference in mortality or AKI. The administration of crystalloids without any colloid was associated with less blood loss, transfusion, and mechanical ventilation having similar effects on MAP and ICU LOS. The administration of colloids in cardiac surgery should be considered critically due to the lack of evidence for benefits but severe concerns. Because of the restriction of hydroxyethylstarch and the subsequent GELA use in many places in cardiac surgery, further studies should investigate the effects of GELA in comparison to crystalloids.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-450/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-450/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-450/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of the University of Ulm (approval ID 50-17) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Christensen MC, Dziewior F, Kempel A, et al. Increased chest tube drainage is independently associated with adverse outcome after cardiac surgery. J Cardiothorac Vasc Anesth 2012;26:46-51. [Crossref] [PubMed]
- Lobo DN, Stanga Z, Aloysius MM, et al. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med 2010;38:464-70. [Crossref] [PubMed]
- Koponen T, Musialowicz T, Lahtinen P. Gelatin and the risk of bleeding after cardiac surgery. Acta Anaesthesiol Scand 2020;64:1438-45. [Crossref] [PubMed]
- EMA EMA. Hydroxyethyl-starch solutions (HES) no longer to be used in patients with sepsis or burn injuries or in critically ill patients 2013. Available online: http://www.ema.europa.eu/docs/de_DE/document_library/Referrals_document/Solutions_for_infusion_containing_hydroxyethyl_starch/Euro-pean_Commission_final_decision/WC500162361.pdf. March 2017.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367:1901-11. [Crossref] [PubMed]
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124-34. [Crossref] [PubMed]
- Jacob M, Fellahi JL, Chappell D, et al. The impact of hydroxyethyl starches in cardiac surgery: a meta-analysis. Crit Care 2014;18:656. [Crossref] [PubMed]
- Reinhart K, Perner A, Sprung CL, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med 2012;38:368-83. [Crossref] [PubMed]
- Winterhalter M, Malinski P, Danzeisen O, et al. Prospective observational study for perioperative volume replacement with 6% HES 130/0,42, 4% gelatin and 6% HES 200/0,5 in cardiac surgery. Eur J Med Res 2010;15:383-9. [Crossref] [PubMed]
- Schramko AA, Kuitunen AH, Suojaranta-Ylinen RT, et al. Role of fibrinogen-, factor VIII- and XIII-mediated clot propagation in gelatin haemodilution. Acta Anaesthesiol Scand 2009;53:731-5. [Crossref] [PubMed]
- Tabuchi N, de Haan J, Gallandat Huet RC, et al. Gelatin use impairs platelet adhesion during cardiac surgery. Thromb Haemost 1995;74:1447-51. [Crossref] [PubMed]
- Marx G, Zacharowski K, Ichai C, et al. Efficacy and safety of early target-controlled plasma volume replacement with a balanced gelatine solution versus a balanced electrolyte solution in patients with severe sepsis/septic shock: study protocol, design, and rationale of a prospective, randomized, controlled, double-blind, multicentric, international clinical trial: GENIUS-Gelatine use in ICU and sepsis. Trials 2021;22:376. [Crossref] [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [Crossref] [PubMed]
- Koponen T, Musialowicz T, Lahtinen P. Gelatin and the risk of acute kidney injury after cardiac surgery. Acta Anaesthesiol Scand 2022;66:215-22. [Crossref] [PubMed]
- Heringlake M, Berggreen AE, Reemts E, et al. Fluid Therapy With Gelatin May Have Deleterious Effects on Kidney Function: An Observational Trial. J Cardiothorac Vasc Anesth 2020;34:2674-81. [Crossref] [PubMed]
- Gurbuz HA, Durukan AB, Salman N, et al. Hydroxyethyl starch 6%, 130/0.4 vs. a balanced crystalloid solution in cardiopulmonary bypass priming: a randomized, prospective study. J Cardiothorac Surg 2013;8:71. [Crossref] [PubMed]
- Lee JS, Ahn SW, Song JW, et al. Effect of hydroxyethyl starch 130/0.4 on blood loss and coagulation in patients with recent exposure to dual antiplatelet therapy undergoing off-pump coronary artery bypass graft surgery. Circ J 2011;75:2397-402. [Crossref] [PubMed]
- Tiryakioğlu O, Yildiz G, Vural H, et al. Hydroxyethyl starch versus Ringer solution in cardiopulmonary bypass prime solutions (a randomized controlled trial). J Cardiothorac Surg 2008;3:45. [Crossref] [PubMed]
- Akkucuk FG, Kanbak M, Ayhan B, et al. The effect of HES (130/0.4) usage as the priming solution on renal function in children undergoing cardiac surgery. Ren Fail 2013;35:210-5. [Crossref] [PubMed]
- Alavi SM, Ahmadi BB, Baharestani B, et al. Comparison of the effects of gelatin, Ringer's solution and a modern hydroxyl ethyl starch solution after coronary artery bypass graft surgery. Cardiovasc J Afr 2012;23:428-31. [Crossref] [PubMed]
- Chakravarthy M, Muniraj G, Patil S, et al. A randomized prospective analysis of alteration of hemostatic function in patients receiving tranexamic acid and hydroxyethyl starch (130/0.4) undergoing off pump coronary artery bypass surgery. Ann Card Anaesth 2012;15:105-10. [Crossref] [PubMed]
- Hans GA, Ledoux D, Roediger L, et al. The effect of intraoperative 6% balanced hydroxyethyl starch (130/0.4) during cardiac surgery on transfusion requirements. J Cardiothorac Vasc Anesth 2015;29:328-32. [Crossref] [PubMed]
- Vanhoonacker J, Ongenae M, Vanoverschelde H, et al. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for cardiopulmonary bypass priming: the effects on postoperative bleeding and volume expansion needs after elective CABG. Acta Anaesthesiol Belg 2009;60:91-7. [PubMed]
- Kimenai DM, Bastianen GW, Daane CR, et al. Effect of the colloids gelatin and HES 130/0.4 on blood coagulation in cardiac surgery patients: a randomized controlled trial. Perfusion 2013;28:512-9. [Crossref] [PubMed]
- Schramko A, Suojaranta-Ylinen R, Kuitunen A, et al. Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Br J Anaesth 2010;104:691-7. [Crossref] [PubMed]
- Van der Linden PJ, De Hert SG, Deraedt D, et al. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesth Analg 2005;101:629-34. [Crossref] [PubMed]
- Miyao H, Kotake Y. Renal Morbidity of 6% Hydroxyethyl Starch 130/0.4 in 9000 Propensity Score Matched Pairs of Surgical Patients. Anesth Analg 2020;130:1618-27. [Crossref] [PubMed]
- Magder S, Potter BJ, Varennes BD, et al. Fluids after cardiac surgery: a pilot study of the use of colloids versus crystalloids. Crit Care Med 2010;38:2117-24. [Crossref] [PubMed]
- Vives M, Callejas R, Duque P, et al. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: a prospective multicentre cohort. Br J Anaesth 2016;117:458-63. [Crossref] [PubMed]
- Soares RR, Ferber L, Lorentz MN, et al. Intraoperative volume replacement: crystalloids versus colloids in surgical myocardial revascularization without cardiopulmonary bypass. Rev Bras Anestesiol 2009;59:439-51. [Crossref] [PubMed]
- Jiang Y, Yuan IH, Dutille EK, et al. Preventing iatrogenic gelatin anaphylaxis. Ann Allergy Asthma Immunol 2019;123:366-74. [Crossref] [PubMed]


