Video-assisted thoracoscopic surgery segmentectomy by non-intubated or intubated anesthesia: a comparative analysis of short-term outcome
Introduction
Before the introduction of the double-lumen tube ventilation, the non-intubated anesthesia has been extensively applied in thoracic surgery. In 1950, Buckingham et al. first proposed the use of thoracic epidural anesthesia (TEA) to perform awake thoracic procedures, describing his experience of 617 thoracic surgery procedures using this method (1). In 1954, Vischnevski applied a multi-step analgesia protocol in more than 600 cases of thoracic surgery, including major lung resections and even esophagectomies (2,3). Since the development of double-lumen tracheal tube, intubated general anesthesia with one-lung ventilation (OLV) has become the standard anesthesia method in both open and thoracoscopic surgery, since it could provide a quiet surgical environment with good pulmonary collapse and a wide visual field (4). Subsequently, the local or regional anesthesia in awake thoracic surgery has been gradually eliminated. However, the adverse effects of conventional intubated general anesthesia have arrested more and more attention these years, including intubation-related airway trauma, ventilation-induced lung injury, residual neuromuscular blockade, impaired cardiac performance and postoperative nausea and vomiting, etc. (5-8). In addition, postoperative residual neuromuscular blockade might increase the rate of respiratory system related complications and lead to impaired clinical recovery (8,9). To minimize these adverse effects, non-intubated video-assisted thoracoscopic surgery (VATS) has been recently employed. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of spontaneous pneumothorax was initially reported by Nezu et al. in 1997 (10). Since then, more and more complex non-intubated thoracoscopic surgeries have been reported, with excellent postoperative results. In 2004, Pompeo et al. reported the feasibility of awake thoracoscopic resection of solitary pulmonary nodules, and found better patient satisfaction, less nursing care and shorter in-hospital stay than procedures performed under general anesthesia (11). In 2011, Chen et al. described non-intubated thoracoscopic lobectomy was technically feasible and as safe as lobectomy performed with intubation in early stage non-small cell lung cancer (NSCLC) patients (12). Liu et al. conducted a randomized control study comparing two groups of patients who received thoracic surgery under epidural anesthesia and those under general anesthesia with double lumen tube. This study has demonstrated the advantages of VATS under non-intubated anesthesia, including shorter postoperative fasting time, shorter duration of antibiotic use, and shorter hospital stay (13).
The non-intubated thoracoscopic anatomical segmentectomy, using the combination of TEA, intrathoracic vagal blockade, and appropriate sedation, was proved to be technically feasible and safe by two single-arm studies (14,15). However, comparison between non-intubated and intubated VATS segmentectomy has never been performed. The aim of this study was to examine the short-term outcome of patients undergoing non-intubated VATS segmentectomy using non-intubated anesthesia or intubated general anesthesia at our institution during the last 5 years. We hypothesized that patients would benefit from non-intubated anesthesia approach, with comparable short-term outcomes compared with those undergoing intubated general anesthesia.
Materials and methods
This study was reviewed and approved by the First Affiliated Hospital of Guangzhou Medical University Research Ethics Committee. All patients has signed the “consent to epidural anesthesia with nontracheal intubation” and “consent to general anesthesia with tracheal intubation” prior to anesthesia.
Study subjects
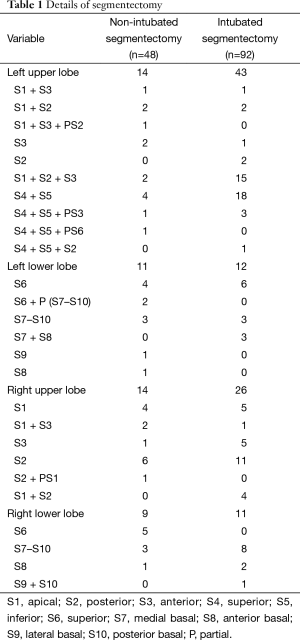
A retrospective review of the thoracic surgery database from July 2011 to June 2015 identified 140 consecutive patients who underwent VATS anatomic segmentectomy, which was defined as the individual dissection through stapling of the involved segmental pulmonary vein (PV), pulmonary artery (PA) and bronchus. All patients had the same indication for VATS segmentectomy, including: (I) a lung tumor close to the hilum in which wedge resection could not be performed; (II) the previous history of lung lobectomy, leading to the consideration for an second primary NSCLC; (III) a solitary metastasis; (IV) multiple pulmonary ground-glass shadows, suspected as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA); (V) a combination with any cardiopulmonary disease that makes lobectomy intolerable; (VI) peripheral early lung cancer ≤2 cm in diameter; or (VII) the benign lesions limited to the interiors of pulmonary segment (15). Before operation, all patients received pre-operative chest high-resolution thin-slice enhanced CT scans and pulmonary function tests. For those suspected of lung malignant tumor, additional upper abdomen CT, head MRI, whole body bone scintigraphy or whole body PET-CT examination was needed to exclude distant metastases. For some small nodules, three-dimensional (3D) CT reconstruction was performed to ascertain the nodule position and to find whether variation occurred to the target segment of vessels and bronchi. The patients in combination with pulmonary lobectomy or resection of multiple pulmonary segments of different pulmonary lobes were excluded. Indications for patients considered appropriate for non-intubated thoracoscopic segmentectomy included ASA I–III, BMI <25, Mallampati grade I–II, little airway secretion and absence of epidural puncture contraindication. Prior to operation, surgeons and anesthetists carefully evaluated patient’s physical condition. The eligible patients made the choice after they were explained about the pros and cons of the two anesthesia methods by surgeons and anesthetists. On the other hand, if patients did not meet the criteria, they would be allocated to receive intubated thoracoscopic segmentectomy. Patients in both groups had the same preoperative preparation, and all surgeries were completed by the same thoracic surgeons and anesthetists team. The distribution of the segmentectomy was shown in Table 1.
Full table
Anesthesia
Non-intubated anesthesia group
Patients received intramuscular midazolam 0.06 mg/kg and atropine 0.01 mg/kg 30 minutes before anesthesia. All patients were routinely monitored electrocardiogram (ECG), heart rate (HR), blood pressure (Bp), pulse oxygen saturation (SpO2), end-tidal carbon dioxide (EtCO2), respiratory rate (RR) and bispectral index (BIS) after entered the operation room. TEA was performed by insertion of an epidural catheter at the T7–8 or T8–9 space. On the supine position, 2–3 mL of 2% lidocaine was injected through the epidural catheter. If signs of spinal anesthesia didn’t present in 5 minutes, a fractionated injection of 12–15 mL 0.375% ropivacaine was administered. Prior to the initiation of surgery, the anesthesia level should reach between T2 and T10.
After the anesthesia plane was determined, the target controlled infusion (TCI) of propofol (target plasma concentration ranged from 2 to 3 ug/mL) and sufentanil 0.1–0.2 ug/kg was adopted for intravenous anesthesia induction. During surgical process, laryngeal mask airway (LMA) was given to provide supplementary oxygen (oxygen flow at the rate of 2 to 3 L/min) to keep oxygen saturation above 90% and the spontaneous respiration rate was sustain on 12 to 20/min. Anesthesia was maintained with TCI of propofol (target plasma concentration of 1 to 2 ug/mL) and dexmedetomidine 0.5–1 ug/kg/h was given, and BIS was monitored and maintained at 40 to 60. According to the surgical needs, the anesthesia depth was adjusted to relieve the mediastinal movement during surgical operation. When the pleural cavity was closed and the wound was sutured, administration of intravenous drugs was suspended. After removal of the epidural catheter and LMA, patient was sent to the ward or ICU according to the evaluation of patient’s preoperative cardio-pulmonary function and intraoperative conditions.
Tracheal intubation anesthesia group
All patients were at the contralateral position and the anesthesia monitoring was the same as that in non-intubated group. TCI of propofol (target plasma concentration 2 to 3 ug/mL), sufentanil 0.4–0.6 ug/kg and cisatracurium 0.2–0.3 mg/kg was adopted for the anesthesia induction. Once the patients lost consciousness, a double-lumen tracheal tube was inserted and the location was examined by fiberoptic bronchoscope. The tidal volume of the ventilator was set to 8–10 mL/kg, RR was 15/min, inspiratory to expiratory ratio is 1:2, inspired oxygen concentration is 50%, EtCO2 was maintained at 30–35 mmHg, and OLV was performed at the beginning of the operation. Anesthesia was maintained with TCI of propofol (target plasma concentration of 1 to 2 ug/mL), sufentanil (target plasma concentration of 4 to 6 ug /mL), sevoflurane 1% to 2% and intermittent IV bolus of cisatracurium 0.1 to 0.2 mg/kg. BIS was monitored and maintained at 40 to 60. During the operation, the SpO2 was maintained ≥90%. After the operation, patients were normally extubated in the operating theater or sometimes remained intubated and were sent to recovery room. The timing of the tracheal extubation was decided by anesthetists. After the patient was fully awake, he (she) would return to the ICU.
Surgical process
Based on the identification of the nodules related to the branches of the PA, PV and bronchus by means of a multi-detector CT scanning or 3D reconstruction, a single or a combined segmentectomy was performed by sole VATS with no hybrid approaches. Patients were placed in the lateral position with the upper arms extended and fixed on the hand support. All procedures were performed with 3-port method. The thoracoscope was inserted in the 7th or 8th intercostal space anterior axillary line through a 1 cm incision with a soft incision protector covering skin, subcutaneous tissue, rib and parietal pleura. An anterior 2.5–3.5 cm incision was placed at the fifth intercostal space and the working port was generally at the 7th intercostal space in the posterior axillary line, to form a triangle on the chest wall. The distance between the incisions should be as far as possible, and they should be away from the lesion sites, which can facilitate the operation. Operative procedure began with the introduction of grasping forceps through operating incisions to help for surgical implementation and to locate the pulmonary nodule.
During operation, all patients in non-intubated group maintained spontaneous breathing. Surgeon could have a satisfactory view from the half compressed lung. It was naturally achieved in most instances by the pneumothorax that takes place when intrapleural and atmospheric pressure equilibrate through the incisions unless there are diffuse pleural adhesions. Approximately 6 mL of 2% lidocaine was sprayed onto the lung surface, then local vagus nerve was blocked with 2 mL of 2% lidocaine under thoracoscopic guidance in the chest cavity to reduce cough for a stable operation environment.
The surgical methods of thoracoscopic segmentectomy under different anesthesia modes were same as our previous study, which has been described in details (15). After deflating the lung, the arteries, veins and bronchial structure of the targeted lung segments were completely divided and sectioned with endoscopic stapling devices (Echelon 45 Endopath stapler; Ethicon Endo surgery Corp, Cincinnati, OH, USA). The root of the intersegmental veins was preserved and could be used as landmark for identification of the intersegmental plane, which was divided by an endoscopic stapler and electrocautery. For patients with suspected primary NSCLC, sampling or dissection of segmental, lobar, and hilar nodes followed by frozen-section analysis was required to confirm the indication of segmentectomy. If SpO2 dropped below 90% during the operation, assisted ventilation via LMA was needed to improve oxygenation. If hemodynamic status was unstable or intraoperative uncontrolled bleeding, the operation should be suspended to conversion from non-intubated anesthesia to intubated general anesthesia, which was decided by both of the surgeon and anesthetist (16). When the intrathoracic procedure was completed, an 18 Fr chest tube was routinely introduced through the inferior under direct camera visualization placed in the pleural apex. When the pleural cavity was closed and the wound was sutured, the collapsed lung would be re-expanded with a mild negative-pressure suction through the chest tube.
Fluid and chest tube management was the same in both groups. Chest tube could be removed when the fluid less than 200 mL in 24 hours, lung well re-expansion demonstrated on chest radiography, and no air leak. After patients discharged home, no activity restrictions or precautions were needed.
Statistical methods
Statistical analysis was performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as means ± standard deviations. Differences between two groups were assessed by the Student t-test. Categorical variables such as sex and smoking status were presented as frequencies (%). Differences between groups with continuous variables were assessed by χ2 test or Fisher exact test. Statistical significance was accepted as a P value was less than 0.05.
Results
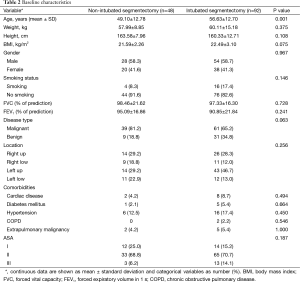
Between July 2011 and June 2015, a total of 140 patients with lung disease underwent VATS segmentectomy. Among them, 48 patients received surgery without tracheal intubation and 92 patients were treated under intubated general anesthesia with OLV. Significant difference was observed between two groups in terms of age. The demographic data and baseline characteristics were summarized in Table 2.
Full table
The surgical and postoperative outcomes were shown in Table 3. During the operation, the detectable mean peak EtCO2 of non-intubated group was higher (44.81 vs. 33.15 mmHg, P<0.001). Patients who underwent non-intubated segmentectomy also had lower △WBC (6.08×109vs. 7.75×109, P=0.004) and anesthesia cost (¥5,757.19 vs. ¥7,401.85, P<0.001), shorter duration of postoperative chest tube drainage (2.25 vs. 3.16 days, P=0.047), earlier resumption of oral intake (6.76 vs. 17.58 hours, P<0.001), and a trend toward shorter postoperative hospital stays (6.04 vs. 7.83 days, P=0.057) than those of intubated group. Two groups had comparable surgical duration (2.81 vs. 2.74 hours, P=0.643), intraoperative blood loss (75.10 vs. 56.65 mL, P=0.441), numbers of dissected lymph nodes of primary NSCLC patients (8.06 vs. 8.02, P=0.969), chest tube drainage volume (383.46 vs. 626.98 mL, P=0.145), △NEU% (neutrophil percentage changes before and after surgery) (31.5% vs. 32.6%, P=0.57), and intraoperative lowest SpO2 (98.0% vs. 96.8%, P=0.422). In the non-intubated group, one patient (2.1%) required conversion to intubated single-lung ventilation because of vigorous mediastinal movement. One patient in intubated group with uncontrolled bleeding was needed to convert from thoracoscopy to thoracotomy.

Full table
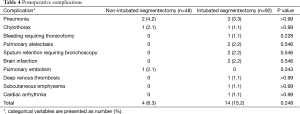
The postoperative complication result was shown in Table 4. The complication incidence in two groups was comparable (P=0.248). Four complications developed in four patients (8.3%) in the non-intubated group, including pneumonia, chylothorax and pulmonary embolism, whereas 14 complications were developed in 14 patients (15.2%) in the intubated group, including pneumonia, chylothorax, bleeding requiring thoracotomy, pulmonary atelectasis, sputum retention requiring bronchoscopy, brain infarction, deep venous thrombosis, subcutaneous emphysema and cardiac arrhythmia. There was no mortality in both groups.
Full table
Discussion
Thoracoscopic surgery without tracheal intubation has been applied successfully in managing selected patients with lung diseases by the cooperation between thoracic surgeons and anesthetists, which propels the thoracic surgery from the apparently minimally invasive surgical incision to the overall minimally invasive procedures that cover anesthesia-associated micro-injuries. By optimizing surgical treatment and anesthetic technique, we could minimize the adverse effects of tracheal intubation and general anesthesia, reduce surgical access trauma, reserve lung function, improve pain relief, and shorten the recovery time, etc. (13,15-17). By now, non-intubated thoracoscopic segmentectomy was considered to be one of the most minimally invasive procedures for major lung resection. To our knowledge, this was the first report to compare the short-term outcomes in patients undergoing non-intubated thoracoscopic segmentectomy with intubated patients, indicating that non-intubated thoracoscopic segmentectomy was technically feasible and could be performed as safe as the intubated surgery.
Patients could benefit from non-intubated thoracoscopic segmentectomy. One of the most important advantages was the avoidance of tracheal intubation and muscle relaxants, which could help patients exempt from anesthesia associated complications. The residual effect of muscle relaxants was considered as one of the most major issues that affect patient’s postoperative recovery and cause respiratory muscle weakness, hypoxemia and hypercapnia. Also, the throat muscle weakness might subsequently trigger upper respiratory tract obstruction and increase the risk of regurgitation and aspiration, etc. (8,18,19). As previous studies described, after TEA surgery, patients could achieve early ambulation and cough vigorously, which may facilitate sputum emission, pulmonary lobe expansion, better respiratory function and rapid recovery (20,21). Our data also indicated that non-intubated anesthesia could prompt patient’s recovery, including shorter postoperative chest drainage duration and hospital stay, lower △WBC, and earlier resumption of oral intake as well. The relatively early postoperative oral intake would improve the recovery of gastrointestinal peristalsis and the nutritional status of patient in a quick way, which was helpful to the early rehabilitation.
Patients could achieve rapid recovery when the stress and inflammation response was attenuated as well. Liu et al. indicated that in terms of VATS surgery under the non-intubated anesthesia, the level of postoperative inflammatory factors TNF-α and hs-CRP was significantly lower than those of intubated general anesthesia group (13). Some authors reported that TEA could inhibit sympathetic system via the blockade of both the afferent and efferent neural pathways, reduce the postsurgical stress response and the influence on postoperative lymphocyte responses (22,23). Our result also confirmed that △WBC was significantly lower in non-intubated group than that in the intubated group. However, TEA could not reduce the stress response completely. The most possible explanation was that the phrenic nerves which convey noxious stimuli to the central nervous system are not blocked by TEA. We speculated the avoidance of general anesthesia and OLV-related stress may account for the difference between two groups (24).
Four factors may affect the extensive application of the non-intubated VATS segmentectomy, which are intraoperative epidural anesthesia-associated cough reflex, respiratory movement including lung and mediastinum, possibility of conversion to intubated general anesthesia, and hypoxia and/or hypercapnia during OLV under spontaneous breathing. Firstly, in our experience, the cough reflex induced by surgical manipulation for the lung and bronchial trees often occurred during the non-intubated segmentectomy, while it was indeed infrequent during intubated segmentectomy. Heavy cough reflex might hamper the dissection of the hilar vessels and lymphadenectomy, leading to dangerous complications more prone to occur. The main predisposing factor was using TEA to inhibit sympathetic system and increase airway hyperreactivity. To abolish the cough reflex during the operation, we sprayed approximately 6 mL of 2% lidocaine onto the surface of lung and used simple intrathoracic vagal blockade, which was easy and effective to implement without affecting the haemodynamic status. In our study, there was no significant difference in numbers of dissected lymph nodes between the two types of anesthesia, which indicated that non-intubated anesthesia has no influence on lymph nodes dissection during the operation.
Secondly, respiratory movement and inadequate lung collapse might also increase the risk of morbidity and mortality. The manipulation for the pulmonary hilum is more difficult in the non-intubated segmentectomy than that in intubated general anesthesia with a quiet and wide-operating field. However, iatrogenic pneumothorax could induce a naturally collapse of the non-dependent lung and lead to an enough space for surgical maneuvering in most instances. In our study, only one patient converted to intubated general anesthesia due to significant mediastinal movement, which could not be effectively and completely controlled, and would make bronchovascular division difficult and dangerous. This case occurred at the beginning of the learning curve. The non-intubated technique is technically demanding for the surgeons and anesthetists who should be familiar with the surgical and anesthetic maneuvering under spontaneous breathing after an accumulation of experiences. However, caution of conversion from spontaneous breathing to intubated general anesthesia should be constantly alerted during the operation.
Thirdly, in our experience, the indication of the conversion from non-intubated anesthesia to intubated general anesthesia includes significant mediastinal or lung movement, persistent hypoxaemia, unstable hemodynamic status or uncontrolled bleeding (16). For conversion, surgeons should seal the wounds with transparent waterproof dressings after the placement of a chest tube to the apex through the camera port incision. This method has contributed to re-expand the lung for optimal oxygenation. At the same time, anesthetists need to perform intubation with a single-lumen endotracheal tube under guidance of fiber bronchoscopy or visual laryngoscopy, followed by insertion of a bronchial blocker, without changing the lateral position of patient. The conversion to intubated general anesthesia and mechanical ventilation by an expert and skilled anesthetist is safe and could be accomplished within a few minutes. In case of an uncontrollable emergency, surgeon should convert to intubated general anesthesia without hesitation. That is the key to reduce the rate of complications and the risk of emergency intubation.
Fourthly, another concern is the occurrence of hypercapnia and/or hypoxemia, especially when the duration of surgery is long. After the thorax was opened, intrapleural and atmospheric pressure equilibrate through the incision and that would lead to the collapse of the non-dependent lung. Our data showed that patients in non-intubated group breathed oxygen through LMA made it possible to maintain a good oxygen saturation during the operation. The lowest intraoperative SpO2 level was above 90%, which was comparable to the intubated group, indicating that simple administration of oxygen could prevent hypoxemia for most patients, except some temporary permissive hypercapnia (PHC).
PHC is a common phenomenon in prolonged non-intubated operations. The pathophysiology of hypercapnia might be associated with hypoventilation due to partial collapse of the non-dependent lung and a rebreathing effect caused by between-lung pendular ventilation. In our non-intubated group, the peak EtCO2 level was significantly higher than that of intubated group, which was consistent with previous report (17). Dong et al. reported that EtCO2 would rapidly fall toward normal value after the operation (25). Generally, hypercapnia that occurred in this procedure was well-tolerated, and it rarely had adverse effect on the hemodynamics (12). As was reported by some experts, in case of pH ≥7.15 and PaCO2<80 mmHg, adverse physiological effects of hypercapnia were reversible and often mild, and the PHC tolerance was relatively acceptable. In some patients, they could still tolerate well while their PaCO2 was higher than 150 mmHg and even 200 mmHg (26,27). In addition, central nervous mechanism would balance the increased carbon dioxide tension by raising RR. In our experience, if patients under non-intubated anesthesia experience significant respiratory depression, manual control or synchronized intermittent mandatory ventilation (SIMV) could be used to assist the ventilation. During the assisted ventilation, the ideal RR is generally controlled at about 12–20 bpm and the tidal volume should be at about 250 mL. Under this status, the patient’s respiratory maneuvers would not be too large, which facilitates surgeon’s surgical maneuvering, especially when it is close to the pulmonary hilus.
Some limitations should be acknowledged. First, our retrospective analysis with non-randomized design might introduce the possibility of bias in patient selection. Second, this study is limited by a single institutional experience with small sample size might have effects on the outcome and value of the study. Third, there is still not enough long-term evidence to support the benefits of non-intubated thoracoscopic segmentectomy over intubated thoracoscopic segmentectomy. Prospective studies in larger patient population are warranted to further validate the application of non-intubated anesthesia. Above factors and others out of our consideration or control may to some extent affect the convincingness of our research, we hope we could address these issues by further study.
Conclusions
In summary, this study reveals that non-intubated thoracoscopic segmentectomy is a safe and technically feasible option for experienced thoracoscopic surgeons. This technique seems to lead to comparable postoperative outcomes when compared with intubated segmentectomy. Proper non-intubated anesthesia indications and a multidisciplinary approach are key factors to success. As non-intubated thoracoscopic segmentectomy could promote rapid recovery and reduce anesthesia cost, it may become a valid alternative to intubated thoracoscopic segmentectomy in the future. In addition, as the experience of the surgical team accumulates, minimally invasive strategies with non-intubated anesthesia could be extended to even more challenging operations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Buckingham WW, Beatty AJ, Brasher CA, et al. The technique of administering epidural anesthesia in thoracic surgery. Dis Chest 1950;17:561-8. [Crossref] [PubMed]
- Petrovsky BV. Role of local anesthesia according to Vichnevsky in thoracic surgery. Anesth Anal 1952;9:75-9. [PubMed]
- Vischnevski AA. Local anesthesia in thoracic surgery: lungs, heart and esophagus. Minerva Anestesiol 1954;20:432-5. [PubMed]
- Bjork VO, Carlens E. The prevention of spread during pulmonary resection by the use of a double-lumen catheter. J Thorac Surg 1950;20:151-7. [PubMed]
- Hofmann HS, Rettig G, Radke J, et al. Iatrogenic ruptures of the tracheobronchial tree. Eur J Cardiothorac Surg 2002;21:649-52. [Crossref] [PubMed]
- Schneider T, Storz K, Dienemann H, et al. Management of iatrogenic tracheobronchial injuries: a retrospective analysis of 29 cases. Ann Thorac Surg 2007;83:1960-4. [Crossref] [PubMed]
- Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol (1985) 2000;89:1645-55. [PubMed]
- Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013;117:133-41. [Crossref] [PubMed]
- Sauer M, Stahn A, Soltesz S, et al. The influence of residual neuromuscular block on the incidence of critical respiratory events. A randomised, prospective, placebo-controlled trial. Eur J Anaesthesiol 2011;28:842-8. [Crossref] [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest 1997;111:230-5. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Guo Z, Shao W, Yin W, et al. Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis 2014;6:37-44. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Sundman E, Witt H, Olsson R, et al. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology 2000;92:977-84. [Crossref] [PubMed]
- Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand 1997;41:1095-1103. [Crossref] [PubMed]
- Li S, Cui F, Liu J, et al. Nonintubated uniportal video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Chin J Cancer Res 2015;27:197-202. [PubMed]
- Yang JT, Hung MH, Chen JS, et al. Anesthetic consideration for nonintubated VATS. J Thorac Dis 2014;6:10-3. [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109-17. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med 1994;150:1722-37. [Crossref] [PubMed]
- Mutlu GM, Factor P, Schwartz DE, et al. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia. Crit Care Med 2002;30:477-80. [Crossref] [PubMed]


