Survival and prognostic factors in patients undergoing the resection of solitary brain metastasis from non-small cell lung cancer: a retrospective cohort study
Introduction
Non-small cell lung cancer (NSCLC) is the most common malignancy that metastasizes to the brain. Brain metastasis (BM), which is considered to be terminal and has a poor prognosis, occurs in about 20–56% of NSCLC patients (1,2). Owing to advancements in imaging technologies and novel treatment regimens, including targeted therapy and immune therapy, primary tumors are generally well controlled and patient survival has been prolonged. Yet, the incidence of BM in NSCLC patients will still increase and BM could be the most significant disease associated with patient morbidity and mortality (3,4).
The treatment of BM in NSCLC patients is still controversial. Neurosurgical extirpation of metastatic lesions is the standard of care in cases where the BM is large and symptomatic or where histological confirmation is required (5). In particular, for patients with solitary or limited BM that can be surgically accessed, intracranial tumor resection can improve prognosis (6). Unfortunately, resection alone generally results in a high BM recurrence rate and poor outcomes, with a reported median postoperative survival time of 10–13.3 months (6,7).
To avoid local and distant intracranial recurrences, postoperative radiotherapy is traditionally recommended. Two randomized controlled trials have demonstrated that postoperative Whole-brain radiotherapy (WBRT) results in a marked decrease in local recurrence in patients with 1–3 BMs (8,9). However, whether adjuvant radiotherapy following BM resection can improve the overall survival (OS) of these patients remains unclear. Limited data from early randomized trials have indicated that postoperative radiotherapy has no significant effect on OS (8). A recent retrospective study demonstrated that in NSCLC patients with resected BM, those who received postoperative radiotherapy mainly of WBRT had markedly longer survival (10).
Meanwhile, Tendulkar et al. evaluated the prognostic factors for surgically-resected single BM and concluded that age, primary tumor/extracranial metastases, and SRS (but not WBRT) were independent factors for OS (11). Studies also identified other possible prognostic factors, including BM number, BM location, resection extent, pathology of adenocarcinoma, Recursive Partitioning Analysis classification, Karnofsky performance scale (KPS) score, postoperative treatment, etc. (12,13). In clinical trials investigating systemic treatments, the presence of BM was often an exclusion criterion, and in studies where it was not, included patients without neural symptoms were not routinely considered for BM diagnosis, as baseline brain imaging was not always performed (14-17). Moreover, tissue-based parameters in BM, such as the Ki67 tumor cell proliferation index (PI), were reportedly associated with OS (18). The positive expression of immune checkpoints in tumor cells and infiltrating immune cells might suggest an immunosuppressive microenvironment, which may also influence tumor growth and survival outcomes (19,20). We conducted immunohistochemical (IHC) staining in resected BM samples to examine the Ki67 PI and molecular expression levels of relevant targets and checkpoints, including epidermal growth factor receptor (EGFR), programmed death-1 (PD-1) and its ligand (PD-L1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and the T cell immunoglobulin domain and mucin domain-3 (TIM3), which were also analyzed as potential prognostic factors.
The present study aims to evaluate postoperative WBRT and other potential prognostic factors including patients’ demographic characteristics, clinical features, histopathological indicators and supplementary systemic treatments for OS after solitary BM resection in NSCLC patients with a controlled primary and extracranial disease. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1279/rc).
Methods
This is a retrospective cohort study. After approval by the Institutional Review Board of Huashan Hospital, Fudan University (approved No. 2020-670), the study was registered in the Chinese Clinical Trial Registry (ChiCTR2000036922) in August 2020. Informed consent was taken from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
From the medical records database, we retrospectively scanned all NSCLC patients with BM undergoing surgical resection of metastatic brain tumors in the Neurosurgery Department of our institute between January 2014 and December 2018. This period was chosen because targeted therapy was becoming more widely accepted and routinely recommended during this interval. Patients were recruited according to the following inclusion criteria: (I) patients with a confirmed diagnosis of BM from NSCLC; (II) patients with a solitary BM lesion; (III) patients receiving neurosurgery of complete excision for BM; (IV) cases in which the precise survival time was available, or those with a follow-up time of at least 2 years for patients whose survival time was censored; and (V) patients with stable or controlled primary lung tumor for more than 1 year after neurosurgery, unless they died of BM recurrence or neurological causes in the first year. The exclusion criteria were as follows: (I) patients with other tumors; (II) patients with other brain diseases, such as brain trauma, massive cerebral infarction, cerebral hemorrhage, encephalitis, or parasitic infection; (III) patients with severe cardiopulmonary diseases, including chronic obstructive pulmonary disease, interstitial pneumonia, coronary heart disease, or heart failure; and (IV) patients with liver or renal failure.
The demographic and clinical data of patients were collected from the medical records, outpatient follow-up records and telephone interviews (if necessary). Patients were usually followed up every 3–6 months in the outpatient clinic of the neurosurgery and/or respiratory departments. Brian and pulmonary imaging examinations were performed as required by physicians, and the electronically stored results could be easily retrieved according to the patient’s name and identification (ID). For those followed up in other hospitals, clinical data including postoperative treatments and regular imaging examinations were also obtained by contacting the patients and their relatives. Patients with insufficient follow-up time or unclear outcome were not included for analysis. Some patients were initially diagnosed with NSCLC via the pathological reports of resected BM tissues due to asymptomatic or silent primary lung cancer. After undergoing surgical extirpation of BM, these patients were also transferred to a lung cancer specialties department for further evaluation and standard treatment according to the accepted treatment guidelines. Other patients with original lung onset were found to have BM during or after lung-based treatments. Thus, postoperative local or systemic treatments were given as needed in this real-world cohort. Typically, WBRT was performed 1 month after neurosurgery with a dose of 30 Gy in ten daily fractions or 40 Gy in twenty daily fractions. The primary endpoint was OS, which was calculated as the time from the day of neurosurgical resection of BM to death or last follow-up. The second endpoint was brain progression-free survival (PFS), which was defined as the period from the day of neurosurgery to the day that local or distal relapse was seen on brain computer tomography (CT) or magnetic resonance imaging (MRI) scans.
Resected BM tissue samples were obtained for each patient. After formalin-fixation and paraffin-embedding according to standard laboratory practice, tissue blocks were stored and later cut into 3-µm-slices. IHC was performed using an automated slide processing and staining system. For the EGFR and immune checkpoints of PD-1, CTLA-4, and TIM3, colored staining within the anticipated expression sites was determined in contrast to the positive and negative controls. For PD-L1, a tumor proportion score <1% was regarded as negative; otherwise, it was considered positive. All of the above tissue-based parameters were estimated and confirmed by two pathologists. The Ki67 PI result, which was calculated according to the assessment method that counted 500 cells within the area of strongest staining to determine the percentage of positive cells (0–100%), was directly obtained from the routine pathological report of each patient. Protein expression was considered positive when Ki67 PI was more than 20%.
Statistical analysis
The baseline characteristics between the WBRT group (patients who received WBRT after surgical resection of BM) and the control group (patients who did not receive postoperative WBRT or other radiotherapy) were compared using the χ2 test. The OS of patients was estimated with the Kaplan-Meier product limit method, and group differences were assessed using the log-rank test. Univariate and multivariate analyses of potential prognostic factors were conducted using the Cox proportional hazards model. In the subgroup analysis, the hazard ratio (HR) and its 95% confidence interval (CI) were calculated for each variable comparing WBRT treatment with the untreated control. P<0.05 (two sided) was considered statistically significant. All statistical analyses were performed using the R software package (version 4.0.3, https://cran.r-project.org/bin/windows/base/old/4.0.3/, The R Foundation for Statistical Computing).
Results
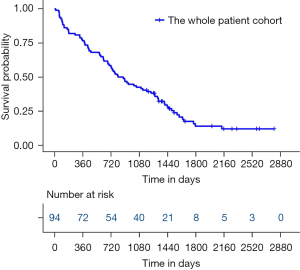
A total of 105 eligible patients diagnosed as NSCLC with a single BM who underwent BM resection were screened. Among them, 11 patients were excluded due to a loss of follow-up (three cases), extracranial disease progression in the first year after neurosurgery (four cases), and concomitant disorders (two cases of brain diseases, one case of heart disease, and one case of thyroid tumor). Finally, a total of 94 patients were recruited and analyzed. The median OS of this cohort was 812 days (Figure 1), with 1-, 2-, 3-, and 5-year survival rates of 76.6%, 56.4%, 42.6%, and 14.0%, respectively.
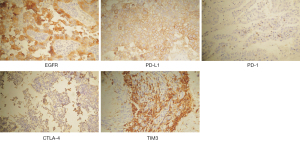
The patients’ baseline characteristics at the research starting point (the day of surgical treatment of BM) are listed in Table 1. Our cohort comprised 41 female and 53 male patients. Among them, 21 cases were elderly patients (≥65 years old) and 72 cases were independent with a KPS score ≥70. Most of the patients had a large BM (≥3 cm) that was located supratentorially. In the resected BM specimens, KI67 PI ≥20 was seen in 39.3% of patients. The percentages of patients with positive staining for EGFR, PD-L1, PD-1, CTLA-4, and TIM3 were 88.3%, 42.6%, 25.5%, 16.3%, and 87.2%, respectively. Examples of positive staining were illustrated in Figure 2. There were 34 patients (36.2%) who received postoperative WBRT, 46 patients (48.9%) who received postoperative chemotherapy, and 31 patients (33.0%) who received postoperative targeted therapy. Targeted therapy, according to the gene-sequencing test of the BM or primary lung samples, included EGFR tyrosine kinase inhibitors (Gefitinib, Erlotinib, and Afatinib) and an anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (Crizotinib). Twenty-four patients also underwent lung cancer surgery either before or after craniotomy depending on the onset disease, while the remaining patients did not (Table 1).
Table 1
| Variables | Level | WBRT (n=34) | Control (n=60) | P value |
|---|---|---|---|---|
| Gender (%) | Female | 11 (32.4) | 30 (50.0) | 0.149 |
| Male | 23 (67.6) | 30 (50.0) | ||
| Age (%) | <65 years | 26 (76.5) | 47 (78.3) | 1 |
| ≥65 years | 8 (23.5) | 13 (21.7) | ||
| KPS (%) | <70 | 7 (20.6) | 15 (25.0) | 0.817 |
| ≥70 | 27 (79.4) | 45 (75.0) | ||
| Tumor size (%) | <3.0 cm | 11 (32.4) | 21 (35.0) | 1 |
| ≥3.0 cm | 22 (64.7) | 38 (63.3) | ||
| Unknown | 1 (2.9) | 1 (1.7) | ||
| Tumor location (%) | Infratentorial | 6 (17.6) | 14 (23.3) | 0.7 |
| Supratentorial | 28 (82.4) | 46 (76.7) | ||
| Pathology (%) | Adenocarcinoma | 21 (61.8) | 38 (63.3) | 1 |
| Squamous carcinoma | 8 (23.5) | 14 (23.3) | ||
| Large cell carcinoma | 2 (5.9) | 2 (3.3) | ||
| Other | 3 (8.8) | 6 (10.0) | ||
| Status (%) | Censor | 7 (20.6) | 11 (18.3) | 1 |
| Dead | 27 (79.4) | 49 (81.7) | ||
| Ki67 PI (%) | <20 | 23 (67.6) | 31 (51.7) | 0.163 |
| ≥20 | 9 (26.5) | 26 (43.3) | ||
| Not reported | 2 (5.9) | 3 (5.0) | ||
| EGFR (%) | Negative | 4 (11.8) | 7 (11.7) | 1 |
| Positive | 30 (88.2) | 53 (88.3) | ||
| PD-L1 (%) | Negative | 19 (55.9) | 35 (58.3) | 0.989 |
| Positive | 15 (44.1) | 25 (41.7) | ||
| PD-1 (%) | Negative | 25 (73.5) | 45 (75.0) | 1 |
| Positive | 9 (26.5) | 15 (25.0) | ||
| CTLA-4 (%) | Negative | 25 (73.5) | 52 (86.7) | 0.212 |
| Positive | 8 (23.5) | 7 (11.7) | ||
| Unknown | 1 (2.9) | 1 (1.7) | ||
| TIM3 (%) | Negative | 4 (11.8) | 8 (13.3) | 1 |
| Positive | 30 (88.2) | 52 (86.7) | ||
| Post-operative chemotherapy (%) | No | 15 (44.1) | 33 (55.0) | 0.424 |
| Yes | 19 (55.9) | 27 (45.0) | ||
| Post-operative targeted therapy (%) | No | 25 (73.5) | 38 (63.3) | 0.434 |
| Yes | 9 (26.5) | 22 (36.7) | ||
| Lung cancer surgery (%) | No | 24 (70.6) | 46 (76.7) | 0.687 |
| Yes | 10 (29.4) | 14 (23.3) |
WBRT, whole-brain radiotherapy; KPS, Karnofsky Performance Scale; PI, proliferation index; EGFR, epidermal growth factor receptor; PD-L1, programmed death-ligand 1; PD-1, programmed death-1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; TIM3, T cell immunoglobulin domain and mucin domain-3.
Postoperative brain PFS was evaluated in 49 patients with precise brain progression imaging records. The median brain PFS was 809 (interquartile range, 562–1,079) days in the WBRT group (n=20) and 378 (interquartile range, 162–691) days in the non-WBRT control group (n=29), and the difference was statistically significant (P<0.001). The 1-year brain control rates (without local or distal intracranial relapse) were 85% in the WBRT group and 52% in the control group, and their 3-year control rates were 20% and 10%, respectively.
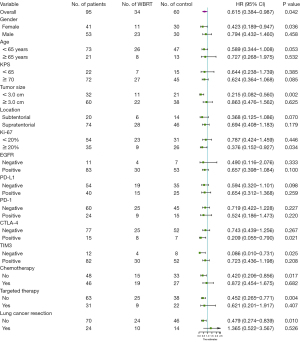
Using the Cox proportional hazards model, potential prognostic factors for OS were analyzed in 87 patients, due to missing data in 7 patients (Table 2). Univariable analysis showed that postoperative WBRT and targeted therapy were associated with OS (P<0.05). According to the backward linear regression multivariable analysis, postoperative WBRT (P<0.001, HR 0.357, 95% CI: 0.207–0.615), chemotherapy (P=0.008, HR 0.512, 95% CI: 0.313–0.839), targeted therapy (P<0.001, HR 0.265, 95% CI: 0.148–0.475), and smaller tumor size (P=0.018, HR 0.553, 95% CI: 0.338–0.904) were independent prognostic factors associated with prolonged OS. However, Ki67 PI, EGFR level, and each checkpoint level were found to be statistically insignificant factors.
Table 2
| Variables | Univariable Cox regression | Multivariable Cox regression | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| Gender | 0.576 | 0.879 (0.559–1.382) | – | – | |
| Age | 0.373 | 1.279 (0.744–2.199) | – | – | |
| KPS | 0.338 | 0.772 (0.455–1.311) | – | – | |
| Tumor size | 0.096 | 0.669 (0.418–1.073) | 0.018 | 0.553 (0.338–0.904) | |
| Tumor location | 0.330 | 0.768 (0.452–1.306) | – | – | |
| Pathology (other) | – | – | |||
| Adenocarcinoma | 0.626 | 0.830 (0.393–1.755) | |||
| Squamous carcinoma | 0.427 | 0.708 (0.302–1.660) | |||
| Large cell carcinoma | 0.484 | 0.575 (0.122–2.710) | |||
| Ki67 PI | 0.080 | 1.513 (0.951–2.408) | 0.719 | 1.092 (0.675–1.768) | |
| EGFR | 0.783 | 1.103 (0.547–2.224) | – | – | |
| PD-L1 | 0.619 | 0.890 (0.562–1.409) | – | – | |
| PD-1 | 0.878 | 0.959 (0.562–1.636) | – | – | |
| CTLA-4 | 0.688 | 0.877 (0.461–1.668) | – | – | |
| TIM3 | 0.469 | 0.789 (0.415–1.500) | – | – | |
| WBRT | 0.044 | 0.615 (0.384–0.987) | 0.000 | 0.357 (0.207–0.615) | |
| Chemotherapy | 0.180 | 0.733 (0.465–1.154) | 0.008 | 0.512 (0.313–0.839) | |
| Targeted therapy | 0.000 | 0.344 (0.199–0.595) | 0.000 | 0.265 (0.148–0.475) | |
| Lung cancer surgery | 0.286 | 0.745 (0.434–1.279) | – | – | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; KPS, Karnofsky Performance Scale; PI, proliferation index; EGFR, epidermal growth factor receptor; PD-L1, programmed death-ligand 1; PD-1, programmed death-1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; TIM3, T cell immunoglobulin domain and mucin domain-3; WBRT, whole-brain radiotherapy.
Kaplan-Meier curves were respectively drawn for the independent prognostic factors (Figure 3). The log-rank analyses demonstrated a statistically significant OS difference for postoperative adjuvant WBRT (P<0.05) and targeted therapy (P<0.01). For evaluating the confounding effects of other clinical factors on prognostic value of WBRT, the baseline variables were comparable between the WBRT and the control group, with no statistical significance for each variable (Table 1).
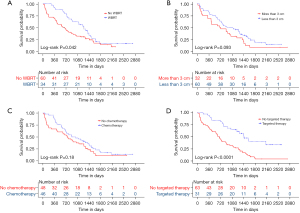
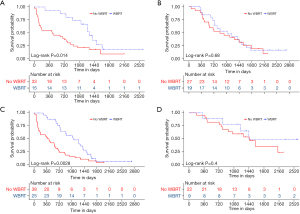
In the subgroup analysis, variable-related survival outcomes were compared between the WBRT treatment group and the untreated controls using the univariable Cox model (Figure 4), with a HR <1 preferring WBRT. A significance with P<0.05 was observed in selected patients with the following characteristics: female, tumor size <3 cm, Ki67 PI >20%, positive CTLA-4, negative TIM3, without chemotherapy, without targeted therapy, and without lung cancer resection. These results indicated that patients with the aforementioned characteristics might be more likely to benefit from postoperative WBRT. Specifically, for the two systemic treatments, Kaplan-Meier analyses also showed that postoperative WBRT significantly improved the survival outcomes in the subgroup that did not receive systemic treatments (chemotherapy and targeted therapy), but not in the subgroup that did receive either of these treatments (Figure 5).
Discussion
This retrospective study demonstrated that adjuvant WBRT after resection of solitary BM improved the prognosis of NSCLC patients with a stable or controlled extracranial disease. Other positive prognostic factors for these patients included the use of chemotherapy or targeted therapy and BM tumor size less than 3 cm. The beneficial effect of WBRT was especially seen in the patients who did not receive the two systemic treatments (chemotherapy and targeted therapy).
Radiotherapy can be delivered in the form of WBRT, single-fraction stereotactic radiosurgery (SRS), or multiple-fraction stereotactic radiation therapy (SRT). Prospective trials have demonstrated that postoperative radiotherapy using either WBRT or SRS resulted in a marked decrease in local recurrence (8,9,21). Although WBRT was associated with a long-term decline in cognitive function, postoperative WBRT had a similar or marginally better effect on local control compared to SRS/SRT (22,23). As shown in this study, the beneficial local effect of WBRT could be further expanded to improve OS in patients with controlled extracranial disease. A recent retrospective study also found that in NSCLC patients with resected BM, postoperative radiotherapy (65% with WBRT, 25% with SRS, and 10% with both) had a significantly positive effect on OS, while multiple brain metastases and extracerebral metastases of NSCLC were negative prognostic factors (10). Limited early data has indicated no significant effect of WBRT on OS, probably because the survival time at that time was relatively shorter due to the uncontrolled primary and metastatic disease. Considering the detrimental effect of WBRT on cognitive function, postoperative SRS/SRT for the residual cavity after BM resection was recently discussed and recommended (24,25). Emerging data has also suggested that SRT may provide superior local control to SRS (25,26). However, whether adjuvant SRS/SRT after BM resection could result in a prolonged survival needs to be further investigated. With the development of hippocampus-avoiding and other techniques, WBRT will likely still be widely used as a standard of care, before SRS/SRT could, if possible, replace WBRT.
Another retrospective study including patients who underwent resection of BM from different original malignancies demonstrated that the significant factors associated with improved OS included a higher KPS score, use of targeted treatment, controlled primary disease, and single BM (27). NSCLC with oncogenic driver mutations, such as EGFR or ALK, had a high predilection for BM compared to unselected patients (3,28). In this study, targeted treatment was again identified as an independent prognostic factor, which could be partly attributed to the use of new generation targeted drugs with a higher blood-brain barrier (BBB) penetrating rate and an improved central nervous system (CNS) efficacy. Similarly, this study also identified chemotherapy as a positive prognostic factor, because cytotoxic therapy has a controlling effect on BM (29). BM of lung cancer disrupts the structure of the BBB, forming a blood-tumor barrier (BTB). Recent research found that chemotherapeutic drugs can cross the BTB of BM despite their difficulty in crossing the normal BBB (30). However, in the subgroup of patients who received either targeted therapy or chemotherapy, we found that WBRT did not demonstrate survival benefits, which is consistent with the result of a meta-analysis that found no OS benefit with the addition of targeted therapy to radiotherapy (31). However, despite previous studies demonstrating that the KPS score is associated with survival outcomes (27,32), we did not observe this in the present study, which might attributable to the high overall KPS scores of our patients. Combining the beneficial features of high KPS, controlled primary disease, and single BM, it is not surprising that the median OS in this patient cohort was 27.1 months, which was longer than that reported previously (11.6–21.1 months) (10,22,27).
Unexpectedly, we failed to observe the effect of pathological parameters on the OS. A previous retrospective study reported that low Ki67 PI and administration of adjuvant WBRT were independently associated with a favorable OS (18); yet, such results were not fully reproduced in our study. However, sub-group analysis indicated that WBRT might be more beneficial for patients with a high Ki67 PI. Although EGFR signaling is critical for BM and aggressive NSCLC behavior, EGFR-targeted therapies are effective for both primary and metastatic lesions. In this cohort, patients with positive EGFR expression in their BM tissue did not fully match those receiving targeted therapy, which is due to the inconsistent IHC staining and gene sequencing results (33) as well as the heterogeneity between the primary lung cancer and BM (34). This might partially explain why the EGFR expression level was not identified as a prognostic factor. Similarly, checkpoint levels, which can reflect the landscape of tumor-infiltrating immune cells, were also not found to be associated with prognosis.
This study has several limitations that should be noted. Firstly, due to its single-center retrospective design, this study has an inherent selection bias. Also, the sample size might be not sufficient for discovering statistical significance in certain factors. Secondly, the number of variables with complete medical records that could be included in the analysis was limited. Other possible factors, such as concomitant disorders and immune checkpoint therapy, were not taken into account. Furthermore, immune therapy was still not routinely administrated for the patient cohort in our institute during the study period. Moreover, the effect of WBRT on the brain PFS was only evaluated in some cases, and the WBRT-associated cognitive decline was not investigated. Finally, by multivariable Cox regression, we could only identify independent prognostic factors; however, the interaction between factors, such as the synergistic anti-tumor effect of combined radiotherapy and targeted therapy, was not evaluated. Therefore, more studies are warranted to confirm our results.
Conclusions
This retrospective study demonstrated that postoperative WBRT after BM resection resulted in a prolonged OS for NSCLC patients with a single BM and the controlled primary lung tumor. The adjuvant systemic treatments of chemotherapy and targeted therapy after neurosurgery also improved the prognosis for these patients. Postoperative WBRT could be considered, especially for patients who do not receive systemic treatments, as they might be more likely to benefit from it.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81000489) and the Medical Project of the Shanghai Municipal Science and Technology Commission (No. 16411954100).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1279/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1279/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1279/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approval by the Institutional Review Board of Huashan Hospital, Fudan University (approved No. 2020-670). Informed consent was taken from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sudmeier L, Tian S, Higgins KA. Multidisciplinary Management of Brain Metastases from Non-Small Cell Lung Cancer in the Era of Immunotherapy. Curr Treat Options Oncol 2021;22:77. [Crossref] [PubMed]
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers 2019;5:5. [Crossref] [PubMed]
- Lee J, Ahn MJ. Brain metastases in patients with oncogenic-driven non-small cell lung cancer: Pros and cons for early radiotherapy. Cancer Treat Rev 2021;100:102291. [Crossref] [PubMed]
- Wrona A, Dziadziuszko R, Jassem J. Management of brain metastases in non-small cell lung cancer in the era of tyrosine kinase inhibitors. Cancer Treat Rev 2018;71:59-67. [Crossref] [PubMed]
- Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 2014;11:203-22. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Bougie E, Masson-Côté L, Mathieu D. Comparison Between Surgical Resection and Stereotactic Radiosurgery in Patients with a Single Brain Metastasis from Non-Small Cell Lung Cancer. World Neurosurg 2015;83:900-6. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Fuchs J, Früh M, Papachristofilou A, et al. Resection of isolated brain metastases in non-small cell lung cancer (NSCLC) patients - evaluation of outcome and prognostic factors: A retrospective multicenter study. PLoS One 2021;16:e0253601. [Crossref] [PubMed]
- Tendulkar RD, Liu SW, Barnett GH, et al. RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys 2006;66:810-7. [Crossref] [PubMed]
- She C, Wang R, Lu C, et al. Prognostic factors and outcome of surgically treated patients with brain metastases of non-small cell lung cancer. Thorac Cancer 2019;10:137-42. [Crossref] [PubMed]
- Nakao T, Okuda T, Yoshioka H, et al. Clinical Outcomes of Surgical Resection for Brain Metastases from Non-small Cell Lung Cancer. Anticancer Res 2020;40:4801-4. [Crossref] [PubMed]
- Levy A, Faivre-Finn C, Hasan B, et al. Diversity of brain metastases screening and management in non-small cell lung cancer in Europe: Results of the European Organisation for Research and Treatment of Cancer Lung Cancer Group survey. Eur J Cancer 2018;93:37-46. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
- Liu L, Gao Q, Jiang J, et al. Randomized, multicenter, open-label trial of autologous cytokine-induced killer cell immunotherapy plus chemotherapy for squamous non-small-cell lung cancer: NCT01631357. Signal Transduct Target Ther 2020;5:244. [Crossref] [PubMed]
- Peters S, Dziadziuszko R, Morabito A, et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial. Nat Med 2022;28:1831-9. [Crossref] [PubMed]
- Berghoff AS, Ilhan-Mutlu A, Wöhrer A, et al. Prognostic significance of Ki67 proliferation index, HIF1 alpha index and microvascular density in patients with non-small cell lung cancer brain metastases. Strahlenther Onkol 2014;190:676-85. [Crossref] [PubMed]
- Vilariño N, Bruna J, Bosch-Barrera J, et al. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat Rev 2020;89:102067. [Crossref] [PubMed]
- Hulsbergen AFC, Mammi M, Nagtegaal SHJ, et al. Programmed Death Receptor Ligand One Expression May Independently Predict Survival in Patients With Non-Small Cell Lung Carcinoma Brain Metastases Receiving Immunotherapy. Int J Radiat Oncol Biol Phys 2020;108:258-67. [Crossref] [PubMed]
- Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1040-8. [Crossref] [PubMed]
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1049-60. [Crossref] [PubMed]
- Rusthoven CG, Yamamoto M, Bernhardt D, et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol 2020;6:1028-37. [Crossref] [PubMed]
- Redmond KJ, De Salles AAF, Fariselli L, et al. Stereotactic Radiosurgery for Postoperative Metastatic Surgical Cavities: A Critical Review and International Stereotactic Radiosurgery Society (ISRS) Practice Guidelines. Int J Radiat Oncol Biol Phys 2021;111:68-80. [Crossref] [PubMed]
- Akanda ZZ, Hong W, Nahavandi S, et al. Post-operative stereotactic radiosurgery following excision of brain metastases: A systematic review and meta-analysis. Radiother Oncol 2020;142:27-35. [Crossref] [PubMed]
- Eitz KA, Lo SS, Soliman H, et al. Multi-institutional Analysis of Prognostic Factors and Outcomes After Hypofractionated Stereotactic Radiotherapy to the Resection Cavity in Patients With Brain Metastases. JAMA Oncol 2020;6:1901-9. [Crossref] [PubMed]
- Koo J, Roh TH, Lee SR, et al. Whole-Brain Radiotherapy vs. Localized Radiotherapy after Resection of Brain Metastases in the Era of Targeted Therapy: A Retrospective Study. Cancers (Basel) 2021;13:4711. [Crossref] [PubMed]
- Fujita Y, Kinoshita M, Ozaki T, et al. The impact of EGFR mutation status and single brain metastasis on the survival of non-small-cell lung cancer patients with brain metastases. Neurooncol Adv 2020;2:vdaa064. [Crossref] [PubMed]
- Wang B, Guo H, Xu H, et al. Research Progress and Challenges in the Treatment of Central Nervous System Metastasis of Non-Small Cell Lung Cancer. Cells 2021;10:2620. [Crossref] [PubMed]
- Ye LY, Sun LX, Zhong XH, et al. The structure of blood-tumor barrier and distribution of chemotherapeutic drugs in non-small cell lung cancer brain metastases. Cancer Cell Int 2021;21:556. [Crossref] [PubMed]
- Singh R, Lehrer EJ, Ko S, et al. Brain metastases from non-small cell lung cancer with EGFR or ALK mutations: A systematic review and meta-analysis of multidisciplinary approaches. Radiother Oncol 2020;144:165-79. [Crossref] [PubMed]
- Chen XR, Hou X, Li DL, et al. Management of Non-Small-Cell Lung Cancer Patients Initially Diagnosed With 1 to 3 Synchronous Brain-Only Metastases: A Retrospective Study. Clin Lung Cancer 2021;22:e25-34. [Crossref] [PubMed]
- Machado-Rugolo J, Fabro AT, Ascheri D, et al. Usefulness of complementary next-generation sequencing and quantitative immunohistochemistry panels for predicting brain metastases and selecting treatment outcomes of non-small cell lung cancer. Hum Pathol 2019;83:177-91. [Crossref] [PubMed]
- Han CH, Brastianos PK. Genetic Characterization of Brain Metastases in the Era of Targeted Therapy. Front Oncol 2017;7:230. [Crossref] [PubMed]
(English Language Editor: A. Kassem)