Development and validation of a new prediction model for calcific aortic valve stenosis
Introduction
Calcific aortic valve stenosis (CAVS) is the most common heart valve disease in developed countries (1). It is characterized by slowly progressing calcification and remodeling of valve leaflet fibers, which leads to a decrease in valvular activity, a gradual narrowing of valvular leaflets, and finally, progressive blood flow obstruction (2). CAVS is the prevailing form of acquired valve disease requiring surgical treatment and has the highest mortality rate among valvular diseases in the United States (3). Moreover, it is strongly associated with mortality and morbidity in elderly patients (4). Indeed, studies have reported that CAVS is the third leading cause of cardiovascular disease (CV) after coronary artery disease and systemic arterial hypertension (2), with a prevalence of 12% in the elderly (>75 years), and that its prevalence increases with age (2,5).
Similar to atherosclerosis, CAVS is generally characterized by lipid accumulation, inflammation, and calcification (6). Most patients have no obvious clinical symptoms in the early stages, but as the disease advances, the aortic valve leaflets progressively calcify, remodel, and thicken, which, in turn, restricts valve opening and leads to severe obstruction of the left ventricular outflow tract. Due to hemodynamic changes, the left ventricular afterload gradually increases, causing left ventricular hypertrophy. In advanced stages, patients usually present with life-threatening clinical symptoms such as syncope, angina pectoris, and heart failure (7). The incidence of the above symptoms represents decompensation of heart function; the disease progresses rapidly and the prognosis is extremely poor. Nearly 50% of patients have a natural life span of fewer than 2 years (8). Due to the prolongation of average life expectancy and the rapid aging of the global population, the prevalence and mortality of CAVS are also expected to increase further (9).
CAVS is generally considered a degenerative disease that occurs with age and is primarily caused by passive calcium deposition in the valves. However, scientists have discovered that the disease is also driven by interactions between genetic factors, lipid permeation, and chronic inflammation. These factors are triggered by hemodynamic stress-related injury and are modulated by risk factors such as hypertension and hypercholesterolemia (10,11). Studies have demonstrated that the development of CAVS is associated with immune cell infiltration, inflammatory response, abnormal lipid metabolism, abnormal signaling pathway transduction, coronary atherosclerosis, and vascular fibrosis (12-15). Disease prevention and drug targets for CAVS have become new research hotspots. Therefore, it is of great clinical significance to clarify the risk factors associated with the occurrence of calcific aortic stenosis in patients, further explore the correlation between calcific aortic stenosis, inflammatory response, and lipid levels. In addition, it is also crucial to lay the groundwork for an in-depth study of the underlying disease mechanisms and develop effective target-specific interventional therapies for the treatment and prevention of this disease. At present, there are limited reports on the construction of prediction models for CAVS. In this study, we screened the risk factors and constructed a nomogram that may predict the occurrence of CAVS to help with early clinical screening of potential CAVS risk groups. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1157/rc).
Methods
Patients
The data obtained from the medical data intelligent platform were processed anonymously to protect the privacy of the patients. This study was performed in line with the Helsinki Declaration (as revised in 2013) and was approved by the Institutional Ethical Committee of the Affiliated Hospital of Qingdao University (No. QYFY WZLL 26950). Due to the retrospective retrieval of the patients' data, the requirement for informed consent was waived. We enrolled patients from the cardiovascular surgery department and physical examination center who were admitted to our hospital from September 2016 to September 2020. The complete medical records of the patients were retrieved from the electronic medical record system of the hospital. They were divided into a CAVS group and a normal group depending on the presence or absence of aortic stenosis, as determined by cardiac ultrasound.
Variables
The clinical data regarding the patient’s first visit to our hospital or their first admission were recorded, including, but not limited to, the following: gender, age, height, weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, as well as a history of hypertension, diabetes, drinking, smoking, carotid atherosclerosis, and chronic kidney disease (CKD). The laboratory test results encompassed routine blood, blood biochemistry, liver and renal function, and other common clinical indicators. Echocardiography results were also considered.
Inclusion and exclusion criteria
Inclusion criteria
(I) Patients included in the case group were eligible for surgery for simple degenerative aortic stenosis according to the 2020 American College Of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Management of Patients With Valvular Heart Disease (16); (II) echocardiography results in the control group were characterized by no abnormalities or only decreased left ventricular diastolic function; and (III) individuals with complete clinical data.
Exclusion criteria
(I) Patients considered to have rheumatic heart disease based on clinical history, cardiac ultrasound results, or surgical exploration findings; (II) patients diagnosed with congenital bicuspid aortic valve malformation; (III) patients with infective endocarditis; (IV) patients with a previous history of valve surgery; (V) patients with hypothyroidism or parathyroid disease; (VI) patients with a clear history of myocardial infarction; (VII) patients with concurrent tissue or organ infections; and (VIII) patients diagnosed with a connective tissue disease.
Diagnostic criteria for aortic stenosis
According to the 2020 ACC/AHA Guidelines for the Management of Patients with Valvular Heart Disease (16), the area of normal aortic valve orifices in adults ranges from 3.0 to 4.0 cm2. Hemodynamic changes are observed when the mean transvalvular pressure difference exceeds 40mmHg. Mild aortic stenosis is defined as aortic stenosis with a maximum transvalvular flow (Vmax) of 2.0–2.9 m/s or a mean transvalvular pressure difference of <20 mmHg. To be defined as moderate aortic stenosis, the Vmax of transvalvular blood has to be from 3.0 to 3.9 m/s, or the mean transvalvular pressure difference has to be from 20 to 39 mmHg. Severe aortic stenosis is defined as aortic stenosis involving obvious thickening and fusion with calcification in the valvular lobe, accompanied by a significantly reduced valvular activity, a maximum blood flow velocity ≥4 m/s across the aortic valve, and an aortic valve orifice area ≤1.0 cm2.
Statistical analysis
The Least Absolute Shrinkage Selection Operator (LASSO) method for high-dimensional data regression was used to select the most useful predictors from the original dataset (17). To further improve the clinical applicability of the model and reduce the sample size requirements of the subsequent logistics regression, we set the λ value to one standard error when implementing the LASSO regression. Next, univariate and multivariate logistic regression analyses were performed to analyze the possible risk factors of CAVS, and a nomogram was prepared to visualize the regression results. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to evaluate the diagnostic efficiency of the nomogram. The Youden index is equal to sensitivity plus specificity minus one, so the optimal sensitivity and specificity can be calculated based on the maximum Youden index. In addition, a calibration curve and clinical decision curve analysis (DCA) were used to evaluate the goodness of fit and clinical practicability of the nomogram. The statistical software utilized included the SPSS software (version 26.0, IBM, Armonk, New York, United States) and R software (version 4.0.5) (The R Foundation for Statistical Computing, Vienna, Austria), and the R packages (https://www.R-project.org) included “glmnet”, “RMS”, “Foreign”, “caret”, “nricens”, etc. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Patients
A total of 548 patients were eligible according to the inclusion and exclusion criteria, including 442 in the control group and 106 in the CAVS group. To build and validate the model correctly, the patients were first grouped randomly based on the following proportions: 70% in the training set and 30% in the validation set. After random grouping, there were 384 patients in the training set and 164 patients in the validation set.
Screening of clinical characteristics
Although there were only 384 patients in the training set, the number of indicators was as high as 48. Therefore, the LASSO regression analysis for high-dimensional data regression selected the most useful predictors from the original dataset (17). After screening, the 48 clinical-pathological indicators were reduced to 15 potential predictors (Figure 1A,1B) among the 384 patients in the training set. These 15 predictors were as follows: history of hypertension, carotid atherosclerosis, age, diastolic blood pressure, C-reactive protein, direct bilirubin, alkaline phosphatase, aspartate aminotransferase, albumin/globulin ratio, low-density lipoprotein (LDL), lipoprotein(a) [Lp(a)], uric acid, creatinine, blood urea nitrogen/creatinine ratio, and cystatin C.

Establishment of the nomogram
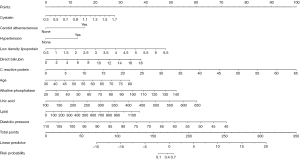
The 15 clinical indicators selected by LASSO regression were assessed by univariate analysis, and the results are shown in Table 1. The history of hypertension, carotid atherosclerosis, age, diastolic blood pressure, C-reactive protein, direct bilirubin, alkaline phosphatase, aspartate aminotransferase, LDL, Lp(a), uric acid, blood urea nitrogen/creatinine, and cystatin C were related to the occurrence of diseases (all P<0.05). Subsequently, all of the above meaningful indicators were included in the multivariate analysis of binary logistic regression. The results revealed that the history of hypertension, carotid atherosclerosis, age, diastolic blood pressure, C-reactive protein, direct bilirubin, alkaline phosphatase, LDL, Lp(a), uric acid, and cystatin C were independent influencing factors (all P<0.05). Therefore, a nomogram model was constructed to predict the occurrence of aortic stenosis in patients according to the above 11 indicators (Figure 2).
Table 1
| Characteristics | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||||
| Upper limit | Lower limit | Upper limit | Lower limit | ||||||
| Hypertension | 4.116 | 2.404 | 7.048 | <0.001 | 6.056 | 1.597 | 22.97 | 0.008 | |
| Carotid atherosclerosis | 15.402 | 7.573 | 31.324 | <0.001 | 10.577 | 2.59 | 43.197 | 0.001 | |
| Age | 1.196 | 1.148 | 1.246 | <0.001 | 1.109 | 1.033 | 1.191 | 0.004 | |
| Diastolic pressure | 0.915 | 0.889 | 0.942 | <0.001 | 0.858 | 0.801 | 0.92 | <0.001 | |
| C-reactive protein | 1.127 | 1.034 | 1.227 | 0.006 | 1.299 | 1.022 | 1.652 | 0.033 | |
| Direct bilirubin | 1.224 | 1.1 | 1.362 | <0.001 | 1.35 | 1.08 | 1.687 | 0.008 | |
| Alkaline phosphatase | 1.029 | 1.014 | 1.045 | <0.001 | 1.06 | 1.017 | 1.105 | 0.006 | |
| AST | 1.035 | 1.01 | 1.061 | 0.006 | 1.05 | 0.998 | 1.105 | 0.062 | |
| A/G ratio | 1.857 | 0.757 | 4.552 | 0.176 | |||||
| Low density lipoprotein | 4.432 | 2.605 | 7.541 | <0.001 | 3.414 | 1.024 | 11.385 | 0.046 | |
| Lp(a) | 1.006 | 1.004 | 1.009 | <0.001 | 1.004 | 1.001 | 1.008 | 0.043 | |
| Uric acid | 1.012 | 1.009 | 1.016 | <0.001 | 1.016 | 1.008 | 1.025 | <0.001 | |
| Creatinine | 0.994 | 0.982 | 1.007 | 0.394 | |||||
| BUN/CREA ratio | 1.069 | 1.036 | 1.103 | <0.001 | 1.07 | 0.993 | 1.153 | 0.077 | |
| Cystatin C | 156.31 | 40.906 | 597.298 | <0.001 | 17.755 | 1.325 | 237.88 | 0.03 | |
OR, odds ratio; CI, confidence interval; AST, aspartate aminotransferase; A/G ratio, Albumin/Globulin ratio; Lp(a), lipoprotein(a); BUN/CREA ratio, blood urea nitrogen/creatinine ratio.
Validation of the nomogram
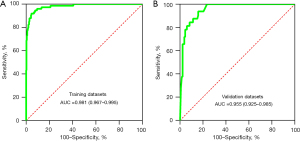
The calibration curve of the nomogram for predicting aortic stenosis showed good agreement between the predicted and actual observed probability values, and the same was observed in the validation set (Figure 3). In addition, in the training set of the model, the χ2 of the Hosmer-Lemeshow test was 2.086 and the P value was 0.978. In the independent validation set of the model, the χ2 was 6.315 and the P value was 0.612, indicating that the nomogram had a good fitting degree and did not deviate from a perfect fit with the actual value. In addition, the AUCs of the model in the training was 0.981 [95% confidence interval (CI): 0.967–0.995], with a sensitivity of 91.89% and a specificity of 95.48%. The nomogram also performs well in the validation set, with an AUC of 0.955 (95% CI: 0.925–0.985), sensitivity of 93.75%, and specificity of 84.09% (Figure 4), which proved that the prediction accuracy of the model was very high.

Clinical practicability of the nomogram
Figure 5 illustrates the DCA of the nomogram in predicting clinical aortic stenosis. The DCA curve demonstrates that when the prediction probability was 0.01–0.99, the net benefit was positive in the training set, and when the prediction probability was 0.01–0.86, the net benefit was positive in the validation set (Table 2). Therefore, in a large probability interval, undergoing an early intervention according to the nomogram could help prevent the occurrence of aortic stenosis.

Table 2
| Subgroup | Net benefit interval |
|---|---|
| Training set | 0.01–0.99 |
| Validation set | 0.01–0.86 |
DCA, decision curve analysis.
Discussion
CAVS represents a growing medical burden for the aging population, yet to date, there are no effective drugs for the prevention or delay of the occurrence and progression of CAVS, and the only viable treatments are surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) (18). However, both of these techniques are inevitably accompanied by serious complications. First, the implantation of a mechanical heart valve increases the risk of thrombosis, leading to the requirement for lifelong anticoagulation therapy. Second, bioprosthetic valves are prone to deterioration, limiting their durability and possible reoperation within less than 15 years (19). Therefore, early prevention of CAVS is particularly important. Since multiple factors and mechanisms influence CAVS, previous univariate stratified risk prediction studies have been unable to meet the needs of clinicians. In the current study, regression analysis demonstrated that the history of hypertension, history of carotid atherosclerosis, age, diastolic blood pressure, C-reactive protein, direct bilirubin, alkaline phosphatase, LDL, lipoprotein(a), uric acid, and cystatin C were independent factors impacting the occurrence of the disease, which is consistent with the results of previous studies (20-26). Based on these variables, a nomogram capable of individually predicting the risk of developing CAVS in patients was constructed, which included 11 clinical characteristic variables that can be easily obtained from clinical records and routine laboratory tests and have a high clinical utility. We hope that this model will aid in the early screening of potential CAVS risk groups and the implementation of secondary prevention interventions to improve patient prognosis.
Endothelial injury on the aortic side of the valve represents the initiating event of CAVS due to the increased mechanical stress and decreased shear stress. The loss of endothelial integrity triggers inflammation and lipid accumulation by promoting infiltration by monocytes, mast cells, T cells, and lipoproteins, which include LDL and Lp(a) (27). This study showed that LDL and Lp(a) were significantly associated with the development of CAVS (P=0.046, P=0.043). When inflammation enters the subcutis, LDL in cells is oxidized to oxidized low-density lipoprotein (oxLDL), which is recognized by macrophage clearance receptors, generating foam cells and inducing further oxidative stress and inflammatory responses (28). In addition to the above mechanisms, Lp(a) and LDL are also involved in lipid metabolism (29). Studies have shown that the Lp(a) and LDL levels play an important role in the pathophysiology of valvular calcification (30). Lp(a) is composed of LDL and apolipoprotein B (ApoB), and as a major carrier of oxidized phospholipids, Lp(a) can induce a series of cascade reactions that ultimately lead to calcification and stiffness of the aortic valve (31). In addition, inflammation plays a crucial role in the pathogenesis of CAVS. The concentration of C-reactive protein, a commonly used inflammatory index in clinical practice, can reflect the degree of inflammation in the aortic valve to some extent. Researchers have discovered that the role of C-reactive protein in aortic valve calcification in an in vitro model was to exert an indirect effect on the blood vessels, with the rate of calcification in the aortic wall increasing with the increasing C-reactive protein concentration (32,33). The presence of local and systemic C-reactive protein is further evidence that inflammation plays an important role in valve degeneration (34,35). In the present study, C-reactive protein was associated with the development of CAVS and was an independent risk factor for the occurrence of CAVS (odds ratio =1.299, 95% CI: 1.022–1.652, P=0.033).
Carotid atherosclerosis is defined as carotid intima-media thickness (CIMT) ≥1.0 mm or the presence of carotid plaque (36). A previous study reported an association between the presence of carotid atherosclerosis and degenerative aortic stenosis (37); the risk of carotid atherosclerosis was 2.1 times higher in patients with aortic valve sclerosis than in control patients (38). In the current study, the risk of carotid atherosclerosis was 10 times higher in patients with aortic valve stenosis. This finding further strengthens the hypothesis that carotid atherosclerosis and degenerative aortic stenosis are related. This study also revealed that hypertension was a significant predictor of CAVS (odds ratio =6.056, 95% CI: 1.597–22.97, P=0.008), which is consistent with previous findings (39). Moreover, hypertension has been associated with increased left ventricular structural abnormalities in patients with early asymptomatic mild to moderate aortic stenosis and has been identified as a major cause of the increased morbidity and mortality rates of CV in the general population (40). The Mendelian randomization study suggested an association between elevated systolic blood pressure and an increased risk of aortic stenosis (41). Large arteries in elderly individuals have poor elasticity, which is usually coupled with elevated systolic blood pressure and low diastolic blood pressure. Interestingly, however, the present study demonstrated that elevated diastolic blood pressure was associated with a reduced risk of CAVS and was a protective factor against the development of CAVS. A large pulse pressure difference in patients with poor systemic vascular conditions and limited left ventricular ejection fraction in patients with severe CAVS could explain the lower diastolic blood pressure; further studies on the hemodynamics of CAVS are warranted to confirm this theory. Additionally, age was also an independent risk factor for CAVS in previous studies, which was validated by our results. For every 1-year increase in age in this study, the risk of developing CAVS increased by 1.109 times (P=0.004). Lindroos et al. reported that calcific degeneration of the aortic valves becomes more common with advancing age (4), indicating that age is an important factor in CAVS development and can help to effectively predict the occurrence of CAVS (42).
A study has shown that the serum uric acid level is an independent risk factor for the development of CV (6). In the present study, the occurrence of CAVS was significantly and positively correlated with serum uric acid levels (odds ratio =1.016, 95% CI: 1.008–1.025, P<0.001). Elevated serum uric acid levels may induce CAVS and accelerate its progression by causing endothelial dysfunction and accelerating LDL oxidation. Similarly, the deposition of urate crystals on the aortic valve may also promote valve degeneration and accelerate CAVS progression (43). Therefore, lowering serum uric acid levels may, to some extent, delay or prevent the progression of CAVS. Alkaline phosphatase is a phosphate monoester hydrolase that is widely present in the liver and bones; numerous studies have demonstrated that it is highly correlated with calcium deposition in the interstitial component of the valve (44,45). Inhibition of transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein 2 (BMP-2) activities can lead to reduced expression of alkaline phosphatase, which in turn reduces calcium deposition in aortic valve interstitial cells, suggesting that alkaline phosphatase plays an important role in the process of aortic valve fibrosis (46). Increased alkaline phosphatase activity is associated with increased calcified nodules and calcium and phosphorus deposition (47). In another study, Liu et al. discovered that alkaline phosphatase plays an important role in the development of aortic valve fibrosis (48). In the present study, alkaline phosphatase was also found to be associated with the pathogenesis of CAVS (P=0.006), which was consistent with the abovementioned findings, suggesting that it could be involved in the occurrence of CAVS through various mechanisms and could be an important predictor of the disease.
In stenotic valves, a significant increase in messenger RNA (mRNA) and protein expression of cystatin C is usually detected in the infiltrated area of inflammatory cells (49). The expression of cystatin C and TGF-β1 is also significantly increased in non-rheumatic calcific aortic valve tissue compared with normal aortic valve tissue (50). It has been shown that TGF-β1 has a significant regulatory effect on the expression of cystatin C in vascular smooth muscle cells (51). However, the specific mechanism of action of cystatin C and TGF-β1 in human aortic valve calcification is unclear and requires further clarification. Modulating the possible interactions between these two molecules could allow us to stop the progression of aortic valve calcification in the early stages of the disease (50). In addition, a study has found that higher bilirubin levels are negatively correlated with cardiometabolic risk factors, including obesity, dyslipidemia, and hypertension (52). This study revealed that direct bilirubin was an independent risk factor for CAVS (P=0.008). However, as a major contributor to CAVS, the mechanism of action of direct bilirubin remains unclear, and further basic research is required to elucidate the physiological mechanism between direct bilirubin and CAVS.
Nonetheless, there are some limitations to this study that should be noted. Although this is a real-world study, the CAVS case group is small, and only 74 cases were used to establish the model. The model could be improved by increasing the sample size. Also, given the increasing use of bioinformatics and machine learning in clinical research, it would have been preferable to incorporate the relationship between the expression of some genes and CAVS into the model (53,54). Finally, because the verification work of this study is limited to the internal population and lacks independent external data set verification, it may have certain limitations. We will further improve this problem in the future work.
The model’s overall accuracy in predicting the occurrence of CAVS was high in both the training and validation sets (both AUCs >0.9), and the clinical decision curves indicated that the model has a broad range of applicability, with its predictions providing clinical benefits across a wide range of probability intervals. Therefore, appropriate preventive measures and specific interventions for the high-risk groups identified by this nomogram will hopefully provide important evidence for the timely initiation of clinical interventions to treat CAVS patients.
Conclusions
In general, the treatment of CAVS is limited, surgical intervention is complicated, and the prognosis is poor. This study successfully established and validated a nomogram for predicting the occurrence of CAVS based on 11 clinically indicators, which may achieve early prediction in CAVS patients. Variables used in the model were easy to obtain clinically and the effectiveness of the model was good.
Acknowledgments
The Affiliated Hospital of Qingdao University supported the completion of the study. We would like to express our gratitude to the Department of Cardiovascular Surgery for sharing large volumes of data.
Funding: This study was sponsored by fund from the Qingdao West Coast New Area Science and Technology Project (No. 2020-51).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1157/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1157/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1157/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021. [Crossref] [PubMed]
- Lindman BR, Clavel MA, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006. [Crossref] [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-603. [Crossref] [PubMed]
- Lindroos M, Kupari M, Heikkilä J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21:1220-5. [Crossref] [PubMed]
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002-12. [Crossref] [PubMed]
- Demir B, Caglar IM, Ugurlucan M, et al. The relationship between severity of calcific aortic stenosis and serum uric acid levels. Angiology 2012;63:603-8. [Crossref] [PubMed]
- Myasoedova VA, Ravani AL, Frigerio B, et al. Novel pharmacological targets for calcific aortic valve disease: Prevention and treatments. Pharmacol Res 2018;136:74-82. [Crossref] [PubMed]
- Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol 2006;47:2141-51. [Crossref] [PubMed]
- Donato M, Ferri N, Lupo MG, et al. Current Evidence and Future Perspectives on Pharmacological Treatment of Calcific Aortic Valve Stenosis. Int J Mol Sci 2020;21:8263. [Crossref] [PubMed]
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. [Crossref] [PubMed]
- Carità P, Coppola G, Novo G, et al. Aortic stenosis: insights on pathogenesis and clinical implications. J Geriatr Cardiol 2016;13:489-98. [PubMed]
- Miller JD, Weiss RM, Serrano KM, et al. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation 2009;119:2693-701. [Crossref] [PubMed]
- Owens DS, Katz R, Takasu J, et al. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA). Am J Cardiol 2010;105:701-8. [Crossref] [PubMed]
- Mahabadi AA, Kahlert P, Kahlert HA, et al. Comparison of Lipoprotein(a)-Levels in Patients ≥70 Years of Age With Versus Without Aortic Valve Stenosis. Am J Cardiol 2018;122:645-9. [Crossref] [PubMed]
- Ljungberg J, Holmgren A, Bergdahl IA, et al. Lipoprotein(a) and the Apolipoprotein B/A1 Ratio Independently Associate With Surgery for Aortic Stenosis Only in Patients With Concomitant Coronary Artery Disease. J Am Heart Assoc 2017;6:007160. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72-e227. [PubMed]
- Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med 2007;26:5512-28. [Crossref] [PubMed]
- Siontis GCM, Overtchouk P, Cahill TJ, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J 2019;40:3143-53. [Crossref] [PubMed]
- Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017;38:2183-91. [Crossref] [PubMed]
- Ye T, Ma T, Wang Q, et al. Prevalence and risk factors of aortic valve calcification among the elderly residents of Wuxi city, Jiangsu province. Zhonghua Xin Xue Guan Bing Za Zhi 2019;47:544-8. [PubMed]
- Mercier N, Pawelzik SC, Pirault J, et al. Semicarbazide-Sensitive Amine Oxidase Increases in Calcific Aortic Valve Stenosis and Contributes to Valvular Interstitial Cell Calcification. Oxid Med Cell Longev 2020;2020:5197376. [Crossref] [PubMed]
- Sanchez PL, Mazzone AM. C-reactive protein in aortic valve disease. Cardiovasc Ultrasound 2006;4:37. [Crossref] [PubMed]
- Fox CS, Guo CY, Larson MG, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol 2006;97:1502-5. [Crossref] [PubMed]
- Oikawa M, Owada T, Yamauchi H, et al. Predominance of Abdominal Visceral Adipose Tissue Reflects the Presence of Aortic Valve Calcification. Biomed Res Int 2016;2016:2174657. [Crossref] [PubMed]
- Huk DJ, Hammond HL, Kegechika H, et al. Increased dietary intake of vitamin A promotes aortic valve calcification in vivo. Arterioscler Thromb Vasc Biol 2013;33:285-93. [Crossref] [PubMed]
- Mohty D, Pibarot P, Després JP, et al. Age-related differences in the pathogenesis of calcific aortic stenosis: the potential role of resistin. Int J Cardiol 2010;142:126-32. [Crossref] [PubMed]
- Hulin A, Hego A, Lancellotti P, et al. Advances in Pathophysiology of Calcific Aortic Valve Disease Propose Novel Molecular Therapeutic Targets. Front Cardiovasc Med 2018;5:21. [Crossref] [PubMed]
- Peeters FECM, Meex SJR, Dweck MR, et al. Calcific aortic valve stenosis: hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur Heart J 2018;39:2618-24. [Crossref] [PubMed]
- Mata P, Alonso R, Pérez de Isla L, et al. Dyslipidemia and aortic valve disease. Curr Opin Lipidol 2021;32:349-54. [Crossref] [PubMed]
- Pérez de Isla L, Watts GF, Alonso R, et al. Lipoprotein(a), LDL-cholesterol, and hypertension: predictors of the need for aortic valve replacement in familial hypercholesterolaemia. Eur Heart J 2021;42:2201-11. [Crossref] [PubMed]
- Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res 2008;49:2230-9. [Crossref] [PubMed]
- Sanchez PL, Mazzone A. C-reactive protein in degenerative aortic valve stenosis. Cardiovasc Ultrasound 2006;4:24. [Crossref] [PubMed]
- Warrier B, Mallipeddi R, Karla PK, et al. The functional role of C-reactive protein in aortic wall calcification. Cardiology 2005;104:57-64. [Crossref] [PubMed]
- Skowasch D, Schrempf S, Preusse CJ, et al. Tissue resident C reactive protein in degenerative aortic valves: correlation with serum C reactive protein concentrations and modification by statins. Heart 2006;92:495-8. [Crossref] [PubMed]
- Galante A, Pietroiusti A, Vellini M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol 2001;38:1078-82. [Crossref] [PubMed]
- Wang X, Li W, Song F, et al. Carotid Atherosclerosis Detected by Ultrasonography: A National Cross-Sectional Study. J Am Heart Assoc 2018;7:008701. [Crossref] [PubMed]
- Novo G, Guarneri FP, Ferro G, et al. Association between asymptomatic carotid atherosclerosis and degenerative aortic stenosis. Atherosclerosis 2012;223:519-22. [Crossref] [PubMed]
- Rossi A, Faggiano P, Amado AE, et al. Aortic valve sclerosis is a marker of atherosclerosis independently of traditional clinical risk factors. Analysis in 712 patients without ischemic heart disease. Int J Cardiol 2012;158:163-4. [Crossref] [PubMed]
- Yan AT, Koh M, Chan KK, et al. Association Between Cardiovascular Risk Factors and Aortic Stenosis: The CANHEART Aortic Stenosis Study. J Am Coll Cardiol 2017;69:1523-32. [Crossref] [PubMed]
- Rieck ÅE, Cramariuc D, Boman K, et al. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension 2012;60:90-7. [Crossref] [PubMed]
- Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, et al. Systolic Blood Pressure and Risk of Valvular Heart Disease: A Mendelian Randomization Study. JAMA Cardiol 2019;4:788-95. [Crossref] [PubMed]
- Eveborn GW, Schirmer H, Lunde P, et al. Assessment of risk factors for developing incident aortic stenosis: the Tromsø Study. Eur J Epidemiol 2014;29:567-75. [Crossref] [PubMed]
- Patterson RA, Horsley ET, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. J Lipid Res 2003;44:512-21. [Crossref] [PubMed]
- Yu Z, Seya K, Chiyoya M, et al. Warfarin calcifies human aortic valve interstitial cells at high-phosphate conditions via pregnane X receptor. J Bone Miner Metab 2019;37:944-56. [Crossref] [PubMed]
- Yu C, Wu D, Zhao C, et al. CircRNA TGFBR2/MiR-25-3p/TWIST1 axis regulates osteoblast differentiation of human aortic valve interstitial cells. J Bone Miner Metab 2021;39:360-71. [Crossref] [PubMed]
- Song R, Fullerton DA, Ao L, et al. BMP-2 and TGF-β1 mediate biglycan-induced pro-osteogenic reprogramming in aortic valve interstitial cells. J Mol Med (Berl) 2015;93:403-12. [Crossref] [PubMed]
- Yu B, Hafiane A, Thanassoulis G, et al. Lipoprotein(a) Induces Human Aortic Valve Interstitial Cell Calcification. JACC Basic Transl Sci 2017;2:358-71. [Crossref] [PubMed]
- Liu Z, Dong N, Hui H, et al. Endothelial cell-derived tetrahydrobiopterin prevents aortic valve calcification. Eur Heart J 2022;43:1652-64. [Crossref] [PubMed]
- Helske S, Syväranta S, Lindstedt KA, et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol 2006;26:1791-8. [Crossref] [PubMed]
- Yetkin E, Tchaikovski V, Erdil N, et al. Increased expression of cystatin C and transforming growth factor β-1 in calcific aortic valves. Int J Cardiol 2014;176:1252-4. [Crossref] [PubMed]
- Shi GP, Sukhova GK, Grubb A, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest 1999;104:1191-7. [Crossref] [PubMed]
- Seyed Khoei N, Wagner KH, Sedlmeier AM, et al. Bilirubin as an indicator of cardiometabolic health: a cross-sectional analysis in the UK Biobank. Cardiovasc Diabetol 2022;21:54. [Crossref] [PubMed]
- Xiong T, Han S, Pu L, et al. Bioinformatics and Machine Learning Methods to Identify FN1 as a Novel Biomarker of Aortic Valve Calcification. Front Cardiovasc Med 2022;9:832591. [Crossref] [PubMed]
- Song C, Wei S, Fan Y, et al. Bioinformatic-based Identification of Genes Associated with Aortic Valve Stenosis. Heart Surg Forum 2022;25:E069-78. [Crossref] [PubMed]
(English Language Editor: A. Kassem)