
Predictive factors for relapse in corticosteroid-treated patients with chronic eosinophilic pneumonia
Introduction
Chronic eosinophilic pneumonia (CEP) was first recognised as a unique pulmonary entity by Carrington et al. in 1969 (1). It is an idiopathic disorder with no known infectious, drugs or toxic aetiology and is characterised by an abnormal and marked accumulation of eosinophils in the interstitium and alveolar spaces of the lungs (2). CEP is a rare disease, representing 3% of the cases of various interstitial diseases (3,4) Peripheral blood eosinophilia and a high eosinophilic proportion in bronchoalveolar lavage fluid (BALF) are often included as diagnostic criteria for CEP. In approximately half of the cases, total serum immunoglobulin E (IgE) levels are elevated (5,6). Spirometry in patients with CEP is normal in up to one-third of patients but can demonstrate either a restrictive or obstruction pattern (4). Obstruction is more likely to occur in patients with underlying asthma. The characteristic computed tomography (CT) manifestations of CEP include bilateral peripheral non-segmental consolidation and ground-glass opacities in the upper lobes (4,7). Peripheral distribution of consolidation is seen in only about 25% of the cases (8,9).
Systemic corticosteroid (CS) therapy is the standard treatment for CEP and leads to marked improvement (6,10-12). However, a relapse often occurs during the clinical course of CEP when the CS is tapered or after cessation (4,7). Although previous studies have reported that smoking status or underlying asthma are possible risk factors for CEP relapse (11,13), they have not been sufficiently established because a few studies have reviewed these two factors (10). In a prospective study comparing CEP relapse rates between patients with CEP who underwent 3 and 6 months of CS treatment, recurrence rates were not significantly associated with the duration of CS therapy. They were not an independent factor for recurrence (14). Therefore, this study aimed to investigate the predictive factors for CEP relapse. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-511/rc).
Methods
This single-center, retrospective study was approved by the Institutional Review Board of the National Hospital Organization Kinki-Chuo Chest Medical Center (KCCMC) (approval No. 684; approval date: 14 February 2019), and performed in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. We used an opt-out method, allowing the patients and their families to refuse to participate in the study.
Study population
We checked the database of bronchoalveolar lavage (BAL) of KCCMC between 1999 and 2019 and selected consecutive cases of CEP. CEP diagnosis was based on the criteria (Table 1) established by Mochizuki et al. (15). The criteria included cases which fulfil two of the following three situations: eosinophilia in BALF ≥10%, eosinophilia in peripheral blood ≥6%, and eosinophil infiltration in the transbronchial lung biopsy (TBLB) specimens. The percentages of eosinophilia in BALF and peripheral blood in the criteria were consistent with the previous reports (9,16).
Table 1
| Inclusion in this study required fulfilment of both criteria |
| (A) CEP was suspected because of clinical symptoms and abnormal chest shadows that had existed for more than 1 month, with the exclusion of other diseases (e.g., infection) and eosinophilic pneumonias of determined origin |
| (B) At least one of the following conditions was satisfied: |
| (I) Histopathological diagnosis of CEP as determined by a surgical lung biopsy |
| (II) Eosinophilia in BALF or blood ≥30% |
| (III) At least two of the following conditions have to be met: |
| Many eosinophils in transbronchial lung biopsy specimens |
| Eosinophilia in BALF ≥10% |
| Eosinophilia in blood ≥6% |
*, diagnostic criteria of CEP in Japanese shown in ref. (15) was translated and assembled into the table by N Takeuchi. CEP, chronic eosinophilic pneumonia; BALF, bronchoalveolar lavage fluid.
Since some cases of CEP go into spontaneous remission, we enrolled the CS-treated cases of CEP in this study.
Clinical parameters
We reviewed demographic data, including patient age, sex, smoking history, the coexistence of asthma, and clinical data, including laboratory data, BALF data, pulmonary function, high-resolution CT pattern, and systemic CS therapy.
The serum levels of Krebs von den Lungen-6 (KL-6) and surfactant protein-D (SP-D) were measured using commercially available ELISA kits (ED046; Eizai, Tokyo, Japan; SP-D ELISA; Yamasa, Tokyo, Japan, respectively). The cut-off levels of serum KL-6 and SP-D were 500 U/mL and 110 ng/mL, respectively.
BAL was performed by instilling 150 mL of normal saline from three 50 mL aliquots and retrieved using a handheld syringe. This procedure has been previously described in detail (17). All pulmonary function tests were performed using CHESTAC-8800 or 8900 (CHEST, Bunkyo-ku, Tokyo, Japan).
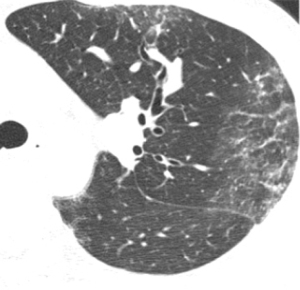
High-resolution CT patterns at diagnosis were reviewed by an expert radiologist (M Akira) and two expert pulmonologists (N Takeuchi and T Arai). The high-resolution CT patterns included consolidation, ground-glass opacity, centrilobular opacity, and pleural effusion. Centrilobular opacities on high-resolution CT generally comprise small 5–10 mm lung nodules anatomically located centrally within secondary pulmonary lobules (Figure 1) (18).
Outcome measures
The observational period of CEP was defined as the time from the start of the CS treatment to the last observation (December 2020) or relapse. Relapse was defined as the resumption of or an increased dose of CS when the shadow worsened during the clinical course after starting CS treatment, while clinically ruled out infection, pulmonary edema or embolism, etc. to the extent clinically possible.
Statistical analysis
We investigated the predictive factors for relapse in CS-treated cases of CEP using univariate and multivariate Cox proportional hazard regression analyses. Factors with P<0.1 in univariate analysis were used for multivariate analysis. We devised a model to predict the relapse of CS-treated CEP by using significant categorical parameters. Relapse incidence curves were described using the Kaplan-Meier method, and the log-rank test was used to compare the curves. Statistical significance was set at P<0.05. JMP® 9.0.3 (SAS Institute, Cary, NC, USA) and SPSS for Macintosh (version 26; IBM Corp., Armonk, NY, USA) were used for all statistical analyses.
Results
We identified 73 consecutive patients with CEP at our institution between 1999 and 2019. We excluded patients with insufficient information (n=6) and those without CS treatment (n=31). The remaining 36 CS-treated patients with CEP were included in the study. Patient characteristics are shown in Table 2. All patients underwent TBLB. As for the CS treatment, 12 patients were treated with 500–1,000 mg/day of methylprednisolone for three successive days (8 patients received maintenance prednisolone therapy of 0.5–1.0 mg/kg/day and 4 patients receive no maintenance therapy) and the remaining 24 patients received prednisolone therapy dosing approximately 0.5 mg/kg/day. No other immunosuppressive drugs were used. CEP relapse was observed in 20 patients (55.6%), and the median observation period until relapse was 404 days [interquartile range (IQR), 201–868 days]. The median observation period of the 36 CS-treated cases with CEP was 595 days (IQR, 304–1,220 days) (Table 2).
Table 2
| Parameters | N=36 |
|---|---|
| Age, years | 59.5 (47.8–70.0) |
| Gender (male/female) | 13/23 |
| Smoking history (yes/no) | 11/25 |
| Asthma (yes/no) | 15/21 |
| Laboratory values | |
| WBC, μL | 11,050 (9,525–14,800) |
| Eosinophils, % | 35.0 (15.6–55.8) |
| Eosinophils, μL | 4,000 (1,172.7–7,117.8) |
| IgE*, IU/mL | 488.5 (178–1,089.5) |
| CRP, mg/dL | 4.2 (1.5–10.7) |
| KL-6*, U/mL | 273 (206.3–350.5) |
| SP-D**, ng/mL | 135 (82.2–176.7) |
| BALF | |
| Neutrophils, % | 1.3 (0.6–3.2) |
| Lymphocyte, % | 8.5 (3.5–14.7) |
| Eosinophils, % | 40.8 (10.7–68.5) |
| CD4/CD8 | 1.7 (0.96–2.7) |
| Pulmonary function test | |
| %FVC*** | 73.9 (62.9–88.5) |
| FEV1%*** | 77.4 (67.8–84.0) |
| Radiological findings on high-resolution CT | |
| Consolidation (yes/no) | 35/1 |
| Ground-glass opacity (yes/no) | 35/1 |
| Centrilobular opacity (yes/no) | 23/13 |
| Pleural effusion (yes/no) | 11/25 |
| Relapse (yes/no) | 20/16 |
| Observation period, days | 595 (304–1,220) |
Each parameter was expressed as number or median (interquartile range). *, n=34; **, n=33; ***, n=30 for the other parameters. WBC, white blood cell; IgE, immunoglobulin E; CRP, C-reactive protein; KL-6, Krebs von den Lunge-6; SP-D, surfactant protein-D; BALF, bronchoalveolar lavage fluid; FVC, forced vital capacity; FEV1, forced expiratory volume in one second, CT; computed tomography.
Predictive factors for relapse
Univariate and multivariate analyses showed that higher levels of serum SP-D [hazard ratio (HR), 1.008; 95% confidence interval (CI): 1.001–1.014, P=0.018] and centrilobular opacity (HR, 3.203; 95% CI: 1.182–10.293, P=0.021) were significantly associated with relapse of CS-treated CEP (Table 3). In these cases, higher serum SP-D levels and centrilobular opacities showed significantly earlier relapse than those without lower serum SP-D levels (HR, 1.007; 95% CI: 1.001–1.012, P=0.017) and centrilobular opacities (HR, 3.141; 95% CI: 1.126–10.396, P=0.028) by multivariate Cox proportional hazard regression analysis (Table 4).
Table 3
| Parameters | HR | 95% CI | P value |
|---|---|---|---|
| Age, years | 0.996 | 0.970–1.025 | 0.770 |
| Gender (male vs. female) | 0.991 | 0.379–2.877 | 0.985 |
| Smoking history (yes vs. no) | 1.312 | 0.458–3.343 | 0.592 |
| Asthma (yes vs. no) | 0.573 | 0.220–1.412 | 0.227 |
| WBC, ×100/μL | 1.006 | 0.999–1.012 | 0.106 |
| Eosinophils, % | 1.009 | 0.987–1.032 | 0.426 |
| Eosinophils, ×100/μL | 1.007 | 0.999–1.013 | 0.091 |
| IgE, ×100 U/L | 1.016 | 0.968–1.056 | 0.477 |
| CRP, mg/dL | 1.057 | 0.958–1.162 | 0.262 |
| KL-6, ×100 U/mL | 1.088 | 0.888–1.247 | 0.359 |
| SP-D, ng/mL | 1.008 | 1.001–1.014 | 0.018 |
| BALF | |||
| Neutrophils, % | 1.047 | 0.853–1.236 | 0.627 |
| Lymphocytes, % | 0.981 | 0.934–1.018 | 0.343 |
| Eosinophils, % | 0.996 | 0.981–1.011 | 0.580 |
| CD4/CD8 | 1.284 | 0.919–1.737 | 0.137 |
| %FVC | 1.008 | 0.982–1.033 | 0.524 |
| FEV1% | 1.010 | 0.971–1.050 | 0.609 |
| Centrilobular opacity (yes vs. no) | 3.203 | 1.182–10.293 | 0.021 |
| Pleural effusion (yes vs. no) | 2.501 | 0.986–6.261 | 0.054 |
HR, hazard ratio; CI, confidence interval; WBC, white blood cell; IgE, immunoglobulin E; CRP, C-reactive protein; KL-6, Krebs von den Lunge-6; SP-D, surfactant protein-D; BALF, bronchoalveolar lavage fluid; FVC, forced vital capacity; FEV1, forced expiratory volume in one second.
Table 4
| Parameters | HR | 95% CI | P value |
|---|---|---|---|
| SP-D (ng/mL) | 1.007 | 1.001–1.012 | 0.017 |
| Centrilobular opacity (yes vs. no) | 3.141 | 1.126–10.396 | 0.028 |
HR, hazard ratio; CI, confidence interval; SP-D, surfactant protein-D.
Serum SP-D levels were divided into higher (>135 ng/mL) and lower (≤135 ng/mL) levels by their median values. Multivariate analysis was performed using two categorical parameters (the presence of centrilobular opacities and serum levels of SP-D, >135/≤135 ng/mL), and the two parameters were also significant (Table 5). Hence, we devised a relapse prediction model for CS-treated CEP using these two parameters. Based on these parameters, cases were scored 2, 1, or 0. Patients with a score of 2 experienced relapses earlier than those with scores of 1 and 0 (log-rank test; P=0.006, P=0.003, respectively). The median relapse time of patients with a score of 1 was between those with scores of 2 and 0 (Figure 2).
Table 5
| Parameters | HR | 95% CI | P value |
|---|---|---|---|
| SP-D (ng/mL) (high vs. low)* | 4.014 | 1.575–11.600 | 0.003 |
| Centrilobular opacity (yes vs. no) | 3.498 | 1.251–11.769 | 0.016 |
*, median levels of serum SP-D were 135 ng/mL and we defined higher levels as >135 ng/mL, and lower levels as ≤135 ng/mL. SP-D, surfactant protein-D; HR, hazard ratio; CI, confidence interval.

Discussion
Our findings showed that centrilobular opacities on high-resolution CT and higher SP-D suggest a higher probability of relapse in CEP.
Centrilobular opacities on high-resolution CT suggested a higher probability of CEP relapse. A retrospective study reported that centrilobular nodules within ground-glass opacities were observed in 18% of patients with CEP (19). Thus, centrilobular opacities may be a feature of high-resolution CT in patients with CEP. Centrilobular opacities generally indicate the presence of a lesion in the bronchioles (20).
Since CEP is an eosinophilic lung disease, centrilobular opacities may reflect bronchiolar eosinophilic inflammation and suggest eosinophilic bronchiolitis (21). The first case of eosinophilic bronchiolitis was reported in Japan (22). Although airflow obstruction and peripheral blood eosinophilia were observed, the patient was not diagnosed with asthma. Centrilobular opacities were observed on the high-resolution CT images. TBLB specimens revealed pathological findings of eosinophilic bronchiolitis, and the number of eosinophils in the BALF increased. Airway obstruction improved with CS therapy but relapsed during CS tapering. Following this report, Cordier et al. proposed hypereosinophilic obliterative bronchiolitis (HOB) to describe a similar disease entity in case reports including 6 patients showing centrilobular nodules on high-resolution CT (23). In these cases, HOB manifestations recurred when the oral prednisone dose was decreased to 10–20 mg per day. These two reports demonstrate that eosinophilic bronchiolitis may relapse easily with a medium dose of CS. Similar to these two reports, the presence of centrilobular opacity on high-resolution CT, possibly suggesting eosinophilic bronchiolitis in CEP, may be associated with early relapse of CEP.
We also found that higher serum SP-D levels were associated with relapse in CS-treated patients with CEP. SP-D is a hydrophilic protein mainly secreted by alveolar type II cells and plays an important role in the innate immunity of the lung (24). SP-D has beneficial effects in infectious diseases because it functions as an opsonin and participates in pathogen phagocytosis. Its pathophysiological role in non-infectious diseases associated with eosinophilia has been examined. Serum SP-D levels are elevated in patients with allergic asthma (25). Mackay et al. found that serum SP-D was increased in severe asthma with mixed eosinophil and neutrophil inflammation and was enriched in SP-D degradation products (26). As for acute eosinophilic pneumonia, elevated serum SP-D reflects disease activity, and its serum levels decrease according to improvement after CS therapy (27,28). These findings could explain why higher levels of serum SP-D were associated with relapse in CS-treated patients with CEP.
What is the physiological role of SP-D in eosinophilic inflammation? In mouse models of allergic bronchopulmonary aspergillosis, SP-D expression in BALF was enhanced, and ablation of SP-D led to enhanced eosinophilia and IgE production. In vitro investigations have also shown that SP-D inhibits eosinophil chemotaxis, binds eosinophils, and attenuate degranulation (29). Hence, SP-D production can be induced to resolve eosinophilic inflammation (30).
Merchand et al. retrospectively reviewed 53 patients with CEP and reported that patients with asthma at the time of CEP diagnosis had more relapses during follow-up than those without asthma (11). In another retrospective study of 73 patients with CEP by Ishiguro et al., a history of smoking was a negative predictor of CEP relapse (13). In contrast, in other previous prospective and retrospective studies of patients with CEP, there were no significant differences in asthma status or smoking habits (14,31,32), similar to the results of our study. Hence, further large-scale prospective studies are needed to determine whether smoking history or asthma status is associated with CEP relapse.
The frequent occurrence of relapse with CEP during tapering or discontinuation of CS demands the need for alternative therapy in case of relapse with CEP. Mepolizumab is a fully humanized antibody against interleukin 5, an important growth factor of eosinophils. Brenard et al. reported that treatment with mepolizumab for CEP relapse significantly reduced another relapse (33). This treatment may contribute to future treatment of CEP with centrilobular opacities on high-resolution CT and higher SP-D.
This study had some limitations. It was retrospective study that took over 20 years, and included a limited number of cases. Therefore, the treatment and timing of assessment varied for each patient. Also, this study was conducted in a single centre. However, we reviewed consecutive patients with CEP, and the CEP criteria for the included studies are conventional and have not been changed. Further large, multicentre studies are warranted.
Conclusions
Centrilobular opacities on high-resolution CT and higher serum SP-D levels at diagnosis may be predictive factors for early relapse in CS-treated patients with CEP. We should cautiously observe CEP relapse in CS-treated patients with these two factors at diagnosis.
Acknowledgments
The preliminary report of this study with part of the data was presented as a poster at the 2018 ERS International Congress in Paris, September 15-19, 2018 [abstract: European Respiratory Journal 2018;52(Suppl. 62):PA3025]. We are grateful to Tomoaki Teramoto, a clinical laboratory technician, for data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-511/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-511/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-511/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-511/coif). YI has received grants from Japanese Ministry of Health, Labour, and Welfare and Japan Agency for Medical Research and Development regarding interstitial lung diseases. TA has received lecture fees from Boehringer Ingelheim and Shionogi for activities not connected with the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carrington CB, Addington WW, Goff AM, et al. Chronic eosinophilic pneumonia. N Engl J Med 1969;280:787-98. [Crossref] [PubMed]
- Matsuda Y, Tachibana K, Sasaki Y, et al. Tracheobronchial lesions in eosinophilic pneumonia. Respir Investig 2014;52:21-7. [Crossref] [PubMed]
- Cordier JF, Cottin V. Eosinophilic pneumonias. In: Schwarz MI, King TE Jr. editors. Interstitial lung disease. 5th edition. Shelton: People’s Medical Publishing House-USA, 2011:833-93.
- Cottin V. Eosinophilic Lung Diseases. Clin Chest Med 2016;37:535-56. [Crossref] [PubMed]
- Stoller JK. Eosinophilic lung diseases. In: Broaddus VC, Ernst JD, Lazarus SC, et al. editors. Murray and Nadel’s Textbook of Respiratory Medicine. 6th edition. Elsevier/Saunders, 2016:1221-28.
- Crowe M, Robinson D, Sagar M, et al. Chronic eosinophilic pneumonia: clinical perspectives. Ther Clin Risk Manag 2019;15:397-403. [Crossref] [PubMed]
- Marchand E, Reynaud-Gaubert M, Lauque D, et al. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P). Medicine (Baltimore) 1998;77:299-312. [Crossref] [PubMed]
- Jeong YJ, Kim KI, Seo IJ, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics 2007;27:617-37; discussion 637-9. [Crossref] [PubMed]
- Jederlinic PJ, Sicilian L, Gaensler EA. Chronic eosinophilic pneumonia. A report of 19 cases and a review of the literature. Medicine (Baltimore) 1988;67:154-62. [Crossref] [PubMed]
- Suzuki Y, Suda T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int 2019;68:413-9. [Crossref] [PubMed]
- Marchand E, Etienne-Mastroianni B, Chanez P, et al. Idiopathic chronic eosinophilic pneumonia and asthma: how do they influence each other? Eur Respir J 2003;22:8-13. [Crossref] [PubMed]
- Marchand E, Cordier JF. Idiopathic chronic eosinophilic pneumonia. Orphanet J Rare Dis 2006;1:11. [Crossref] [PubMed]
- Ishiguro T, Takayanagi N, Uozumi R, et al. The Long-term Clinical Course of Chronic Eosinophilic Pneumonia. Intern Med 2016;55:2373-7. [Crossref] [PubMed]
- Oyama Y, Fujisawa T, Hashimoto D, et al. Efficacy of short-term prednisolone treatment in patients with chronic eosinophilic pneumonia. Eur Respir J 2015;45:1624-31. [Crossref] [PubMed]
- Mochizuki Y, Kobashi Y, Nakahara Y, et al. Chronic eosinophilic pneumonia--a follow-up study of 12 cases. Nihon Kokyuki Gakkai Zasshi 2002;40:851-5. [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Kurashima K, Mukaida N, Fujimura M, et al. A specific elevation of RANTES in bronchoalveolar lavage fluids of patients with chronic eosinophilic pneumonia. Lab Invest 1997;76:67-75. [PubMed]
- Gruden JF, Webb WR, Warnock M. Centrilobular opacities in the lung on high-resolution CT: diagnostic considerations and pathologic correlation. AJR Am J Roentgenol 1994;162:569-74. [Crossref] [PubMed]
- Furuiye M, Yoshimura N, Kobayashi A, et al. Churg-Strauss syndrome versus chronic eosinophilic pneumonia on high-resolution computed tomographic findings. J Comput Assist Tomogr 2010;34:19-22. [Crossref] [PubMed]
- Boitsios G, Bankier AA, Eisenberg RL. Diffuse pulmonary nodules. AJR Am J Roentgenol 2010;194:W354-66. [Crossref] [PubMed]
- Kobayashi T, Inoue H, Mio T. Hypereosinophilic obliterative bronchiolitis clinically mimicking diffuse panbronchiolitis: four-year follow-up. Intern Med 2015;54:1091-4. [Crossref] [PubMed]
- Takayanagi N, Kanazawa M, Kawabata Y, et al. Chronic bronchiolitis with associated eosinophilic lung disease (eosinophilic bronchiolitis). Respiration 2001;68:319-22. [Crossref] [PubMed]
- Cordier JF, Cottin V, Khouatra C, et al. Hypereosinophilic obliterative bronchiolitis: a distinct, unrecognised syndrome. Eur Respir J 2013;41:1126-34. [Crossref] [PubMed]
- Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol 2001;63:521-54. [Crossref] [PubMed]
- Sorensen GL. Surfactant Protein D in Respiratory and Non-Respiratory Diseases. Front Med (Lausanne) 2018;5:18. [Crossref] [PubMed]
- Mackay RM, Grainge CL, Lau LC, et al. Airway Surfactant Protein D Deficiency in Adults With Severe Asthma. Chest 2016;149:1165-72. [Crossref] [PubMed]
- Daimon T, Tajima S, Oshikawa K, et al. KL-6 and surfactant proteins A and D in serum and bronchoalveolar lavage fluid in patients with acute eosinophilic pneumonia. Intern Med 2005;44:811-7. [Crossref] [PubMed]
- Fujii M, Tanaka H, Kameda M, et al. Elevated serum surfactant protein A and D in a case of acute eosinophilic pneumonia. Intern Med 2004;43:423-6. [Crossref] [PubMed]
- von Bredow C, Hartl D, Schmid K, et al. Surfactant protein D regulates chemotaxis and degranulation of human eosinophils. Clin Exp Allergy 2006;36:1566-74. [Crossref] [PubMed]
- Ledford JG, Addison KJ, Foster MW, et al. Eosinophil-associated lung diseases. A cry for surfactant proteins A and D help? Am J Respir Cell Mol Biol 2014;51:604-14. [Crossref] [PubMed]
- Suzuki Y, Oyama Y, Hozumi H, et al. Persistent impairment on spirometry in chronic eosinophilic pneumonia: A longitudinal observation study (Shizuoka-CEP study). Ann Allergy Asthma Immunol 2017;119:422-428.e2. [Crossref] [PubMed]
- Suzuki Y, Suda T. Long-term management and persistent impairment of pulmonary function in chronic eosinophilic pneumonia: A review of the previous literature. Allergol Int 2018;67:334-40. [Crossref] [PubMed]
- Brenard E, Pilette C, Dahlqvist C, et al. Real-Life Study of Mepolizumab in Idiopathic Chronic Eosinophilic Pneumonia. Lung 2020;198:355-60. [Crossref] [PubMed]


