Stereotactic body radiotherapy: current strategies and future development
Introduction
Lung cancer has been the most common cancer in the world for several decades. In 2015, there will be more than 1,980,000 new cases of lung cancer worldwide. It remains the leading cause of cancer death globally, accounting for about 1 in 5 deaths (1). Surgery is the mainstay of treatment for early-staged non-small cell lung cancer (NSCLC). In the past, radiotherapy (RT) could cure only a small proportion of patients who refuse or are medically unfit for surgery. The local control rate with conventional RT is just around 50% (2-4). In the era of stereotactic body radiotherapy (SBRT), the local control rate with RT is over 90%. The approach to selecting the appropriate techniques for executing SBRT should be personalized to meet the needs and limitations of patients. The applications of SBRT to treatment of lung tumours are evolving. Various techniques for SBRT treatments are under development to make SBRT both an effective and a safe alternative to surgery.
The concept of stereotactic body radiotherapy (SBRT)
SBRT (synonym stereotactic ablative radiotherapy, SABR) is an external beam radiation therapy method that very precisely delivers a high radiation dose to an extracranial target (5). Despite a very high (ablative) radiation dose delivery to the tumour target, SBRT is able to minimise radiation of surrounding normal tissues. The standard dose of SBRT varies from 48 to 60 Gy in five or less treatment sessions given within 2 weeks (in conventional RT, the whole treatment will take 6 to 7 weeks).
A clinical dose-response curve of human malignant lung tumours has been established (6). A high radiation dose is required for complete eradication of all cancer cells within the target. With conventional RT, however, the adjacent normal tissues (normal lung tissues, trachea and main bronchi, heart, great vessels, oesophagus, spinal cord, brachial plexus, ribs and skin) may also receive a significant radiation dose which may result in fatal tissue damage. The success of SBRT relies on its ability to deliver a high radiation dose to the target and yet spare the surrounding normal tissues. This is made possible by its intrinsic physical properties, restriction of tumour movement during treatment (tumour motion management) and meticulous verification of tumour position before and during treatment [image-guided radiation therapy (IGRT)].
Intrinsic physical properties of stereotactic body radiotherapy (SBRT)
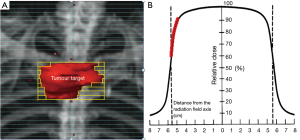
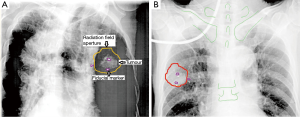
In SBRT, the radiation dose is highly conformed to the target. There will be a rapid radiation dose fall-off outside the target. This can be achieved by aligning the multi-leaf collimators of the linear accelerator (a machine for generation of radiation beam) close to or touching the target (Figure 1A). Moreover, the radiation dose is prescribed to the part of radiation isodose curve with a steep slope i.e., 60–90% isodose levels (Figure 1B). The rapid dose fall-off characteristics of SBRT can be further enhanced by adopting the radiation techniques of intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT) to create a concave radiation dose distribution conformed to the target.
Tumour motion management
A lung tumour will move with respiration. To avoid geographical tumour miss, the radiation field will be much larger than the tumour itself to encompass the tumour at any respiratory phase. This will result in excessive irradiation of normal tissues adjacent to the tumour and subsequent significant or even fatal normal tissue damage. In SBRT, some forms of tumour motion management techniques may be required to limit the extent of lung tumour movement during treatment. This will result in reduction of radiation field size to limit the amount of normal tissue exposed to a high radiation dose.
Image-guided radiation therapy (IGRT)
Even with proper tumour motion management, there will still be some residual tumour displacement from its expected position both during the same treatment session (intra-fractional displacement) and between two different treatment sessions on different days (inter-fractional displacement). Nelson et al. showed that residual tumour motion under respiratory gating during treatment could be substantial (up to 0.5 cm) (7). Seppenwoolde et al. also found that 30% of lung tumours would have a systematic shift of position between radiation treatment sessions in both tidal breathing and breath-holding conditions (8). Therefore daily online verification of tumour position before treatment is essential to avoid geographic tumour miss and to achieve radiation field size reduction. On the other hand, multiple studies have concluded that neither external surrogate [infrared reflective block used in the Real-time Patient ManagementTM (RPM) system for respiratory gating (9,10)] nor internal surrogates [diaphragm (11), bony anatomy e.g., vertebral bodies (12,13)] has a consistent correlation with the tumour position over time. Consequently, direct visualization of the lung tumor itself before each treatment session is required for accurate and precise radiation delivery to the tumour. IGRT is a process of using various imaging techniques (X-ray, CT) to locate target and critical tissues and, if needed, reposition the patient just before or during the delivery of RT (14). Various techniques are available for performance of IGRT.
Current strategies of tumour motion management for Stereotactic body radiotherapy (SBRT)
In the era of personalised medicine, SBRT should be executed with a technique which suits the individual patient most. The requirement of restriction of tumour movement during RT and the adopted technique of tumour motion management depends on:
- Extent of tumour movement at tidal breathing;
- Patient’s ability of breath-holding for about 15 s during RT.
Theoretically, all patients should be treated under breath holding where the tumour is immobilised and the radiation field will be smallest. In practice, many of the candidates of SBRT are elderly and smokers with poor pulmonary functions such that they are contraindicated for surgery. Each SBRT treatment session will take about half an hour and most of these patients cannot tolerate repeated breath-holds for the treatment. If the tumour movement at tidal breathing is less than 1 cm, we can adopt the Tumour Encompassing Targeting technique in which the radiation field is designed to encompass all possible tumour positions at various respiratory phases as shown on the planning 4-dimensional CT (4D-CT, 4D refers to addition of time dimension to the usual 3-dimensions). If tumour excursion at tidal breathing is more than 1 cm, some forms of tumour motion management techniques should be adopted to limit the extent of tumour movement during SBRT.
Active breathing control (ABC)/voluntary breath-hold
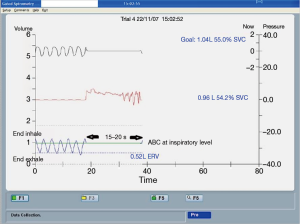
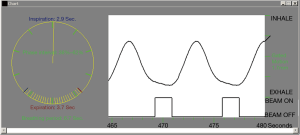
Breath-holding techniques for SBRT include ABC and voluntary breath-hold. The ABC apparatus is a modified spirometer comprising two pairs of flow monitors and scissor valves to control inspiration and expiration, respectively. By closing both scissor valves at a predefined lung volume, patient’s breathing motion will be immobilised for 15 to 20 s. At the same time, radiation beam is switched on. Then the radiation beam will be off temporarily and the patient is allowed to breathe freely until next ABC activation. The cycle will repeat until completion of RT, which typically takes 30 min for each treatment session. As the lungs expand at inspiration, ABC will be activated at inspiration so as to reduce the proportion of normal lung irradiation (Figure 2). ABC is able to reduce normal lungs V20 (volume of normal lung tissues receiving a radiation dose ≥20 Gy) by 34% when compared with free breathing (15). The reproducibility of lung tumour position under ABC is good both intra-fractionally and inter-fractionally. The mean tumour displacement in supero-inferior direction was only 1.1 and 0.3 mm, respectively (16,17). Voluntary breath-holding technique can be used if a patient is unable to hold the mouthpiece of an ABC apparatus without air leakage.
Abdominal compression
In case that the tidal tumour movement is more than one cm, its motion can be restricted by application of an abdominal compressor to the upper abdomen (Figure 3). If the residual tumour movement is less than 1 cm, the Tumour Encompassing Targeting technique can be adopted for SBRT treatment.
Respiratory gating
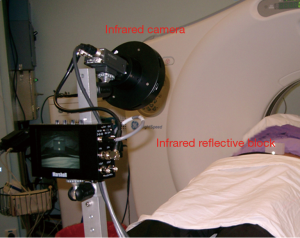
Respiratory gating is a technique to synchronise the delivery of radiation with specified respiratory phases. The respiratory phases at which radiation beam will be turned on (gating window) are usually selected near end expiration because of two reasons. First, a lung tumour will stay in expiratory phase more than in inspiratory phase, resulting in a shorter treatment time. In addition, the tumour position will be more consistent and reproducible at end expiration. A 4D-CT for RT planning purpose is done with the RPM system, which consists of an infrared reflective block and an infrared tracking camera (Figure 4). The reflective block is placed on patient’s upper anterior abdominal wall. Following the breathing movement, the abdominal wall and thus the reflective block will move up and down and such movement will be tracked by the infrared camera. Thus, position of the reflective block can be utilised to estimate the respiratory phase at which a particular set of CT images is captured. After determination of the tumour positions at various phases of respiration, a radiation field size can be chosen in accordance to the range of tumour positions at selected gating window. Respiratory gating can be executed with either the RPM or the ExacTrac® Adaptive Gating systems.
- Real-time Patient ManagementTM (RPM) system. During SBRT treatment, the infrared reflective block is placed on patient’s upper anterior abdominal wall and it serves as an external surrogate to predict the tumour position at a particular time. The infrared camera will track the movement of the reflective block and the radiation beam will be turned on (at gating window) and off according to the position of the reflective block (Figure 5);
- ExacTrac® Adaptive Gating system. The optical infrared tracking system of ExacTrac® consists of several infrared reflective body markers (usually 5 to 8) placed on patient’s anterior abdominal wall and an infrared tracking camera mounted on the ceiling of the radiation treatment room (Figure 6). The radiation beam will be turned on only at the predefined gating window.
Available image-guided radiation therapy (IGRT) systems and devices
An IGRT technique should be selected to match requirements of the selected tumour motion management technique and tumour characteristics. For invisible tumour on X-ray or for tumour tracking SBRT, internal fiducial marker(s) made of pure gold will be implanted into or close to the tumour. This fiducial marker can be recognized clearly on X-ray images and acts as an indicator of tumour position for IGRT treatment. Various types of fiducial markers of different shapes and sizes are available in commercial market e.g., a cylindrical marker of 0.75 mm (diameter) × 10 mm (length). Mostly these fiducial markers are implanted under CT-guidance. Unfortunately, a significant proportion of patients will develop pneumothorax after the procedure necessitating chest tube drainage. Thus, it is not recommended in old and/ or frail patients.
Dynamic targeting image-guided radiation therapy (IGRT) system
There are 2 devices in the system for execution of IGRT:
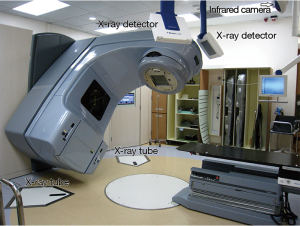
- Onboard Imager® (OBI). OBI is a high resolution X-ray device mounted to the treatment head of a linear accelerator to display real-time tumour location (Figure 7). In RPM respiratory gated SBRT, X-ray images can be taken at gating window both before and during treatment for detection of intra-fractional and inter-fractional tumour movement. In ABC treatment, the tumour position under ABC can also be verified (Figure 8).
- Cone-beam CT (CBCT). CBCT is a 3-dimensional (3D) mode of OBI. It is able to acquire and reconstruct 3D volumetric data in one rotation of treatment head of the linear accelerator in 1 min. Because of the relatively long image acquisition time, CBCT cannot be utilised for treatment verification in breath-holding or respiratory gated treatment. Rather, it is a useful and accurate tool for daily treatment verification of the tumour encompassing targeting SBRT.

ExacTrac® Adaptive Gating system
The ExacTrac® Adaptive Gating system comprises an optical infrared tracking system for respiratory gating and a stereoscopic X-ray imaging system for online detection and correction of tumour position shift. In the stereoscopic X-ray imaging system, there are two X-ray tubes embedded to the linear accelerator room floor and two amorphous silicon flat panel detectors mounted on the ceiling, the angle between the two X-ray tube-detector pairs is approximately 90° (Figure 6). Stereoscopic X-ray can be taken at the gating window for verification of tumour position both before and during treatment. Fiducial marker implantation is necessary for ExacTrac® Adaptive Gating RT.
Real-time Tumour-tracking Radiation Therapy (RT-RT) system
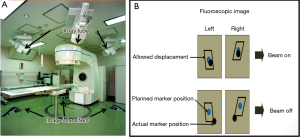
The Hokkaido University of Japan adopts a unique IGRT system called RT-RT. There are four sets of diagnostic X-ray tubes embedded on the floor of the linear accelerator room and corresponding image intensifiers mounted on the ceiling for localization of implanted gold markers at or close to the tumour (Figure 9A). Real-time orthogonal fluoroscopy is repeated every 0.03 s. The fiducial marker is allowed to displace from its expected position for 1 to 3 mm in 3-dimensions. Beyond this tolerance, the radiation beam will be turned off automatically until the marker returns to its allowed zone of displacement (Figure 9B).
Cyberknife®
Cyberknife® is a robotic radiosurgery system in which the small linear accelerator is attached to a robotic arm with 6-dimensional movement (translational xyz and rotational pitch, roll and yaw). Just like the ExacTrac® Adaptive Gating system, it consists of an infrared tracking mechanism and an X-ray imaging device. Internal fiducial markers implantation is essential for Cyberknife® treatment. Infrared emitters are used to record the movement of patient’s skin surface. The position of the implanted fiducial is detected continuously (can be more than 25 Hz) via the X-ray imaging device. The correlation of motion between the external infrared emitters and internal fiducial markers will be updated periodically during treatment. Through a combination of infrared tracking and synchronised X-ray imaging, the tumour movement is tracked and the small linear accelerator will chase along the moving target to direct radiation beam to the target continuously (thus tumour tracking RT). At the moment, it is the most common commercial system for tumour tracking SBRT.
Indications of stereotactic body radiotherapy (SBRT) for lung tumours
Stage I non-small cell lung cancer (NSCLC), medically inoperable or patient refuses surgery
SBRT has established its role as the standard treatment of stage I NSCLC for patients who refuses tumour excision or are unfit for surgery, most commonly due to poor pulmonary function. The reported local control rate in various prospective studies was about 90% and the 5-year overall survival ranged from 43% to 83% (18-23) (Table 1).

Full table
Oligometastasis involving the lungs
Oligometastasis usually refers to presence of 1 to 5 metastatic lesions besides the primary tumour, if any. In some cases of oligometastasis, the disease can be cured after aggressive local treatment of all lesions either by surgery or high dose RT. The data from the International Registry of Lung Metastases revealed that 10- and 15-year survival rates of patients undergoing complete lung metastasectomy were 26% and 22%, respectively. Patients with fewer metastases and longer disease-free interval fared even better (26). Various prospective studies on SBRT for limited lung metastases reported 2-year local control and 2-year survival of 89–96% and 39–84%, respectively, which are not inferior to the surgical results (27-29).
Evolving applications in treating lung tumours
Besides its established role in treating medically inoperable early-stage NSCLC and oligometastases involving lungs, new indications of SBRT are on the horizon although we are in need of further evidence to define its role clearly in each indication.
Stage I operable non-small cell lung cancer (NSCLC)
The success of SBRT in treating medically inoperable stage I NSCLC has prompted investigators to explore its possible role in operable cases. Propensity score matched analyses suggested that survival of the patients with operable stage I NSCLC treated with SBRT paralleled that of lobectomy2 (30-33). Two prospective phase II trials also reported 76–84% 2- to 3-year overall survivals for operable stage I disease after SBRT, which compare favourably with surgical outcomes (24,25). Two phase III randomised trials (STARS and ROSEL) to compare SBRT with surgery in operable stage I NSCLC were prematurely terminated due to poor accrual. Chang et al. conducted a pooled analysis of these two trials. The 3-year recurrence-free survival after SBRT and surgery were 86% and 80% (hazard ratio, 0.69; P=0.54), respectively. The 3-year overall survival were 95% and 79% (hazard ratio, 0.14; P=0.037), respectively. The inferiority of overall survival after surgery could be attributed to its 4% surgical mortality (34). At present, there is one ongoing US phase III randomised trial VALOR which compares sublobar resection with SBRT in stage I disease. Another similar UK phase III trial SABRTooth will be opened soon. The debate on possible role of SBRT in operable early-stage NSCLC will remain till availability of results of these trials.
Locally advanced non-small cell lung cancer (NSCLC) or recurrent disease
SBRT has been investigated as a boost for persistent disease after chemoradiation of locally advanced NSCLC (35,36), or for salvage of recurrent disease (35,37-40). Most of the studies are retrospective, and the reported local control rate at 1 year ranged from 65% to 88%. The treatment toxicity could be appreciable, in particular in centrally located tumours. One retrospective study reported 59% grade three or above toxicities with 10% treatment-related death (39). Clearly, SBRT for persistent or recurrent lung tumour is just in its infancy and should only be performed under clinical trial setting. More data is required to define indications of SBRT in persistent and recurrent NSCLC, and to identify its optimal dose fractionation schedules.
Local treatment of oligo-progressive non-small cell lung cancer (NSCLC) despite targeted therapy
Despite initial good response of activating epidermal growth factor receptor (EGFR) mutated NSCLC to tyrosine kinase inhibitors (TKI), eventually these cancer cells will develop further mutations and will become resistant to TKI. A prospective study suggested that discontinuation of TKI after disease progression would result in even more rapid disease progression (41). The possible explanation is that the oligo-progressive sites harbour TKI-resistant clones. Other cancer cells retain their TKI-sensitive genotype and they will progress without TKI treatment. One of the treatment approaches is continuation of TKI to treat the TKI-sensitive clones while the oligo-progressive sites are treated with local therapy, either surgery or RT. The preliminary results are encouraging with this approach. Two retrospective analyses reported median progression-free survival of 6.2 to 10 months, and the median overall survival after local therapy was 41 months (42,43).
New technology development in stereotactic body radiotherapy (SBRT)
Tumour motion management and image-guided radiation delivery are integral to a successful SBRT treatment without overdosing surrounding normal tissues. The direction of technology advancement for SBRT is integration of innovative imaging systems and radiation delivery techniques into one treatment delivery system. One of such innovation is the VeroTM SBRT system, first launched in 2010, which combines all IMRT, VMAT, IGRT and dynamic tumour tracking techniques into one machine. It consists of a gimbaled X-ray head mounted on a rigid O-ring structure. The gimbaled X-ray head is able to rotate along the two orthogonal gimbals and the O-ring structure can rotate around the treatment table. There are two sets of on-board kilovoltage X-ray tubes and flat panel detectors used for all X-ray imaging, CBCT and real-time fluoroscopic monitoring of moving target during treatment. Vero is able to perform verification of tumour position at any time during treatment which enables dynamic tumour tracking for uninterrupted treatment.
At present, the most common method for implantation of internal fiducial markers for IGRT is through CT-guided trans-thoracic approach. One of the hindrances to internal fiducial implantation is its substantial percentage of pneumothorax after the procedure. The post-procedural pneumothorax rate was as high as 15% to 43% (median 26.5%), and 4% to 18% (median 5%) of patients would require chest tube drainage (44). The latest innovation to reduce risk of pneumothorax after fiducials implantation is electromagnetic navigation bronchoscopy (ENB). ENB utilises electromagnetic technology to localize and guide endoscopic tools or catheters through the bronchial pathway. Before the procedure, a CT scan of thorax is done. With reconstructed virtual, 3D bronchial map from the CT images, bronchoscopists are able to navigate the bronchoscope to a desired location within or close to the lung tumour to insert fiducial markers. With this approach, pneumothorax occurred in only 3.1% of patients after the procedure, and chest tube drainage rate was 1.6% (45). In view of its low risk of post-procedural pneumothorax and increasing practice of tumour tracking SBRT, it is foreseeable that more and more oncology centres all over the world will practise internal fiducials insertion by ENB.
A treatment session of SBRT typically takes about half an hour for both patient set up and radiation delivery. Flattening filter-free (FFF) radiation delivery is able to shorten the treatment time. A flattening filter is used to produce a uniform radiation fluence output from the linear accelerator. Its drawback is that the filter will also attenuate the radiation beam. By removing the flattening filter, a higher radiation dose rate can be achieved to shorten the treatment delivery time, a particular advantage in breath-holding or respiratory-gated SBRT. One study showed that FFF treatment reduced treatment and immobilization time by more than 50% compared to a non-FFF treatment. In addition, it was associated with less physician-ordered image guidance, which contributed to further improvement in treatment delivery efficiency (46).
Proton is a charged particle which possesses a well-defined range of penetration into body tissue. As the proton beam penetrates the body, the particles slow down and deposit radiation dose sharply near end of its range (Bragg peak), leaving minimal dose behind. Modulation of Bragg peaks of different proton beams across target volume will deliver a uniform dose to the target and spares surrounding normal structures, in particular those behind the tumor. Compared to IMRT, proton beam conforms to the target even better. Theoretically, stereotactic body proton therapy (SBPT) will deliver radiation to the target with higher precision than IMRT. At the moment, SBPT remains in its conceptual stage. Technology for execution of SBPT is still deficient to support its evaluation in clinical trials.
Conclusions
SBRT is the standard treatment for medically inoperable stage I NSCLC. Various techniques of tumour motion management and image-guided radiation delivery are available for the practice of personalised medicine. New indications of stereotactic body RT for various lung tumours are emerging. Further development of stereotactic RT is linked to technology advancement.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at: . Accessed on 12 October 2015.http://globocan.iarc.fr
- Haffty BG, Goldberg NB, Gerstley J, et al. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1988;15:69-73. [Crossref] [PubMed]
- Kaskowitz L, Graham MV, Emami B, et al. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1993;27:517-23. [Crossref] [PubMed]
- Krol AD, Aussems P, Noordijk EM, et al. Local irradiation alone for peripheral stage I lung cancer: could we omit the elective regional nodal irradiation? Int J Radiat Oncol Biol Phys 1996;34:297-302. [Crossref] [PubMed]
- American College of Radiology (ACR). ACR-ASTRO practice parameter for the performance of stereotactic body radiation therapy; 2014 p.2. Available online: . Accessed on 15 October 2015.http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/Stereo_body_radiation.pdf
- Fletcher GH. Clinical dose-response curves of human malignant epithelial tumours. Br J Radiol 1973;46:1-12. [Crossref] [PubMed]
- Nelson C, Starkschall G, Balter P, et al. Assessment of lung tumor motion and setup uncertainties using implanted fiducials. Int J Radiat Oncol Biol Phys 2007;67:915-23. [Crossref] [PubMed]
- Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 2002;53:822-34. [Crossref] [PubMed]
- Koch N, Liu HH, Starkschall G, et al. Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part I--correlating internal lung motion with skin fiducial motion. Int J Radiat Oncol Biol Phys 2004;60:1459-72. [Crossref] [PubMed]
- Berbeco RI, Nishioka S, Shirato H, et al. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates. Phys Med Biol 2005;50:3655-67. [Crossref] [PubMed]
- Wang J, Liang J, Hugo G, et al. Distance between thoracic tumor position and diaphragm position during the course of radiotherapy: does it remain constant? Int J Radiat Oncol Biol Phys 2005;63:S525-6. [Crossref]
- Guckenberger M, Meyer J, Wilbert J, et al. Cone-beam CT based image-guidance for extracranial stereotactic radiotherapy of intrapulmonary tumors. Acta Oncol 2006;45:897-906. [Crossref] [PubMed]
- Purdie TG, Bissonnette JP, Franks K, et al. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys 2007;68:243-52. [Crossref] [PubMed]
- American College of Radiology. ACR–AAPM technical standard for medical physics performance monitoring of image-guided radiation therapy (IGRT); 2014 p.2. Available online: . Aaccessed on 15 October 2015.http://www.acr.org/~/media/ACR/Documents/PGTS/standards/IGRT.pdf
- Barnes EA, Murray BR, Robinson DM, et al. Dosimetric evaluation of lung tumor immobilization using breath hold at deep inspiration. Int J Radiat Oncol Biol Phys 2001;50:1091-8. [Crossref] [PubMed]
- Kashani R, Balter JM, Hayman JA, et al. Short-term and long-term reproducibility of lung tumor position using active breathing control (ABC). Int J Radiat Oncol Biol Phys 2006;65:1553-9. [Crossref] [PubMed]
- Cheung PC, Sixel KE, Tirona R, et al. Reproducibility of lung tumor position and reduction of lung mass within the planning target volume using active breathing control (ABC). Int J Radiat Oncol Biol Phys 2003;57:1437-42. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Stereotactic body radiation therapy for T1N0M0 non-small cell lung cancer: first report for inoperable population of a phase II trial by Japan Clinical Oncology Group (JCOG 0403). Int J Radiat Oncol Biol Phys 2012;84:s46. [Crossref]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72-7. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol 2013;31:abstr 7523.
- Nagata Y, Hiraoka M, Shibata T, et al. A phase II trial of stereotactic body radiation therapy for operable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group (JCOG0403). Int J Radiat Oncol Biol Phys 2010;78:s27-8. [Crossref]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012;75:77-81. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [Crossref] [PubMed]
- Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008;72:398-403. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Trovo M, Minatel E, Durofil E, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;88:1114-9. [Crossref] [PubMed]
- Feddock J, Arnold SM, Shelton BJ, et al. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 2013;85:1325-31. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Reyngold M, Wu AJ, McLane A, et al. Toxicity and outcomes of thoracic re-irradiation using stereotactic body radiation therapy (SBRT). Radiat Oncol 2013;8:99. [Crossref] [PubMed]
- Trakul N, Harris JP, Le QT, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol 2012;7:1462-5. [Crossref] [PubMed]
- Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys 2010;78:1387-93. [Crossref] [PubMed]
- Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res 2007;13:5150-5. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Yu HA, Sima CS, Drilon AE, et al. Local therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Clin Oncol 2012;30:abstr 7527.
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?*: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94-107S.
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Prendergast BM, Fiveash JB, Popple RA, et al. Flattening filter-free linac improves treatment delivery efficiency in stereotactic body radiation therapy. J Appl Clin Med Phys 2013;14:4126. [PubMed]