
Should cardiovascular comorbidities be a contraindication for pulmonary metastasectomy?
Introduction
Pulmonary metastasectomy (PM) is a well-established method and is believed to prolong survival in selected patients (1-4). Previous studies on the results of PM focused on the long-term survival and prognostic factors. The data on postoperative morbidity and mortality is scarce, though cardiovascular diseases has been established as significant risk factor for postoperative morbidity and mortality in patients with lung cancer undergoing anatomical lung resections (5,6).
The objective of this study was to compare the postoperative morbidity and mortality as well as the long-term survival in patients with and without cardiovascular comorbidities (CVC) undergoing PM. Furthermore, we aimed to detect predictors for postoperative morbidity in patients with CVC and to identify prognostic factors for survival in these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-409/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Freiburg ethics committee and registered in the German Registry for Clinical Trials (DRKS-ID: DRKS00021251). Informed consent was waived due to the retrospective nature of the study. In this retrospective study, we included all patients with pulmonary metastases from a variety of primary tumors, who underwent PM in curative intent in our institution. Demographic, clinical, and follow up data were extracted from electronic medical records between January 2000 and December 2020. Exclusion criteria were diagnostic interventions, extrathoracic metastases at the time of surgery, pleural carcinomatosis and carcinomatous lymphangiosis. Overall survival (OS) was calculated between first PM and death of the patient from any cause or last date on which the patient was known to be alive. Mortality was subdivided in in-hospital mortality and 30-day mortality.
Video-assisted thoracoscopic surgery (VATS) or open lung resections with thoracotomy as well as extent of resection were decided according to the metastases’ number, size, and location. The PM was conducted with wedge resections, segmentectomies or lobectomies. Patients requiring bilateral metastasectomies were treated in two separate operations. The contralateral side was operated after three weeks after the first operation. The following oncological criteria for PM were employed: (I) The primary cancer was controlled or controllable and (II) all the lung metastases must be resectable and the patient should have adequate cardio-pulmonary reserves. The eligibility criteria for lung resection including preoperative cardiological evaluation were defined according to the European Respiratory Society (ERS)/European Society of Thoracic Surgery (ESTS) clinical guidelines on fitness for radical therapy in lung cancer patients.
Preoperatively all patients were examined by a permanent team consisting of anesthesiologic and surgical consultants. Echocardiography was performed preoperatively only in patients with known CVC or in patients with clinical suspicion of CVC. Because of the retrospective nature of the study a standardized scoring systems such as the Charlson comorbidity index could not be used. Treating of more than 4 lung metastases per patient was defined as resection of a high number of metastases.
Definition of complications
Morbidity or complications were defined as the appearance of new disease during the first 30 days after surgery that required specific treatment and/or implied an increase in the length of hospital stay. Among postoperative complications that merit definition are postoperative pneumonia and persistent air leak. Postoperative pneumonia was diagnosed according to the European Perioperative Clinical Outcome (EPCO) criteria (7), which include new pulmonary infiltrations with associated leukocytosis, fever, new purulent sputum, required antibiotic therapy and increased oxygen demand via face mask. Air leaks were considered persistent if present for >7 days. The distinction between minor and major complications was made according to the Clavien-Dindo classification with minor complications being defined as Clavien-Dindo ≤ II. In this case, treatment consisted of medication or a minor intervention. Major complications (Clavien-Dindo grade ≥III) required surgical, radiologic, endoscopic intervention, multitherapy, general anesthesia or intensive care and life support or had led to single or multiorgan dysfunction (8).
CVC
The following CVC were separately investigated considering their influence on morbidity, mortality, and OS: peripheral artery disease, reduced left ventricular function, arrythmias, coronary heart disease, valvular heart disease, history of cerebrovascular accident or transient ischemic attack (CVA/TIA), history of previous cardiac operations, history of myocardial infarction, and the existence of general vascular comorbidity. Reduced left ventricular function was defined as an ejection fraction (EF) lower than 35% (EF <35%). Coronary artery disease was diagnosed with exercise electrocardiogram (ECG), myocardial scintigraphy, echocardiography, or coronary angiography. For peripheral artery disease ankle-brachial index <0.9, history of claudication, ischemic pain in rest and/or abnormally low extremity pulse, nonhealing lower extremity wound or gangrene were applied. General vascular comorbidity included patients with peripheral artery disease, thoracic or abdominal aneurysm with or without surgical correction, patients with aneurysms in other blood vessels. Multiple cardiovascular comorbidities (MCC) are defined as the presence of ≥2 cardiovascular diseases.
Statistical analysis
Morbidity, mortality, and possible risk factors for postoperative morbidity were analyzed using cross tables, χ2 test and Fisher’s exact test. Survival was estimated by the Kaplan-Meier method. The log-rank test was used to calculate survival differences between the groups. A stepwise backward multivariate Cox proportional hazard model was performed to evaluate prognosticators on survival benefit. All the analyses were performed using the SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Results were considered statistically significant if the P value was less than 0.05.
Results
Analysis of characteristics of CVC
The details of the patients’ preoperative characteristics are shown in Tables 1,2. Data of a total of 760 patients were assessed. A total of 164 patients with CVC (21.6%) were identified. CVC were more often detected in male (P=0.01) and older patients (P<0.001). Most of these patients had preoperative arrythmia (N=48, 6.3%), coronary heart disease (N=39, 5.1%) or history of myocardial infarction (N=23, 3.0%). 8 patients suffered from reduced left ventricular function. General vascular comorbidities were identified in 52 patients (6.8%) and MCC in 45 patients (27.8%).
Table 1
| Parameter | N patients (%) |
|---|---|
| CVC | 164 (21.6) |
| Vascular comorbidities generally | 52 (6.8) |
| Peripheral artery disease | 26 (3.4) |
| Reduced left ventricular function | 8 (1.1) |
| Arrythmia | 48 (6.3) |
| Coronary heart disease | 39 (5.1) |
| Other cardiopathies | 4 (0.5) |
| Pulmonary embolism | 20 (2.6) |
| Ischemic heart disease | 42 (5.5) |
| Valvular heart disease | 8 (1.1) |
| Previous cardiac disease | 12 (1.6) |
| Myocardial infarction | 23 (3.0) |
| Coronary stenting | 23 (3.0) |
| CVA/TIA | 21 (2.8) |
| More than two CVC per patient | 45 (27.8) |
CVC, cardiovascular comorbidities; CVA/TIA, cerebrovascular accident or transient ischemic attack.
Table 2
| Parameter | Patients with CVC | Patients without CVC | P value |
|---|---|---|---|
| Patients, n (%) | 164 (21.6) | 596 (78.4) | – |
| Sex, n (%) | 0.01 | ||
| Males | 113 (68.9) | 352 (59.1) | |
| Females | 51 (31.1) | 244 (40.9) | |
| Age (years), mean (± SD) | 66.88 (±12.1) | 58.91 (±14.1) | 0.001 |
| Primary tumor, n (%) | |||
| Colorectal cancer | 77 (47.0) | 279 (46.8) | 0.5 |
| Renal cell cancer | 28 (17.1) | 56 (9.4) | 0.01 |
| Other tumor entities | 59 (36.0) | 261 (43.8) | 0.04 |
| VATS, n (%) | 45 (27.4) | 147 (24.7) | 0.5 |
| Open surgery, n (%) | 119 (72.6) | 449 (75.3) | 0.5 |
| Lobectomy, n (%) | 8 (4.9) | 32 (5.4) | 0.8 |
| Wedge resection/segmentectomy, n (%) | 156 (95.1) | 564 (94.6) | 0.8 |
| Number of resected lung metastases, mean (± SD) | 3.44 (±3.3) | 3.60 (±5.1) | 0.6 |
| Single lung metastasis, n (%) | 65 (39.6) | 271 (45.5) | 0.2 |
| More than four lung metastases, n (%) | 56 (34.1) | 160 (26.8) | 0.06 |
CVC, cardiovascular comorbidities; SD, standard deviation; VATS, video-assisted thoracoscopic surgery.
Histology of the primary tumors
In most cases, PM was performed for colorectal carcinoma (N=356, 46.8%) with comparable number of patients in both groups (P=0.5). The rest of the tumor entities are described in Table 2.
Number of resected lung metastases and surgical approach
A total of 1,000 first-time PM in curative intention were performed in 760 patients with various primary tumors. There was no significant difference between the two groups regarding the method of resection (VATS: P=0.5; open lung resection: P=0.5), major (P=0.8), minor lung resections (P=0.8) Or the resected lung metastases (P=0.6). Above data are described in Table 2.
Postoperative morbidity
Patients with CVC suffered more postoperative complications than patients without CVC (28.7% vs. 20.5%, P=0.02). However, most of them were minor (N=37, 22.6% with CVC vs. N=93, 15.6%, P=0.03). No difference was shown between the two cohorts for major postoperative complications (N=8, 4.9% with CVC vs. N=29, 4.9%, P=0.05). Postoperative atrial fibrillation was more frequent in patients with CVC (N=7, 4.3% vs. N=4, 0.7%, P=0.001). In addition, in the CVC-group, one patient suffered an acute ST-elevation myocardial infarction (STEMI) postoperatively and another one presented with angina pectoris. For respiratory complications (P=0.1) including postoperative pneumonia (P=0.1), pleural empyema (P=0.6), pulmonary embolism (P=0.4) and prolonged air leak (P=0.6) no statistical significance was shown between the two groups. In addition, wound healing disorders (P=0.2) was not affected from CVC. Patients with CVC required a prolonged hospital stay (P=0.001). The data is summarized in Table 3.
Table 3
| Parameter | With CVC | Without CVC | P value |
|---|---|---|---|
| Postoperative comorbidities, n (%) | 47 (28.7) | 122 (20.5) | 0.02 |
| Postoperative cardiovascular complications, n (%) | 9 (5.5) | 8 (1.3) | 0.001 |
| Postoperative atrial fibrillation, n (%) | 7 (4.3) | 4 (0.7) | 0.001 |
| Major complication, n (%) | 8 (4.9) | 29 (4.9) | 0.05 |
| Minor complication, n (%) | 37 (22.6) | 93 (15.6) | 0.03 |
| Respiratory complications, n (%) | 19 (11.6) | 49 (8.2) | 0.1 |
| Pneumonia, n (%) | 12 (7.3) | 27 (4.5) | 0.1 |
| Postoperative pulmonary embolism, n (%) | 0 (0) | 2 (0.3) | 0.4 |
| Pleural empyema, n (%) | 1 (0.6) | 6 (1.0) | 0.6 |
| Prolonged air leak, n (%) | 2 (1.2) | 5 (0.8) | 0.6 |
| Wound healing disorder, n (%) | 0 (0) | 5 (0.8) | 0.2 |
| Hospital stay (days), mean (± SD) | 10.82 (±5.9) | 9.50 (±4.0) | 0.001 |
| 30-day mortality, n (%) | 1 (0.6) | 5 (0.8) | 0.7 |
| 90-day mortality, n (%) | 5 (3.0) | 13 (2.2) | 0.4 |
| 5-year survival | 68.0% | 75.8% | 0.03 |
CVC, cardiovascular comorbidities; SD, standard deviation.
Risk factors for postoperative morbidity in patients with CVC
MCC (N=18 patients, 40.0% with two or more CVC vs. N=28, 23.9% with only one CVC, P=0.04) and reduced left ventricular function (N=5 patients, 62.5% with reduced left ventricular function vs. N=42, 27.1% without reduced left ventricular function, P=0.03) were identified as risk factors for postoperative morbidity among patients with CVC. Moreover, reduced left ventricular function was identified as risk factor to develop major postoperative complications (N=2, 25.0% with heart failure vs. N=6, 3.9% without heart failure, P=0.01).
Age >70 (P=0.5), sex (P=0.5) vascular comorbidities (P=0.8), previous myocardial infarction (P=0.9), preoperative arrythmias (P=0.8), coronary heart disease (P=0.7), history of CVA/TIA (P=0.6), previous cardiac operations (P=0.06) and number of resected metastases with CVC were not be identified as risk factor for postoperative complications. The data is summarized in Table 4.
Table 4
| Parameter | Postop. morbidity | Postop. atrial fibrillation | Major morbidity | |||||
|---|---|---|---|---|---|---|---|---|
| N patients, % | P value | N patients, % | P value | N patients, % | P value | |||
| Two or more CVC | 18 (40.0) | 0.04 | 4 (8.9) | 0.07 | 3 (6.7) | 0.09 | ||
| Reduced left ventricular function | 5 (62.5) | 0.03 | 0 | 0.5 | 2 (25.0) | 0.01 | ||
| Arrythmias | 14 (29.8) | 0.8 | 2 (4.3) | 0.9 | 1 (2.1) | 0.5 | ||
| Coronary heart disease | 10 (27.0) | 0.7 | 3 (8.1) | 0.1 | 1 (2.7) | 0.7 | ||
| CVA/TIA | 5 (33.3) | 0.6 | 1 (6.7) | 0.6 | 0 | 0.4 | ||
| Previous cardiac operations | 5 (55.6) | 0.06 | 0 | 0.5 | 1 (11.1) | 0.1 | ||
| Myocardial infarction | 6 (30.0) | 0.9 | 0 | 0.3 | 2 (10.0) | 0.5 | ||
| Lobectomy | 4 (50.0) | 0.1 | 2 (25.0) | 0.003 | 0 | 0.1 | ||
| Single lung metastasis | 16 (24.6) | 0.3 | 3 (4.6) | 0.8 | 2 (3.1) | 0.4 | ||
| Resection of more than 4 lung metastases | 11 (25.6) | 0.5 | 2 (4.7) | 1 (2.3) | 0.6 | |||
| Vascular comorbidities | 15 (30.0) | 0.8 | 2 (4.0) | 0.9 | 2 (4.0) | 0.8 | ||
| Age >70 years | 13 (25.5) | 0.5 | 2 (3.9) | 0.8 | 5 (9.8) | 0.06 | ||
| Male | 34 (30.0) | 0.5 | 5 (4.4) | 0.9 | 5 (4.4) | 0.9 | ||
CVC, cardiovascular comorbidities; postop, postoperative; CVA/TIA, cerebrovascular accident or transient ischemic attack.
Postoperative atrial fibrillation
Need for lobectomy for PM in patients with CVC was associated with increased risk of postoperative atrial fibrillation (N=2 patients, 25.0% undergoing lobectomy vs. N=5, 3.2% undergoing wedge resections or segmentectomy, P=0.003).
In-hospital and postoperative mortality
In both groups, zero in-hospital mortality was detected. There was no difference in both cohorts for 30-day mortality (N=1 patient, 0.6% with CVC vs. N=5 patients, 0.8% without CVC, P=0.7).
Overall survival
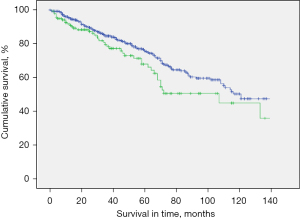
The median survival in the study population was 40 months (mean: 49.6 months; range, 0–223 months). Presence of CVC was associated with reduced OS after PM. Five-year survival rate (5-y-s) was 68.0% in patients with CVC compared to 75.8% in patients without CVC, P=0.03). Patients with colorectal cancer as primary tumor comprised almost half of the study population. However, presence of CVC in patients with colorectal cancer did not show to affect survival rate negatively (5-y-s: 74.5% in patients with CVC vs. 74.2% in patients without CVC and colorectal cancer, P=0.243) (Figure 1).
Risk factors for survival in patients with CVC and multivariate analysis
In the multivariate analysis lobectomy [hazard ratio (HR), 0.3; 95% confidence interval (CI): 0.1–0.8, P=0.02] and general vascular comorbidities (HR, 2.1; 95% CI: 1.1–3.9, P=0.01) were identified as independent negative prognostic factors for survival after PM in patients with CVC. Univariate and multivariate analysis are summarized in Table 5.
Table 5
| Parameter | 5-year survival (%) | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Postoperative comorbidity | 0.9 | 0.4–1.8 | 0.7 | – | – | – | ||
| Yes | 68.0 | |||||||
| No | 68.1 | |||||||
| More than two CVC | 0.6 | 0.3–1.2 | 0.2 | – | – | – | ||
| Yes | 64.0 | |||||||
| No | 67.0 | |||||||
| Vascular comorbidities | 2.1 | 1.1–3.9 | 0.01 | 2.1 | 1.1–3.9 | 0.01 | ||
| Yes | 60.0 | |||||||
| No | 72.0 | |||||||
| Periphery artery disease | 1.0 | 0.4–2.4 | 0.8 | – | – | – | ||
| Yes | 60.0 | |||||||
| No | 60.4 | |||||||
| Reduced left ventricular function | 0.6 | 0.1–2.5 | 0.4 | – | – | – | ||
| Yes | 65.0 | |||||||
| No | 66.0 | |||||||
| Coronary heart disease | 1.6 | 0.8–3.1 | 0.1 | – | – | – | ||
| Yes | 52.9 | |||||||
| No | 73.8 | |||||||
| Lobectomy | 0.3 | 0.1–0.8 | 0.02 | 0.3 | 0.1–0.8 | 0.02 | ||
| Yes | 22.0 | |||||||
| No | 68.3 | |||||||
| Myocardial infarction | 0.5 | 0.2–1.2 | 0.1 | – | – | – | ||
| Yes | 58.0 | |||||||
| No | 69.5 | |||||||
| More than four lung metastases | 1.3 | 0.6–2.5 | 0.3 | – | – | – | ||
| Yes | 62.1 | |||||||
| No | 70.3 | |||||||
| Single lung metastasis | 1.1 | 0.6–2.1 | 0.6 | – | – | – | ||
| Yes | 69.0 | |||||||
| No | 67.6 | |||||||
| Major postop. complication | 0.7 | 0.1–2.9 | 0.6 | – | – | – | ||
| Yes | 65.0 | |||||||
| No | 66.0 | |||||||
| Postop. atrial fibrillation | 0.6 | 0.08–4.4 | 0.6 | – | – | – | ||
| Yes | 66.7 | |||||||
| No | 66.3 | |||||||
PM, pulmonary metastasectomy; CVC, cardiovascular comorbidities; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
Our studies main finding is, that CVC in patients undergoing PM is associated with higher postoperative morbidity (28.7% vs. 20.5% in patients without CVC, P=0.02). However, the complications were mainly minor (23.3% vs. 4.9%) and required mostly pharmacological treatment. This observation led to a prolonged length of hospital stay without affecting the in-hospital mortality, or 30-day mortality (0.7%). The presence of CVC in patients undergoing PM is associated with reduced OS compared to patients without CVC in the long term follow up. However, a prolonged 5-y-s of 68.0% could be achieved.
In contrast, reduced left ventricular function (EF <35%) and MCC were identified as risk factors for postoperative morbidity. Lobectomy in patients with CVC frequently leads to postoperative atrial fibrillation. Adverse prognostic factors for survival in patients with CVC were vascular comorbidities and the need for lobectomy for complete resection of the lung metastases. The resection of a high number of lung metastases in patients with CVC was not associated with increased postoperative morbidity.
Many studies have investigated risk factors for postoperative complications after major lung resection for NSCLC and CVC is an acknowledged risk factor for this operation (5,9,10). Ambrogi et al. reported a significant difference in mortality and morbidity in patients with CVC undergoing minor or major lung resection for NSCLC (postoperative morbidity: 59% vs. 21% and 30-day mortality: 9% vs. 2.5%) (5). However, lung resections for PM are considered safer. PM in general has low mortality (0–2.2%) and low morbidity (0–15.6%) (1,11). In our study, 164 patients with CVC undergoing PM had zero in-hospital mortality and a postoperative morbidity rate of 28.8%.
Risk factors for postoperative complications after PM have not been thoroughly investigated yet. We identified only two studies analyzing postoperative morbidity after PM for CRC. In these two studies, CVC were identified as risk factors for postoperative complications after PM. Rodríguez-Fuster et al. observed a postoperative morbidity of 23% for patients with CVC undergoing PM (vs. 12.28% without CVC), but mortality was minimal (0.37% in all patients). However, in this study there was no distinction between major and minor morbidity (12).
Sponholz et al. investigated possible risk factors for postoperative comorbidity in elderly patients. Preoperative atrial fibrillation was found to be a risk factor for postoperative complications. However, the number of patients was limited and out of 47 patients >70 years with atrial fibrillation, 8 patients suffered from minor complications and 5 patients from major complications. However, coronary heart disease in elderly patients was not identified as risk factor. In our study, MCC multiple CVC and reduced left ventricular function were associated with increased postoperative morbidity. Although the number of patients in the subgroups were limited, we postoperatively observed acute myocardial infarction (N=1), electrolyte imbalance (N=4), atrial fibrillation (N=4), pneumonia (N=6) and angina pectoris (N=1). It seems possible, that the accumulation of CVC in a patient or an EF <35% could lead to heart strain or increased bronchorrhea, which can lead to cardiac complications or pneumonia (13).
For history of coronary artery disease, Benker et al. and Licker et al. showed a negative effect on postoperative morbidity and mortality after lung resection for NSCLC (9,14). More specifically, Benker et al. reported a significant difference in postoperative morbidity in patients with coronary heart disease compared to patients without (15.3% vs. 12.7%) (9). Licker et al. reported similar results for perioperative mortality in patients with coronary heart disease (7.9%) (14). However, for Kanzaki et al. EF <50% and myocardial infarction alone were no contraindication for major lung resection for NSCLC if the patient had preserved exercise tolerance (15). For PM in the elderly, Sponholz et al. considered coronary artery disease no risk factor (3% complications in elderly vs. 4.5% in younger) (13). The findings in our study are similar. Except for the two parameters mentioned above, no other risk factors were identified. We believe that these findings should be interpreted with caution given the limited number of patients in each comorbidity group. In addition, it is possible that these patients were assessed more carefully preoperatively, protected by their medication, or did not suffer severe perioperative stress because they underwent only minor lung resections. These might lead to the non-significant rate of postoperative morbidity in our study.
For previous cardiac surgeries, Senbaklavaci et al. showed that patients undergoing cardiovascular operation including coronary revascularisation did not show any difference in overall morbidity, postoperative pneumonia, prolonged air leak, atrial fibrillation or in-hospital mortality compared to patients without history of heart surgery (16). These findings are similar to our subgroup population. Conversely, our data seems to imply, that previous surgical correction of heart pathology acted protectively against morbidity after PM.
Concerning survival, patients with CVC showed a reduced 5-year survival of 68% in comparison with 75.8% for patients without CVC (P=0.03). However, a prolonged 5-year survival rate of 68.0% could be achieved. We suggest, therefore, that selected patients with a stable cardiovascular disease should not be excluded from PM as it could offer a good survival result. In addition, OS could be affected from the primary tumor. Patients with resected lung metastases from colorectal cancer consisted 46.8% of the study population. Stable cardiovascular disease did not influence survival in patients with colorectal cancer. Because of the reduced number of patients in the other primary tumor groups any further investigation of survival was waived. In our study, general vascular comorbidities were an independent negative prognostic factor for survival. We assume that patients with general vascular problems present with an already reduced general condition before surgery, which subsequently leads to reduced survival postoperatively.
The need for lobectomy to achieve complete resection of the lung metastases in patients with CVC was detected as an adverse factor for OS in the multivariate analysis and was associated with a higher incidence of postoperative atrial fibrillation. Major lung resections in PM have already been identified as risk factors in the past (12,17-20). Similarly, Ambrogi et al. reported that postoperative morbidity was significantly higher in patients with CVC than in patients without, that underwent lobectomy for NSCLC (80% vs. 41%) (5). Stefani et al. also observed that lobectomies for PM were associated with significantly longer operation time, later removal of indwelling chest drains, longer hospital stay and more severe postoperative complications (17). In our study, patients with CVC undergoing lobectomy showed higher postoperative morbidity (50%) and thus postoperative atrial fibrillation (25%). When analyzing 5-year survival, patients with CVC undergoing lobectomy had reduced survival compared to patients with CVC undergoing minor lung resections or patients without CVC undergoing lobectomy (5-year survival: 22% patients with lobectomy and CVC vs. 75.8% general CVC vs. 68% without CVC).
Parameters such as peripheral artery disease, preoperative arrythmia and coronary heart disease also did not influence morbidity.
In our series, only one patient with CVC died one month postoperatively. For this reason, we did not analyze potential risk factors. PM is mostly performed with lung-sparring wedge resections or segmentectomies in contrast to the lobectomies and pneumonectomies preferred for NSCLC, which entail higher mortality and morbidity. In our study, wedge resections and segmentectomies accounted for 94.7% of all resections. Therefore, even the resection of a high number of lung metastases (>4) had no impact on the incidence of postoperative complications or mortality.
Limitations
This is a retrospective single center study that included patients with lung metastases from a variety of tumor entities. Therefore, our findings should be interpreted cautiously, and no definitive conclusions should be drawn.
The number of patients with CVC was low. However, the formation of subgroups and statistical analysis was possible. We believe that our data on 1,000 PM in 760 patients, of which 164 had CVC, reflect everyday clinical practice accurately. Additionally, the presence of a selection bias is possible, as patients with symptomatic CVC or patients with CVC and reduced general condition might not be referred for surgery at all. Furthermore, echocardiography was not performed preoperatively for all patients. Therefore, the number of patients with CVC could be underestimated. Moreover, several important parameters such as preoperative chemotherapy, need for transfusion, postoperative re-admission to the intermediate care or intensive care unit could not be assessed due to unavailable data or the limited number of patients in each category. Further analysis of the subgroups based on the primary tumor was not possible due to the small number of patients and the non-homogeneous distribution. Thus, this study is limited to the examination of the influence of specific comorbidities on the clinical course of patients undergoing first PM. However, we believe that these findings may be useful in planning PM in patients with CVC. We believe that these patients should not be directly excluded from potentially curative PM, but that it is crucial that they undergo careful preoperative evaluation.
Conclusions
Resection of pulmonary metastases can be performed safely in selected patients with CVC, who fulfill general cardiopulmonary criteria of operability, even if they present with a high number of lung metastases. Patients with MCC or reduced left ventricular function <35% should be evaluated cautiously, as well as those, for whom lobectomy is required, as these factors were identified as adverse independent factors for survival.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-409/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-409/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-409/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Medical Center-University of Freiburg and registered in the German Registry for Clinical Trials (DRKS-ID: DRKS00021251). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okumura T, Boku N, Hishida T, et al. Surgical Outcome and Prognostic Stratification for Pulmonary Metastasis From Colorectal Cancer. Ann Thorac Surg 2017;104:979-87. [Crossref] [PubMed]
- Schmid S, Le UT, Zeisel C, et al. Pulmonary metastasectomy in sarcoma-experiences with laser-assisted resection. J Thorac Dis 2018;10:314-20. [Crossref] [PubMed]
- Moneke I, Funcke F, Schmid S, et al. Pulmonary laser-assisted metastasectomy is associated with prolonged survival in patients with colorectal cancer. J Thorac Dis 2019;11:3241-9. [Crossref] [PubMed]
- Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007;84:324-38. [Crossref] [PubMed]
- Ambrogi V, Pompeo E, Elia S, et al. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small-cell lung cancer. Eur J Cardiothorac Surg 2003;23:811-7. [Crossref] [PubMed]
- Mishra PK, Pandey R, Shackcloth MJ, et al. Cardiac comorbidity is not a risk factor for mortality and morbidity following surgery for primary non-small cell lung cancer. Eur J Cardiothorac Surg 2009;35:439-43. [Crossref] [PubMed]
- Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88-105. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Benker M, Citak N, Neuer T, et al. Impact of preoperative comorbidities on postoperative complication rate and outcome in surgically resected non-small cell lung cancer patients. Gen Thorac Cardiovasc Surg 2022;70:248-56. [Crossref] [PubMed]
- Herrero Rivera D, Nieto-Guerrero Gómez JM, Cacicedo Fernández de Bobadilla J, et al. Cardiovascular disease and survival in non-small cell lung cancer: a multicenter prospective assessment. Clin Transl Oncol 2019;21:1220-30. [Crossref] [PubMed]
- Welter S, Jacobs J, Krbek T, et al. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2007;84:203-10. [Crossref] [PubMed]
- Rodríguez-Fuster A, Belda-Sanchis J, Aguiló R, et al. Morbidity and mortality in a large series of surgical patients with pulmonary metastases of colorectal carcinoma: a prospective multicentre Spanish study (GECMP-CCR-SEPAR). Eur J Cardiothorac Surg 2014;45:671-6. [Crossref] [PubMed]
- Sponholz S, Schirren M, Oguzhan S, et al. Morbidity, mortality, and survival in elderly patients undergoing pulmonary metastasectomy for colorectal cancer. Int J Colorectal Dis 2018;33:1401-9. [Crossref] [PubMed]
- Licker M, de Perrot M, Höhn L, et al. Perioperative mortality and major cardio-pulmonary complications after lung surgery for non-small cell carcinoma. Eur J Cardiothorac Surg 1999;15:314-9. [Crossref] [PubMed]
- Kanzaki R, Inoue M, Minami M, et al. Outcomes of lung cancer surgery in patients with coronary artery disease: a decade of experience at a single institution. Surg Today 2017;47:27-34. [Crossref] [PubMed]
- Senbaklavaci Ö, Taspinar H, Hartert M, et al. Impact of previous cardiovascular surgery on postoperative morbidity and mortality after major pulmonary resection for non-small cell lung cancer. Langenbecks Arch Surg 2013;398:903-7. [Crossref] [PubMed]
- Stefani A, Oricchio F, Cinquepalmi A, et al. Is laser-assisted resection preferable to lobectomy for pulmonary metastasectomy? Lasers Med Sci 2020;35:611-20. [Crossref] [PubMed]
- Hayashi K, Tsuchiya H. The role of surgery in the treatment of metastatic bone tumor. Int J Clin Oncol 2022;27:1238-46. [Crossref] [PubMed]
- Williams NR, Patrick H, Fiorentino F, et al. Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) randomized controlled trial: a systematic review of published responses. Eur J Cardiothorac Surg 2022;62:ezac253. [Crossref] [PubMed]
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]


