
Retrospective, observational analysis of cardiac function associated with global preoperative myocardial scar in patients with ischemic cardiomyopathy after coronary artery bypass grafting
Introduction
Coronary artery bypass grafting (CABG) has become the primary treatment for patients with ischemic cardiomyopathy (ICM) (1). However, such patients have a high surgical mortality rate and a high incidence of adverse events, and their prognosis is closely related to the improvement of postoperative cardiac function (2,3). According to a report (4), nearly 40% of ICM patients have no improvement in cardiac function after CABG, which increases their risk of death from any cause. Scars cannot be revascularized, and cardiac magnetic resonance with late gadolinium enhancement (CMR-LGE) is the gold standard for detecting myocardial scarring (5). The goal of this study was to analyze cardiac function associated with global preoperative myocardial scarring assessed by CMR-LGE in patients with ICM after CABG. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-846/rc).
Methods
Participants
The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). Approval from the Ethics Committee of Anzhen Hospital Affiliated to Capital Medical University was obtained before the study (No. 2021104X) and individual consent for this retrospective analysis was waived. This was a single-center, retrospective, observational cohort study. Between September 2017 and September 2021, 129 patients diagnosed with ICM and who received preoperative CMR-LGE at Beijing Anzhen Hospital were enrolled in the study. According to the inclusion and exclusion criteria, 57 patients were finally analyzed. The inclusion criteria were as follows: (I) left ventricular ejection fraction (LVEF) of less than or equal to 40% on transthoracic echocardiography; (II) diagnosis of coronary heart disease by percutaneous coronary angiography and subsequent CABG; (III) preoperative CMR-LGE and 6-month postoperative evaluation of cardiac function by echocardiography; (IV) follow-up coronary computed tomography angiography (CTA) showing patency of the bypass grafts; and (V) complete clinical dataset. The exclusion criteria were as follows: (I) acute myocardial infarction within the previous 3 months; (II) concurrent combined cardiac surgery (such as aortic valve, mitral valve, tricuspid valve, congenital heart disease, great vascular disease, ventricular aneurysm resection, etc.); (III) concomitant arrhythmias, such as atrial fibrillation; (IV) preoperative complications, such as a malignant tumor and chronic renal failure; and/or (V) preoperative emergency surgery performed for cardiogenic shock.
Among the 129 patients, 6 received drug treatment, 33 underwent mitral valve surgery, 4 underwent tricuspid valve surgery, 19 underwent aneurysm resection, and 67 patients received CABG surgery alone. Of those 67 patients, 2 died of postoperative low cardiac output syndrome, 2 failed to follow up 6 months after surgery, 3 had bypass grafts failure 6 months postoperatively, and 3 patients could not be accurately assessed for preoperative myocardial scarring. As a result, 57 patients were included in this study (Figure 1).
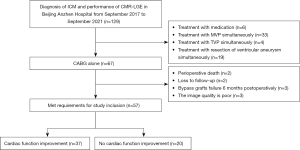
Study protocol
Clinical baseline characteristics, such as demographic information, echocardiography, and CMR-LGE data, were collected retrospectively. The patients’ follow-up information was collected via phone, WeChat, or outpatient clinic records. Based on the results of transthoracic echocardiography 6 months after CABG surgery, the patients were divided into the following 2 groups: the improved cardiac function group, defined as change of LVEF (ΔLVEF) greater than or equal to 5% (6), and the no improved cardiac function group, defined as ΔLVEF less than 5%. The baseline characteristics of the 2 groups were compared to analyze the risk factors for cardiac function failure after CABG.
CMR-LGE image acquisition
The CMR-LGE examination was performed on patients 1 week before CABG surgery, and images were collected in the supine position. A 3.0 T superconducting magnetic resonance system was adopted (Verio; Siemens Medical Solutions, Erlangen, Germany), and 32 channels were dedicated to the heart phase-controlled front coil. All sequences were gated by electrocardiogram. Phase-sensitive inversion recovery (PSIR) magnetic moments were used to prepare rapid small-angle excitation sequences for late enhancement of cardiac imaging approximately 10 minutes after intravenous injection of 0.1 mmol/kg of gadopentetate dimeglumine contrast agent under the following conditions: repeat time/echo time, 4.1 ms/1.56 ms; field of view, 350 mm2; matrix, 2.1 mm × 1.4 mm × 5.0 mm; turn angle, 35°; and, acceleration factor, 2. The thickness of the left ventricular short-axis imaging layer was 8 mm, with an interval of 0 mm. The imaging layers of the left ventricular 2-heart cavity and 4-heart cavities were both 5 mm thick without any separation.
CMR-LGE image post-processing
Left ventricular myocardial activity was analyzed using the cvi42 v 5.14.0 (Circle Cardiovascular Imaging Inc., Calgary, AB, Canada) post-processing software. The analysis was conducted by an associate senior radiologist with at least 5 years of CMR-LGE experience. The radiologist was unaware of the clinical data and information used to group the patients. The endocardium and epicardium (excluding papillary muscle) were delineated at the short axis level of the PSIR sequence, and the interventricular septum insertion point was marked at the short axis level to delineate the region of the normal myocardium. Normal myocardium was defined as having no LGE and being distant from the LGE area, whereas scarred myocardium was defined as being within the LGE area. The LGE region was defined as the myocardial gray threshold 5 standard deviations above the normal myocardium mean. By subtracting the end-diastolic pericardium volume and then multiplying it by 1.05 g/cm3, the software calculated the left ventricular myocardial mass (LV mass) and late gadolinium enhancement mass (LGE mass) and then calculated the percentage of cardiac scar (LGE mass/LV mass × 100%) (Figure 2).
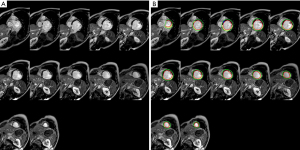
Surgical techniques
All patients underwent medial sternotomy. Depending on the patient’s condition and the surgeon’s experience, off-pump or on-pump CABG was performed. The skeletal or pedicled method was used to obtain the internal mammary artery, and the open technique was used to obtain the great saphenous vein. The left internal mammary artery was preferred for transplantation to the left anterior descending branch, and the great saphenous vein was sequentially branched out to other coronary arteries. All patients had anatomically complete revascularization (7), which implied that revascularization was performed for vessels with a coronary artery diameter of more than 1.5 mm and a stenosis of 50% or more, with at least one location indicated by coronary angiography. The quality of bridge anastomosis was evaluated using transit time flow measurement.
Postoperative management and medical therapy
The patients were returned to the intensive care unit (ICU) with tracheal intubation and given electrocardiogram monitoring, ventilator-assisted breathing, and hemodynamic monitoring. Arterial blood gas analysis was performed immediately after surgery, on the first day after surgery, and before removal of the endotracheal intubation, to facilitate timely adjustment of the water and electrolyte metabolism and acid-base balance disorders. All patients received guideline-directed medical therapy after undergoing CABG (1), including the following: renin-angiotensin system inhibitors, such as an angiotensin-converting enzyme inhibitor, an angiotensin type II receptor blocker, or an angiotensin receptor and neprilysin inhibitor; a beta-blocker; and a mineralocorticoid receptor antagonist, such as spironolactone, in addition to long-term antiplatelet drugs.
Follow-up
These patients were followed up regularly at 3 and 6 months postoperatively and then every 6 months. If patients presented with symptoms of heart failure or coronary heart disease after surgery, they should be followed up at that time to adjust medication and other treatments. At follow-up, cardiac function improvement, New York Heart Association (NYHA) classification, and the occurrence of major adverse cardiovascular and cerebrovascular events (MACCEs) were evaluated. After 6 months, improvement in cardiac function was defined as ΔLVEF greater than or equal to 5% on transthoracic echocardiography. The MACCEs included all-cause death, myocardial infarction, stroke, and readmission for heart failure. Meanwhile, the patency of bridge vessels was evaluated using coronary CTA. The patency of bridge vessels was assessed using the Fitzgibbon classification system (8), with Fitzgibbon-A considered patency and Fitzgibbon-B/O considered occlusion. All patient data were obtained from our institution’s online database and collected using standardized data collection forms by trained staff who were unaware of the purpose of this study.
Statistics analysis
The Student’s t-test presented the normal distribution measurement data as mean ± standard deviation, the Mann-Whitney U test presented the non-normal distribution measurement data as median (M) and quartile spacing [M (P25, P75)], and the chi-square test or Fisher’s exact test as frequency (rate) presented the count data as frequency (rate). Univariate logistic regression analysis examined the relationship between variables and no improvement in cardiac function. The multivariate logistics regression analysis included the univariate analysis of variables with P<0.05 and those thought to be clinically related to endpoint events. The Kaplan-Meier method was used to calculate the MACCE-free survival curves of the 2 groups. The log-rank test was used to check if the survival curves of the 2 groups differed. For the data analysis, the software packages SPSS 23.0 (IBM Corp., Armonk, NY, USA) and Stata 16 (StataCorp, College Station, TX, USA) were used.
Results
Preoperative baseline data of the 2 groups
At 6 months after CABG, 37 patients (64.9%) showed improvement in cardiac function, but 20 patients (35.1%) did not. The average age was 60.5±9.8 years (range, 39–84 years), and 78.9% (45/57) were males. There were no statistical differences in age, body mass index (BMI), body surface area (BSA), medical history, and degree of coronary lesion between the 2 groups (P>0.05). The unimproved group had significantly higher left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), ventricular wall thickness, left ventricular end-diastolic volume index (LVEDVI), and left ventricular end-systolic volume index (LVESVI) than the improved group. The degree of myocardial scarring in the unimproved group was significantly higher than that in the improved group (41.9%±6.4% vs. 27.8%±8.5%; P<0.001; Table 1).
Table 1
| Variables | Improved (n=37) | Unimproved (n=20) | P value |
|---|---|---|---|
| Age (years) | 61.0±10.7 | 59.6±8.0 | 0.591 |
| Male | 26 (70.3) | 19 (95.0) | 0.065 |
| BMI (kg/m²) | 25.3±3.0 | 24.5±2.0 | 0.305 |
| BSA (m²) | 1.78±0.2 | 1.81±0.2 | 0.481 |
| Medical history | |||
| Hypertension | 17 (45.9) | 7 (35.0) | 0.424 |
| Diabetes | 15 (40.5) | 5 (25.0) | 0.241 |
| Hyperlipemia | 15 (40.5) | 6 (30.0) | 0.431 |
| Smoking | 16 (43.2) | 12 (60.0) | 0.227 |
| Drinking | 11 (29.7) | 6 (30.0) | 0.983 |
| Cerebral infarction | 5 (13.5) | 2 (10.0) | 1.000 |
| Echocardiography | |||
| LVEF (%) | 36.4±4.1 | 35.4±3.6 | 0.353 |
| LVEDD (mm) | 57.4±5.8 | 60.9±6.4 | 0.042 |
| LVESD (mm) | 43.7±6.7 | 49.2±7.4 | 0.008 |
| IVST (mm) | 9.9±2.0 | 9.2±1.9 | 0.208 |
| LVPWT (mm) | 8.4±1.8 | 8.3±1.5 | 0.832 |
| LVEDVI (mL/m²) | 112.9±25.4 | 134.9±27.3 | 0.004 |
| LVESVI (mL/m²) | 79.4±23.8 | 100.4±25.7 | 0.003 |
| LVSVI (mL/m²) | 33.5±9.6 | 34.4±9.8 | 0.748 |
| Coronary lesion | 0.780 | ||
| One lesion | 1 (2.7) | 1 (5.0) | |
| Two lesions | 7 (18.9) | 4 (20.0) | |
| Three lesions | 29 (78.4) | 15 (75.0) | |
| SYNTAX score | 41.7±7.6 | 42.8±8.3 | 0.603 |
| NYHA class | 0.556 | ||
| I | 2 (5.4) | 1 (5.0) | |
| II | 17 (45.9) | 8 (40.0) | |
| III | 12 (32.4) | 6 (30.0) | |
| IV | 6 (16.2) | 5 (25.0) | |
| Myocardial scarring (%) | 27.8±8.5 | 41.9±6.4 | <0.001 |
Data are presented as n (%) or mean ± standard deviation. BMI, body mass index; BSA, body surface area; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVST, interventricular septal thickness; LVPWT, left ventricular posterior wall thickness; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVSVI, left ventricular stroke volume index; SYNTAX, Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery; NYHA, New York Heart Association.
Comparison of surgical data between the 2 groups
A bilateral internal mammary artery bypass was not used in either group. There were no significant differences between the 2 groups in the use of cardiopulmonary bypass, left internal mammary artery utilization rate, number of bypass procedures, operation time, and blood product dosage (P>0.05). Although the ICU stay time, ventilator use time, and postoperative hospital stay in the unimproved group were higher than those in the improved group, the differences were not statistically significant (P>0.05; Table 2).
Table 2
| Variables | Improved (n=37) | Unimproved (n=20) | P value |
|---|---|---|---|
| Off-pump | 25 (67.6) | 15 (75.0) | 0.558 |
| LIMA | 36 (97.3) | 19 (95.0) | 0.653 |
| Number of grafts | 3.5±0.6 | 3.7±0.37 | 0.353 |
| Operation time (h) | 4.1±0.9 | 4.1±0.7 | 0.958 |
| Erythrocytes (μ) | 0 (0, 4) | 0 (0, 1.5) | 0.489 |
| Plasma (mL) | 0 (0, 0) | 0 (0, 0) | 0.762 |
| Platelets (μ) | 0 (0, 0) | 0 (0, 0) | 0.473 |
| ICU stay time (h) | 46.0 (22.5, 67.5) | 48.0 (26.9, 87.3) | 0.358 |
| Ventilator use time (h) | 25.0 (18.5, 46.0) | 34.0 (23.8, 55.6) | 0.068 |
| Postoperative hospital time (days) | 7.0 (6.0, 8.0) | 8.0 (6.3, 11.8) | 0.134 |
Data are presented as n (%), mean ± standard deviation or median (first quartile, third quartile). LIMA, left internal mammary artery; ICU, intensive care unit.
Comparison of echocardiographic data between the 2 groups preoperatively and 6 months postoperatively
The results of preoperative and postoperative echocardiographic follow-up revealed that the LVEF and LVSVI were significantly increased 6 months after surgery in the improved group compared with the preoperative results, yet the LVEDD, LVESD, LVESVI, and LVEDVI were significantly decreased. Simultaneously, the LVEF, left ventricular size, and left ventricular volume were not significantly changed in the unimproved group. The differences between the 2 groups before and after the operation showed that the improved group had better improvement in left ventricular function (P<0.05), left ventricular size (P<0.05), and left ventricular volume (P<0.05), as shown in Table 3.
Table 3
| Variables | Improved (n=37) | Unimproved (n=20) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-op | 6 months | Δ | Pre-op | 6 months | Δ | |||
| LVEF (%) | 36.4±4.1 | 51.2±5.6 | 10.2±5.9 | 35.4±3.6 | 37.7±5.6 | 0.7±4.4 | <0.001 | |
| LVEDD (mm) | 57.4±5.8 | 52.7±3.5 | −4.7±5.5 | 60.9±6.4 | 59.4±6.5 | −1.1±6.1 | 0.029 | |
| LVESD (mm) | 43.7±6.7 | 38.4±3.8 | −5.5±6.3 | 49.2±7.4 | 47.3±6.2 | −1.4±6.6 | 0.027 | |
| LVEDVI (mL/m²) | 112.9±25.4 | 85.1±18.6 | −27.8±28.3 | 134.9±27.3 | 126.8±20.7 | −8.1±37.8 | 0.030 | |
| LVESVI (mL/m²) | 79.4±23.8 | 40.7±11.5 | −38.6±23.0 | 100.4±25.7 | 91.3±12.4 | −9.1±28.4 | <0.001 | |
| LVSVI (mL/m²) | 33.5±9.6 | 44.4±13.5 | 10.9±16.2 | 34.4±9.8 | 35.5±13.4 | 1.1±19.7 | 0.048 | |
Data are presented as mean ± standard deviation. Pre-op, preoperative; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVSVI, left ventricular stroke volume index.
Factors associated with failure to improve cardiac function
In ICM patients, univariate analysis showed that left ventricular size, left ventricular volume, and myocardial scarring were factors associated with no improvement in cardiac function after CABG. Multivariate regression analysis of these variables revealed that only myocardial scarring was a risk factor for no improvement in cardiac function (OR =1.44; 95% CI: 1.13–1.83; P=0.003; Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| LVEDD (mm) | 1.11 (1.00–1.22) | 0.048 | 0.81 (0.54–1.22) | 0.318 | |
| LVESD (mm) | 1.12 (1.02–1.22) | 0.014 | 1.28 (0.86–1.92) | 0.227 | |
| LVEDVI (mL/m²) | 1.03 (1.01–1.06) | 0.008 | 1.05 (0.93–1.19) | 0.451 | |
| LVESVI (mL/m²) | 1.04 (1.01–1.06) | 0.007 | 1.00 (0.88–1.13) | 0.999 | |
| Myocardial scar (%) | 1.34 (1.15–1.56) | <0.001 | 1.44 (1.13–1.83) | 0.003 | |
Data are presented as median (first quartile, third quartile). OR, odds ratio; CI, confidence interval; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index.
The incidence of MACCEs
After a median follow-up of 1.6 years (range, 0.6–4.1 years), 3 patients in the improved group and 5 patients in the non-improved group developed MACCE, with 1 patient dying, 1 experiencing a stroke, and 1 being readmitted for heart failure in the improved group, and 5 deaths in the non-improved group. According to the Kaplan-Meier survival analysis, the incidence of MACCE in the unimproved group was significantly lower than in the improved group (P=0.044; Figure 3). Meanwhile, the improved group had a lower NYHA classification and significantly reduced heart failure symptoms (P=0.018; Table 5).
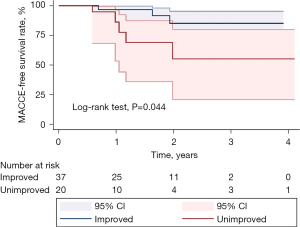
Table 5
| Variables | Improved (n=37) | Unimproved (n=20) | P value |
|---|---|---|---|
| MACCE | 3 (8.1) | 5 (25.0) | 0.044 |
| NYHA class | 0.018 | ||
| I | 6 (16.2) | 2 (10.0) | |
| II | 26 (70.3) | 9 (45.0) | |
| III | 4 (10.8) | 5 (25.0) | |
| IV | 1 (2.7) | 4 (20.0) |
Data are presented as n (%). MACCE, major adverse cardiovascular and cerebrovascular events; NYHA, New York Heart Association.
Discussion
This study yielded the following findings. Firstly, 35.1% of ICM patients had improved cardiac function 6 months after CABG. Secondly, preoperative myocardial scarring was shown to be an independent risk factor for failure to improve cardiac function following CABG. We had hoped to use receiver operating characteristic curves to detect the cut-off value of preoperative myocardial scarring to assist in predicting unimproved cardiac function after CABG, but it was inaccurate as a slight tweaking of endocardial and epicardial contours on CMR-LGE changed the LGE%, and the sample size was also small. Thirdly, we found that no improvement in cardiac function was associated with a poorer mid-term prognosis. The group with improved cardiac function had better outcomes than those without improvement, helping to identify the benefits for CABG patients.
CABG has become the primary treatment for ICM patients (1). However, different studies on whether cardiac function improves after CABG in ICM patients have reported conflicting results. A previous study (9) found that 50% of ICM patients had improved cardiac function 7 days after CABG. Another study (10) found that 35% of patients had improved cardiac function three months after CABG. Another report (11) found that 73% (51/70) of patients had improved cardiac function 1 year after CABG. According to the most recent study (4), nearly 40% of patients had no improvement in cardiac function at an average of 64.5±45.5 months after CABG. There was no significant relationship between improvement in postoperative cardiac function and all-cause mortality. Similar to a previous study, 64.9% of patients had improved cardiac function 6 months after CABG, and 35.1% had no improvement. Patients with unimproved cardiac function had a higher incidence of MACCE than those with improved cardiac function.
Myocardial scarring is more accurate than viable myocardium in predicting the improvement of cardiac function (5,12-15). As a result, we can predict cardiac function improvement after CABG by assessing the degree of myocardial scarring. The myocardium is a non-regenerative cell, hence once myocardial scars are formed, even if blood supply is restored, the function of the scarred myocardium cannot be restored. Further, myocardial scarring may impair the movement of the surrounding myocardium. The tethering of myocardial scar tissue may cancel out the improvement in systolic activity brought on by viable myocardium, preventing an improvement in overall cardiac function. As a result, the degree of myocardial scarring may significantly impact cardiac function.
CMR-LGE imaging is an effective method for detecting and quantifying myocardial scarring (5). In previous research (15), myocardial scar segments (LGE >50%) less than or equal to 4 were found to be predictive of improved cardiac function after CABG in ICM patients. Other studies (11,12) have shown that the number of myocardial scar segments (≤6 or ≤2) can predict the improvement of cardiac function after CABG. In the same way that previous studies have investigated whether the number of segments of myocardial scarring improves cardiac function, the present study quantified the overall degree of myocardial scarring in the left ventricle using CMR-LGE and found that a greater amount of preoperative myocardial scarring was associated with unimproved cardiac function after CABG. The number of segments of myocardial infarction was not used as a predictor in this study because the results of various studies on the number of segments of myocardial scarring have been inconsistent. Simultaneously, Hwang et al. (16) have shown that, although the degree of LGE mural penetration is negatively correlated with the improvement of myocardial segment function, one-third of myocardium scar segments still have a chance of improvement after revascularization.
Myocardial scarring can be used to identify patients at higher risk of cardiovascular events and has some value in predicting patient prognosis (13,17,18). A single-center analysis (19) demonstrated the association between myocardial scarring and prognosis. A total of 631 ICM patients with LVEF ≤40% underwent CMR-LGE and found that myocardial scarring was an independent risk factor for cardiogenic death. Meanwhile, patients with the same degree of myocardial scarring who underwent revascularization had a similar risk of death to those who received only drug therapy. There was no improvement in survival of ICM patients regardless of whether revascularization was performed on myocardial scars. Gerber et al. (20) demonstrated this in a study of 144 patients, in which 86 received complete revascularization (92% CABG) and 58 received drug therapy. Following patients for 3 years, the researchers found that myocardial scarring, regardless of revascularization, was associated with a higher incidence of adverse events, suggesting that drug treatment is likely the best option for such patients. They also found that patients with unimproved cardiac function had a greater degree of myocardial scarring, which was consistent with the findings of this study, and unimproved cardiac function was associated with a less favorable mid-term prognosis (and higher incidence of MACCE).
This study evaluated the degree of myocardial scarring using CMR-LGE to identify whether cardiac function improved after CABG in ICM patients. Since no improvement in cardiac function has a significant adverse impact on patient prognosis, our findings are important for patients who cannot benefit from CABG. Alternative therapies are available for ICM patients who do not benefit from CABG (1,21), such as continued drug therapy, a permanent left ventricular assistance device, heart transplant, or cell therapy.
This study had several limitations. Firstly, the study was a single-center, retrospective, observational cohort study with a small sample size, which may have resulted in selection bias. Secondly, the slice gap during a CMR-LGE scan can affect the accurate measurement and calculation of myocardial scarring and affect the accuracy of predicting the improvement of cardiac function. Thirdly, this study only considered the overall degree of myocardial scarring present in the left ventricle but did not consider the distribution of myocardial scarring. The distribution of cardiac scarring may also be a potential factor influencing the improvement of cardiac function. In follow-up work, we will conduct additional research to address these gaps. Finally, patients failed to undergo another CMR-LGE examination during postoperative follow-up, resulting in a lack of dynamic evolution over time. This was because the outpatient CMR-LGE reexamination took 1–3 months, which affected the postoperative examination.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and Jumatay Biekan (Circle Cardiovascular Imaging, Calgary, AB, Canada) for his assistance in CMR-LGE image post-processing.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81870181).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-846/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-846/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-846/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-846/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). Approval from the Ethics Committee of Anzhen Hospital Affiliated to Capital Medical University was obtained before the study (No.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bakaeen FG, Gaudino M, Whitman G, et al. 2021: The American Association for Thoracic Surgery Expert Consensus Document: Coronary artery bypass grafting in patients with ischemic cardiomyopathy and heart failure. J Thorac Cardiovasc Surg 2021;162:829-50.e1. [Crossref] [PubMed]
- Velazquez EJ, Lee KL, Jones RH, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med 2016;374:1511-20. [Crossref] [PubMed]
- Ryan M, Morgan H, Petrie MC, et al. Coronary evascularization in patients with ischaemic cardiomyopathy. Heart 2021;107:612-8. [Crossref] [PubMed]
- Nakae M, Kainuma S, Toda K, et al. Incidence, determinants and clinical impact of left ventricular function recovery after surgical treatments for ischaemic cardiomyopathy. Eur J Cardiothorac Surg 2021;60:689-96. [Crossref] [PubMed]
- Dhore-Patil AS, Aneja A. Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy. Heart Fail Clin 2021;17:41-56. [Crossref] [PubMed]
- Schinkel AF, Poldermans D, Rizzello V, et al. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg 2004;127:385-90. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Baer FM, Theissen P, Crnac J, et al. Head to head comparison of dobutamine-transoesophageal echocardiography and dobutamine-magnetic resonance imaging for the prediction of left ventricular functional recovery in patients with chronic coronary artery disease. Eur Heart J 2000;21:981-91. [Crossref] [PubMed]
- Bax JJ, Poldermans D, Elhendy A, et al. Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol 1999;34:163-9. [Crossref] [PubMed]
- Hwang HY, Yeom SY, Choi JW, et al. Cardiac Magnetic Resonance Predictor of Ventricular Function after Surgical Coronary Revascularization. J Korean Med Sci 2017;32:2009-15. [Crossref] [PubMed]
- Pegg TJ, Selvanayagam JB, Jennifer J, et al. Prediction of global left ventricular functional recovery in patients with heart failure undergoing surgical evascularization, based on late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:56. [Crossref] [PubMed]
- Chery G, Kamp N, Kosinski AS, et al. Prognostic value of myocardial fibrosis on cardiac magnetic resonance imaging in patients with ischemic cardiomyopathy: A systematic review. Am Heart J 2020;229:52-60. [Crossref] [PubMed]
- Doukas D, Porcaro K, Marot J, et al. Clinical characteristics and outcomes of patients with severe left ventricular dysfunction undergoing cardiac MRI viability assessment prior to revascularization. Int J Cardiovasc Imaging 2021;37:675-84. [Crossref] [PubMed]
- Yang T, Lu MJ, Sun HS, et al. Myocardial scar identified by magnetic resonance imaging can predict left ventricular functional improvement after coronary artery bypass grafting. PloS One 2013;8:e81991. [Crossref] [PubMed]
- Hwang HY, Yeom SY, Park EA, et al. Serial cardiac magnetic resonance imaging after surgical coronary revascularization for left ventricular dysfunction. J Thorac Cardiovasc Surg 2020;159:1798-805. [Crossref] [PubMed]
- Holtackers RJ, Emrich T, Botnar RM, et al. Late Gadolinium Enhancement Cardiac Magnetic Resonance Imaging: From Basic Concepts to Emerging Methods. Rofo 2022;194:491-504. [Crossref] [PubMed]
- Yang T, Lu M, Ouyang W, et al. Prognostic value of myocardial scar by magnetic resonance imaging in patients undergoing coronary artery bypass graft. Int J Cardiol 2021;326:49-54. [Crossref] [PubMed]
- Kwon DH, Obuchowski NA, Marwick TH, et al. Jeopardized Myocardium Defined by Late Gadolinium Enhancement Magnetic Resonance Imaging Predicts Survival in Patients With Ischemic Cardiomyopathy: Impact of Revascularization. J Am Heart Assoc 2018;7:e009394. [Crossref] [PubMed]
- Gerber BL, Rousseau MF, Ahn SA, et al. Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction: impact of revascularization therapy. J Am Coll Cardiol 2012;59:825-35. [Crossref] [PubMed]
- Bolli R, Solankhi M, Tang XL, et al. Cell therapy in patients with heart failure: a comprehensive review and emerging concepts. Cardiovasc Res 2022;118:951-76. [Crossref] [PubMed]
(English Language Editors: K. Gilbert and J. Jones)


