
Feasibility and safety of percutaneous cryoablation under local anesthesia for the treatment of malignant lung tumors: a retrospective cohort study
Introduction
Stage I and II non-small cell lung cancer and metastatic lung tumor without extrathoracic disease are treatable by surgery (1-6).
However, many patients are ineligible for surgical resection because of age, pulmonary dysfunction, comorbidities, multiple tumors, or multiple prior operations, among other reasons. Therefore, minimally invasive therapeutic options are desirable for such patients. Nonsurgical treatment of primary lung cancer and metastatic lung tumor is currently an evolving field that consists of stereotactic body radiation therapy (SBRT) and percutaneous thermal ablative procedures, such as radiofrequency ablation (RFA), microwave ablation, and cryoablation (7-11).
Percutaneous image-guided cryoablation is a minimally invasive treatment for cancers. And recent advancements in cryoablation devices have expanded local treatment options for various malignancies (12-15). Wang et al. first reported the percutaneous cryoablation for lung tumors in 2005 (15). Computed tomography (CT)-guided cryoablation enables visualization of the ablation zone, defined by a consolidative or interstitial shadow or visible ice ball. It delivers complete tumor ablation, while preventing the destruction of adjacent vital structures. In addition, cryoablation can treat tumors adjacent to the visceral pleura without pain or bronchopleural fistula (16). We have performed percutaneous cryoablation for malignant lung tumors since 2002 and reported the preliminary data on its feasibility and safety in 2006 and 2012 (17,18). In our previous studies, we did not report fatal adverse events.
Given this background, the present study aimed to examine the feasibility and safety of percutaneous cryoablation as a method for the treatment of malignant lung tumors based on a 15-year experience at our institution. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-830/rc).
Methods
Patients
A total of 227 patients, who underwent cryoablation for the treatment of malignant lung tumors in our institution between July 2002 and December 2016, were identified retrospectively. And 117 patients of those were included in our previous report (18). No patients were lost to follow-up and had insufficient data. After December 2016, issues with the CRYOcare cryosurgical unit and with domestic maintenance resulted in temporary suspension of its use. Preparations are being carried out for restarting percutaneous cryoablation.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The medical research ethics committee of Keio University School of Medicine (approval No. 20210003) approved the study, and the requirement for informed consent was waived due to the retrospective nature of this study.
The inclusion criteria were as follows: (I) surgical unsuitability due to multiple prior surgeries, multiple tumors, patient’s refusal, poor pulmonary function, comorbidities, or advanced age (75 years or more); (II) absence of active extrapulmonary malignancies; (III) performance status (PS) of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) scale; (IV) platelet count of >50,000 per L; (V) prothrombin time/international normalized ratio of <1.5; (VI) estimated life expectancy longer than 12 months; and (VII) written informed consent.
Age at treatment, sex, smoking status, ECOG PS, comorbidities, history of ipsilateral surgery, history of radiation therapy to the target tumor, history of chemotherapy (cytotoxic chemotherapy and molecular targeted therapy), tumor size on thin-section chest CT, tumor location, histology, tumors treated in one session, number of cryoprobes in one session, number of sessions, cryoablation protocol, duration of post-treatment hospitalization, complication rate, and mortality rate were investigated in 366 treatment sessions targeting 609 lesions. Tumor location was defined as central, if the center of tumor is located in the inner one third of the lung field on thin-section chest CT. The type of interstitial pneumonia [idiopathic pulmonary fibrosis (IPF) or not] was initially determined and recorded on medical charts by board-certified respiratory specialists. Histological classification was performed according to the fifth edition of the World Health Organization Classification of Tumors.
Information about the adverse events was collected on a per-session basis. Adverse events were defined as an unanticipated problem arising within 30 days post-treatment, of grade 2 or higher according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5. The adverse events, including post-procedural pain, were prospectively graded, and recorded on medical charts every day by a thoracic surgeon in charge. Death was defined as 30- and 60-day mortality within 30 and 60 days post-treatment, respectively, or in-hospital mortality.
Percutaneous cryoablation
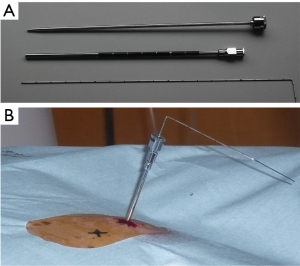
Cryoablation was performed using a CRYOcare cryosurgical unit (Endo-Care, Irvine, CA, USA) with 2.4- and 3.0-mm-diameter probes. CRYOcare is currently manufactured and sold by Varian Medical Systems, Inc. (Palo Alto, CA, USA). Intravenous cefotiam hydrochloride was administered prophylactically to the patients with 1 g three times (before/after the procedure and the postoperative day 1). We administered intramuscular pentazocine (15 mg) before leaving the ward. It was about 20 min before the start of the procedure. Then patients were carried to the CT room on the stretcher for safety. The appropriate patient posture on the CT bed was determined according to the tumor location. Cardiac monitoring was performed. All procedures were performed by two interventional radiologists or surgeons. The chief interventionist (SN) had ablation experience for more than 30 years and performed all the 366 sessions in this series. After the skin was cleansed with iodine, local anesthesia was administered by means of a subcutaneous injection of 1% lidocaine with 10–20 mL from the skin down to the pleura. A 21-gauge guiding needle was inserted into the tumor using three-slice CT fluoroscopic guidance (Aquilion 64; Toshiba, Tokyo, Japan). Next, a customized 8- or 11-gauge stainless steel coaxial needle with an inner guiding sheath and an outer sheath (Silux, Kawaguchi, Japan) (Figure 1A,1B) was introduced over the guiding needle (17). Following the removal of the inner sheath, a cryoprobe was inserted into the outer sheath. The number and size of cryoprobes were determined according to the tumor diameter to secure the appropriate ablation zone with at least a 5-mm margin (Figure 2). If a large pneumothorax (40% of the hemithorax) occurred during the procedure, manual aspiration was performed with an 18-gauge cannula. Cryoablation was performed with three freeze-thaw cycles, and freezing was performed using high-pressure argon gas. Before July 2006, the first and second freezes took 5 min, while the third one took 10 min. After July 2006, the first freeze took 5 min, and the second and third took 10 min (19,20). Active thawing was then performed using high-pressure helium gas until the temperature of the cryoprobe reached 20 ℃. To prevent pneumothorax, Bolheal fibrin glue (Teijin, Tokyo, Japan) was plugged into the outer sheath. CT was performed immediately after the procedure to assess the ablation zone and complications, including intracardial gas and pneumothorax. Patients were discharged on the second post-treatment day unless drainage for pneumothorax was required.

Follow-up
Follow-up chest radiographs performed after three hours and on the following day, and non-contrast CT scan performed on the following day were aimed to aid in the detection of the adverse events. Evaluation of the curative effect was determined by contrast-enhanced CT scans conducted at 1 week, 1 month, and 3- to 4-month intervals. In this study, evaluation of local control or prognosis was not conducted, because those differed among each type of tumor.
Collection of variables
Variables were collected from medical charts, CT images, and procedural records of cryoablation.
Statistical analyses
All data are presented as means, counts, or percentages. A logistic regression model was used to estimate odds ratios (ORs) with 95% confidence intervals (CIs) in the univariate and multivariate analyses to identify clinical factors associated with the development of post-treatment adverse events. All tests were two-sided, and P<0.05 were considered statistically significant. Factors that were significant (P<0.05) in the univariate analysis were entered into the multivariate analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences version 27.0 (IBM, Armonk, NY, USA).
Tumor characteristics after cryoablation treatment
The diagnosis of 227 patients was primary lung cancer in 56 (24.7%) and metastatic lung tumor in 171 (75.3%) (primary focus: colorectal cancer, n=48; lung cancer, n=41; malignant bone and soft tissue tumor, n=28; and others, n=54), respectively. The tumor characteristics of 227 patients treated with cryoablation are shown in Table 1.
Table 1
| Characteristics | Value |
|---|---|
| Primary lung cancer | 56 (24.7) |
| Metastatic lung tumor | 171 (75.3) |
| Colorectal cancer | 48 |
| Lung cancer | 41 |
| Malignant bone and soft tissue tumor | 28 |
| Uterine carcinoma | 10 |
| Salivary gland carcinoma | 9 |
| Renal cell carcinoma | 8 |
| Hepatocellular carcinoma | 6 |
| Esophageal cancer | 6 |
| Ovarian cancer | 3 |
| Breast cancer | 2 |
| Tongue cancer | 2 |
| Testicular cancer | 2 |
| Pancreatic cancer | 1 |
| Thyroid cancer | 1 |
| Laryngeal cancer | 1 |
| Pharyngeal cancer | 1 |
| Prostate cancer | 1 |
| Thymoma | 1 |
Data are presented as number (%) or number.
Patient and tumor characteristics of 366 sessions
As shown in Table 2, the demographic factors of the 366 sessions of treatment were as follows: median age, 64 (16–90) years; male, n=236 (64.5%); smoking history, n=193 (52.7%); comorbid diabetes, n=45 (12.3%); comorbid interstitial pneumonia, n=37 (10.1%); history of lung resection on the treated side, n=134 (36.6%), history of radiotherapy to the tumor, n=48 (13.1%), and history of chemotherapy, n=166 (45.4%). All patients with IPF had usual interstitial pneumonia (UIP) patterns on chest CT. No patients had received the immunotherapy. The median diameter of the targeted tumor was 1.3 (range, 0.2–10.2) cm, and the lesions were central (inner one-third of the lung field) in 104 (28.4%) sessions.
Table 2
| Characteristics | Value |
|---|---|
| Age, years | |
| Mean ± SD | 61.4±15.0 |
| Median | 64 |
| Range | 16–90 |
| Sex | |
| Female | 130 (35.5) |
| Male | 236 (64.5) |
| Smoking status | |
| Nonsmoker | 173 (47.3) |
| Current or former smoker | 193 (52.7) |
| ECOG PS | |
| 0 | 260 (71.0) |
| 1 | 106 (29.0) |
| Diabetes mellitus | |
| Present | 45 (12.3) |
| Absent | 321 (87.7) |
| Interstitial pneumonia | |
| Present | 37 (10.1) |
| Idiopathic pulmonary fibrosis | 15 (4.1) |
| Others | 22 (6.0) |
| Absent | 329 (89.9) |
| History of ipsilateral surgery | |
| Present | 134 (36.6) |
| Absent | 232 (63.4) |
| History of RT to the target tumor | |
| Present | 48 (13.1) |
| Absent | 318 (86.9) |
| History of chemotherapy | |
| Present | 166 (45.4) |
| Absent | 200 (54.6) |
| Freezing time | |
| 5-5-10 min (before July 2006) | 138 (37.7) |
| 5-10-10 min (after July 2006) | 228 (62.3) |
| Tumor size, cm | |
| Mean ± SD | 1.8±1.5 |
| Median | 1.3 |
| Range | 0.2–10.2 |
| Tumor location | |
| Central† | 104 (28.4) |
| Noncentral | 262 (71.6) |
Data are presented as mean ± SD, median, range or number (%). †, center of tumor located in the inner one third of the lung field. SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; PS, performance status; RT, radiation therapy.
Results
Cryoablation procedure characteristics
As shown in Table 3, the median number of tumors punctured during the first treatment was 1 (range, 1–10), the median number of cryoprobes used was 2 (range, 1–5), and the median number of sessions was 1 (range, 1–12). As multiple tumors were ablated in 37.2% (136 of 366) of all sessions, the responsible tumor for adverse events could not be verified in such sessions. Therefore, this study did not assess the association of tumor factors and adverse events. In 12.0% (44 of 366) of all sessions, the same probe was used to treat multiple tumors. Simultaneous tumor biopsy and cryoablation were performed in 8.2% (30 of 366).
Table 3
| Characteristics | Value |
|---|---|
| No. of tumors treated in one session | |
| Mean ± SD | 1.7±1.2 |
| Median | 1 |
| Range | 1–10 |
| No. of cryoprobes in one session | |
| Mean ± SD | 2.2±1.0 |
| Median | 2 |
| Range | 1–5 |
| No. of sessions | |
| Mean ± SD | 2.2±2.5 |
| Median | 1 |
| Range | 1–12 |
Data are presented as mean ± SD, median or range. SD, standard deviation.
Post-treatment results
All the patients tolerated the cryoablation procedure with no severe pain. The post-treatment results are presented in Table 4. The median duration of post-treatment hospitalization was 2 (range, 1–38) days. Overall, adverse events (grade 2 or higher) were observed in 79 (21.6%) sessions, with five (1.4%) of them experiencing an adverse event rate of grade 3. There was no 30-day mortality; however, there were two 60-day mortalities (0.5%) due to acute exacerbation of interstitial pneumonia following prolonged air leakage. Both patients had IPF with UIP pattern on chest CT. They had received home oxygen therapy due to IPF with UIP pattern and developed prolonged air leakage after the cryoablation. A 64-year-old man with primary lung cancer was diagnosed as developing an acute exacerbation on the 30 post-treatment day and died on the 38 post-treatment day. An 85-year-old man with metastatic lung tumor from esophageal cancer developed an acute exacerbation on the 18 post-treatment day and died on the 44 post-treatment day.
Table 4
| Characteristics | Value |
|---|---|
| Duration of post-treatment hospitalization, days | |
| Mean ± SD | 3.8±4.4 |
| Median | 2 |
| Range | 1–38 |
| Adverse event† | |
| Present | 79 (21.6) |
| Grade 2 | 74 (20.2) |
| Grade 3 | 5 (1.4) |
| Absent | 287 (78.4) |
| 30-day mortality | |
| Present | 0 (0.0) |
| Absent | 366 (100.0) |
| 60-day mortality | |
| Present | 2 (0.5) |
| Absent | 364 (99.5) |
Data are presented as mean ± SD, median, range or number (%). †, adverse events were defined as adverse events of grade 2 or higher according to the Common Terminology Criteria for Adverse Events, version 5. SD, standard deviation.
Table 5 shows the results of cryoablation adverse events in malignant lung tumors. Pneumothorax occurred in 18.0% (66 of 366) of the ablation sessions. Among the pneumothorax cases, 39 (10.7%) underwent chest tube placement, and two (0.5%) underwent pleurodesis as a result of a grade 3 adverse event. Hypoxemia occurred after six (1.6%) of the 366 sessions. However, all six patients recovered with oxygen inhalation within 24 hours. Both acute exacerbation of interstitial pneumonia and frostbite occurred after two sessions (0.5%) as a grade 3 adverse event. Wound infection, hematoma, empyema, phrenic nerve paralysis, empyema, and pulmonary embolism were documented after one session (0.3%). The empyema required fenestration as a grade 3 adverse event. Though fibrin glue was plugged into the outer sheath to prevent pneumothorax and bleeding, air embolism such as cerebral or pulmonary artery embolism was not observed in any case. The pain during the procedure was self-limiting, and the patients could accomplish a therapeutic procedure with local anesthesia and intramuscular pentazocine only.
Table 5
| Characteristics | Value |
|---|---|
| Pneumothorax | 66 (18.0) |
| Chest tube insertion | 39 (10.7) |
| Manual aspiration | 25 (6.8) |
| Chest tube insertion + pleurodesis | 2 (0.5) |
| Hypoxemia | 6 (1.6) |
| Acute exacerbation of interstitial pneumonia | 2 (0.5) |
| Frostbite | 2 (0.5) |
| Wound infection | 1 (0.3) |
| Hematoma | 1 (0.3) |
| Empyema | 1 (0.3) |
| Phrenic nerve palsy | 1 (0.3) |
| Pulmonary embolism | 1 (0.3) |
Data are presented as number (%).
Table 6 shows the results of the univariate and multivariate analyses for the clinical predictors of grade 2 or higher adverse events. In the multivariate analysis, comorbid interstitial pneumonia (OR =2.20; 95% CI: 1.04–4.64) and lack of history of pulmonary resection on the treated side (OR =3.04; 95% CI: 1.65–5.62) were identified as predictors of post-treatment adverse events. However, there were no correlations between the central location of the tumor in the lung field or the number of cryoprobes used or the number of sessions and post-treatment adverse events. We performed additional analysis on a per-patient basis to consider the impact of multiple sessions on the same patient. The data was shown in Tables S1-S4. The predictors for adverse events identified by both analyses were the same (Table S4).
Table 6
| Characteristics | No. of patients | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Age at operation, years | ||||||
| <65 | 186 | 1 | – | |||
| ≥65 | 180 | 1.01 (0.61–1.66) | 0.970 | – | – | |
| Sex | ||||||
| Female | 130 | 1 | – | |||
| Male | 236 | 1.69 (0.97–2.94) | 0.063 | – | – | |
| Smoking status | ||||||
| Nonsmoker | 173 | 1 | – | |||
| Current or former smoker | 193 | 1.09 (0.66–1.79) | 0.733 | – | – | |
| ECOG PS | ||||||
| 0 | 260 | 1 | – | |||
| 1 | 106 | 1.01 (0.58–1.75) | 0.973 | – | – | |
| Diabetes mellitus | ||||||
| Absent | 321 | 1 | – | |||
| Present | 45 | 0.89 (0.41–1.95) | 0.783 | – | – | |
| Interstitial pneumonia | ||||||
| Absent | 329 | 1 | 1 | |||
| Present | 37 | 2.16 (1.04–4.47) | 0.038 | 2.20 (1.04–4.64) | 0.039 | |
| History of ipsilateral surgery | ||||||
| Present | 134 | 1 | 1 | |||
| Absent | 232 | 3.02 (1.64–5.56) | <0.001 | 3.04 (1.65–5.62) | <0.001 | |
| History of RT to the target tumor | ||||||
| Absent | 318 | 1 | – | |||
| Present | 48 | 0.38 (0.15–1.01) | 0.051 | – | – | |
| History of chemotherapy | ||||||
| Absent | 198 | 1 | – | |||
| Present | 168 | 1.54 (0.93–2.55) | 0.087 | – | – | |
| Freezing time | ||||||
| 5-5-10 min (before July 2006) | 138 | 1 | – | |||
| 5-10-10 min (after July 2006) | 228 | 0.70 (0.43–1.17) | 0.173 | – | – | |
| Tumor location | ||||||
| Noncentral | 262 | 1 | – | |||
| Central† | 104 | 0.63 (0.35–1.14) | 0.127 | – | – | |
| No. of cryoprobes | ||||||
| 1 | 95 | 1 | – | |||
| ≥2 | 271 | 1.83 (0.97–3.44) | 0.062 | – | – | |
| No. of sessions | ||||||
| 1 | 230 | – | – | |||
| ≥2 | 136 | 0.79 (0.47–1.34) | 0.378 | – | – | |
Data are presented as number, OR (95% CI) or P value. †, center of tumor located in the inner one third of the lung field. OR, odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; RT, radiation therapy.
Discussion
In the present study, all 366 cryoablation procedures for lung tumors were completed under local anesthesia and associated with acceptable adverse event rates. Adverse events were observed in 79 (21.6%) sessions, with pneumothorax (n=66; 18.0%) being the most common, and patients had grade 3 adverse event in five (1.4%) sessions. In this study, 11.2% of pneumothorax cases required chest tube insertion, which is a slightly lower rate than that previously reported for cryoablation and RFA (21-32). In addition, none of the patients required subsequent surgical treatment for persistent pneumothorax or bronchopleural fistula. Therefore, the pneumothorax rate in the present study was acceptable. The adverse event rate in the present study was lower than that of our previous reports (17,18), because we used clearly defined criteria for adverse events as grade 2 or higher according to CTCAE, version 5.
The ablation needle used in this study (8–11 gauges) was thicker than an RFA needle (17 gauge) and, thus, could create a larger needle hole on the visceral pleura. However, this modified coaxial system offers two advantages. First, it enables a more precise penetration of the targeted tumor. Specifically, because the lung parenchyma is soft and lung tumors are easily moved, the cutting ability of the cryoprobe is inadequate to penetrate solid or small tumors. Second, the pathway through the outer sheath can be plugged with fibrin glue, which can potentially reduce the occurrence of pneumothorax and bleeding. Wang et al. (15) reported that preserving collagenous structure in frozen tissue facilitates the rapid natural closure of needle tracts. These factors might contribute to the absence of bronchopleural fistula in this series.
The results of the multivariate analysis indicated that the absence of history of pulmonary resection on the treated side and comorbid interstitial pneumonia were significant predictors of adverse events. The mean number of cryoprobes inserted was 2.2, which was equal to the number of pleural holes and comparable to previously reported values for RFA (15,33-38). These results suggest that the number of cryoprobes used is not a significant predictor of adverse events. It is reasonable to consider the absence of history of ipsilateral pulmonary surgery a risk factor for pneumothorax, since pleural adhesion after surgery prevents large pneumothorax.
Two deaths within 60 days of treatment were due to acute exacerbation of interstitial pneumonia following prolonged pneumothorax. Acute exacerbation of interstitial pneumonia in the postoperative period, which is associated with high mortality and intensive care, including mechanical ventilation, is frequently observed after surgery for primary lung cancer in which interstitial pneumonia coexists (39). In the present study, comorbid interstitial pneumonia was a significant risk factor for post-treatment adverse events. Weak lung parenchyma in patients with interstitial pneumonia can lead to post-treatment pneumothorax, resulting in exacerbation of interstitial pneumonia in cryoablation cases. Therefore, the risk of acute exacerbation after cryoablation for the treatment of malignant lung tumors, in which interstitial pneumonia coexists, should be recognized. Because we did not report mortality in our previous study on the safety of cryoablation (18), detecting interstitial pneumonia as the risk factor for cryoablation-related death is the clinical significance of this additional report on the safety of cryoablation for lung tumors.
Cryoablation offers several advantages over other local therapies. The first advantage is repeatability. SBRT is associated with difficulties treating several lesions with overlapping irradiation fields and retreating local progression after previous SBRT treatment. In contrast, cryoablation can be performed on neighboring lesions or repeated with disease recurrence. The second advantage is the application to the central area where larger bronchi and vessels exist. To date, there are several reports that SBRT and RFA are not suitable for lesions adjacent to hilar structures because of the risk of excessive toxicity (40). Herrera et al. reported death from massive hemoptysis after RFA of a centrally located lung tumor, which resulted in the termination of its use for central nodules (41). Several deaths as a result of massive hemothorax after RFA have also been reported (31,32). In the present study, cryoablation was performed for centrally located tumors in 104 sessions, and no major adverse events were reported. Cryoablation was reported to preserve collagenous architecture such as the bronchial wall (42). Yokomise et al. reported that the bronchial wall could resist ultralow temperatures because tracheas obtained for transplantation from cadavers can be deep-frozen (43). On the other hand, the wall of large blood vessels heated by circulating blood during cryoablation resulted in preserving the vessel wall.
In recent prospective trials, cryoablation was performed under general anesthesia in more than half of the cases (21,22). However, in the present study, all cryoablation procedures were completed under local anesthesia, and none of the patients experienced moderate to severe (grade 2 or higher) pain during the postprocedural period. This low incidence of peri- and post-procedural pain is commonly considered a specific advantage of cryoablation over other thermal therapies (44-53). Moorjani et al. (54) investigated the effects of cryoanalgesia on intercostal nerves and considered cryoablation to be an ideal technique from the perspective of treatment-associated pain. However, some patients complained of dull pain in the anterior chest after treatment, which was likely related to freezing of the intercostal nerves, and typically disappeared within a few months. This may explain why the intercostal nerve pain in most patients in the present study was recoverable. These features also suggest the advantage of cryoablation over RFA, especially for tumors adjacent to large pulmonary vessels, bronchus, or other mediastinal structures. Chipko et al. (55) reported that chest pain occurred in 33 (28.0%) of 118 patients at 1 year after SBRT for malignant lung tumors, eight of whom presented grade 3 pain. Rib fractures were also reported in 29% of the patients.
This study has some limitations. This retrospective study did not include a control group. The patient cohort was heterogeneous in terms of disease presentation and course, which could increase the generalizability of the results to the real-world practice. However, the statistical power was insufficient to draw conclusions on individual disease characteristics in the subgroup analyses. Because poor pulmonary function is not the necessary condition for enrollment, pretreatment forced expiratory volume in one second was unavailable on medical charts in 40% of the patients. The insufficient data about pretreatment pulmonary function should be the study limitation. Furthermore, the efficacy of cryoablation for pulmonary tumors was not assessed because local control rate and survival were different among each type of tumor. We aim to evaluate the efficacy of cryoablations for each type of tumors in the future. Moreover, this study included patients in the early 2000s. Because we did not have enough evidence about the safety of cryoablation at the time, our care might have been overmedication in retrospect, including prophylactic antibiotics or frequent CT checkups after the procedure. The discrepancy with today’s clinical standards would be the study limitation.
We believe that identifying the predictors of adverse events after ablative therapy is helpful in reducing adverse events. In general, the characteristics of candidates for SBRT and ablative procedures, such as medically or technically inoperable patients, are very similar. However, percutaneous cryoablation is expected to be more suitable than SBRT for radiation-resistant tumors, irradiated tumors, and tumors with a history of bilateral surgery. Further clinical studies are required to define the strengths and weaknesses of each modality.
Conclusions
In conclusion, CT-guided percutaneous cryoablation is a minimally invasive treatment option for malignant lung tumors with acceptable adverse event rates. The grade 3 or higher adverse event rate was 1.4%, suggesting that it can be safely performed. However, the possibility of adverse events in patients with comorbid interstitial pneumonia should be considered. Further studies clarifying the efficacy and appropriate candidate selection for cryoablation are required in the future.
Acknowledgments
The authors thank Yasunori Sato, Department of Preventive Medicine and Public Health, Keio University School of Medicine, for his help with statistical analysis.
Funding: This study was funded by the institutional research support grant. The funder had no role in the study design, analysis, and reporting of the study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-830/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-830/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-830/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-830/coif). KK, KA, RN, YO, KM, TH, and HA received scholarship donation from Eli Lilly Japan K.K., Shionogi & Co., Ltd., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kyowa Kirin Co., Ltd., Ethicon, Inc., and Covidien Japan Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The medical research ethics committee of Keio University School of Medicine (approval No. 20210003) approved the study, and the requirement for informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Headrick JR, Miller DL, Nagorney DM, et al. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg 2001;71:975-9; discussion 979-80. [Crossref] [PubMed]
- Dickinson KJ, Blackmon SH. Results of Pulmonary Resection: Colorectal Carcinoma. Thorac Surg Clin 2016;26:41-7. [Crossref] [PubMed]
- Mouli SK, Kurilova I, Sofocleous CT, et al. The Role of Percutaneous Image-Guided Thermal Ablation for the Treatment of Pulmonary Malignancies. AJR Am J Roentgenol 2017;209:740-51. [Crossref] [PubMed]
- Sharma A, Duijm M, Oomen-de Hoop E, et al. Survival and prognostic factors of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol 2019;58:74-80. [Crossref] [PubMed]
- Hess A, Palussière J, Goyers JF, et al. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology 2011;258:635-42. [Crossref] [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643-51. [Crossref] [PubMed]
- Bahn DK, Lee F, Badalament R, et al. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology 2002;60:3-11. [Crossref] [PubMed]
- Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg 1997;225:193-201. [Crossref] [PubMed]
- Gill IS, Novick AC, Soble JJ, et al. Laparoscopic renal cryoablation: initial clinical series. Urology 1998;52:543-51. [Crossref] [PubMed]
- Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology 2005;235:289-98. [Crossref] [PubMed]
- Ahmed A, Littrup P. Percutaneous cryotherapy of the thorax: safety considerations for complex cases. AJR Am J Roentgenol 2006;186:1703-6. [Crossref] [PubMed]
- Kawamura M, Izumi Y, Tsukada N, et al. Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg 2006;131:1007-13. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Yashiro H, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol 2012;23:295-302; quiz 305. [Crossref] [PubMed]
- Nakatsuka S, Yashiro H, Inoue M, et al. On freeze-thaw sequence of vital organ of assuming the cryoablation for malignant lung tumors by using cryoprobe as heat source. Cryobiology 2010;61:317-26. [Crossref] [PubMed]
- Hinshaw JL, Littrup PJ, Durick N, et al. Optimizing the protocol for pulmonary cryoablation: a comparison of a dual- and triple-freeze protocol. Cardiovasc Intervent Radiol 2010;33:1180-5. [Crossref] [PubMed]
- de Baère T, Woodrum D, Tselikas L, et al. The ECLIPSE Study: Efficacy of Cryoablation on Metastatic Lung Tumors With a 5-Year Follow-Up. J Thorac Oncol 2021;16:1840-9. [Crossref] [PubMed]
- Callstrom MR, Woodrum DA, Nichols FC, et al. Multicenter Study of Metastatic Lung Tumors Targeted by Interventional Cryoablation Evaluation (SOLSTICE). J Thorac Oncol 2020;15:1200-9. [Crossref] [PubMed]
- Yuan Z, Wang Y, Zhang J, et al. A Meta-Analysis of Clinical Outcomes After Radiofrequency Ablation and Microwave Ablation for Lung Cancer and Pulmonary Metastases. J Am Coll Radiol 2019;16:302-14. [Crossref] [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [Crossref] [PubMed]
- Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2007;134:857-64. [Crossref] [PubMed]
- Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg 2005;129:639-44. [Crossref] [PubMed]
- Hiraki T, Gobara H, Iishi T, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg 2007;134:1306-12. [Crossref] [PubMed]
- Pennathur A, Abbas G, Schuchert MJ, et al. Image-guided radiofrequency ablation for the treatment of early-stage non-small cell lung neoplasm in high-risk patients. Semin Thorac Cardiovasc Surg 2010;22:53-8. [Crossref] [PubMed]
- Ferguson J, Alzahrani N, Zhao J, et al. Long term results of RFA to lung metastases from colorectal cancer in 157 patients. Eur J Surg Oncol 2015;41:690-5. [Crossref] [PubMed]
- Petre EN, Jia X, Thornton RH, et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer 2013;12:37-44. [Crossref] [PubMed]
- McDevitt JL, Mouli SK, Nemcek AA, et al. Percutaneous Cryoablation for the Treatment of Primary and Metastatic Lung Tumors: Identification of Risk Factors for Recurrence and Major Complications. J Vasc Interv Radiol 2016;27:1371-9. [Crossref] [PubMed]
- Hasegawa T, Takaki H, Kodama H, et al. Three-year Survival Rate after Radiofrequency Ablation for Surgically Resectable Colorectal Lung Metastases: A Prospective Multicenter Study. Radiology 2020;294:686-95. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [Crossref] [PubMed]
- Yamakado K, Hase S, Matsuoka T, et al. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicenter study in Japan. J Vasc Interv Radiol 2007;18:393-8. [Crossref] [PubMed]
- Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol 2008;15:1765-74. [Crossref] [PubMed]
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology 2006;241:275-83. [Crossref] [PubMed]
- Yamagami T, Kato T, Hirota T, et al. Pneumothorax as a complication of percutaneous radiofrequency ablation for lung neoplasms. J Vasc Interv Radiol 2006;17:1625-9. [Crossref] [PubMed]
- Nomura M, Yamakado K, Nomoto Y, et al. Complications after lung radiofrequency ablation: risk factors for lung inflammation. Br J Radiol 2008;81:244-9. [Crossref] [PubMed]
- Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. [Crossref] [PubMed]
- Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012;142:1620-35. [Crossref] [PubMed]
- Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg 2003;125:929-37. [Crossref] [PubMed]
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [Crossref] [PubMed]
- Yokomise H, Inui K, Wada H, et al. Reliable cryopreservation of trachea for one month in a new trehalose solution. J Thorac Cardiovasc Surg 1995;110:382-5. [Crossref] [PubMed]
- McTaggart RA, Dupuy DE. Thermal ablation of lung tumors. Tech Vasc Interv Radiol 2007;10:102-13. [Crossref] [PubMed]
- Zhang X, Tian J, Zhao L, et al. CT-guided conformal cryoablation for peripheral NSCLC: initial experience. Eur J Radiol 2012;81:3354-62. [Crossref] [PubMed]
- Jahangeer S, Forde P, Soden D, et al. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev 2013;39:862-71. [Crossref] [PubMed]
- Yan TD, King J, Sjarif A, et al. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol 2006;13:1529-37. [Crossref] [PubMed]
- Yan TD, King J, Sjarif A, et al. Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol 2007;14:1718-26. [Crossref] [PubMed]
- Chua TC, Sarkar A, Saxena A, et al. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol 2010;21:2017-22. [Crossref] [PubMed]
- Pusceddu C, Sotgia B, Fele RM, et al. CT-guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: a preliminary experience. Eur J Radiol 2013;82:e246-53. [Crossref] [PubMed]
- Balduyck B, Van Thielen J, Cogen A, et al. Quality of life evolution after pulmonary metastasectomy: a prospective study comparing isolated lung perfusion with standard metastasectomy. J Thorac Oncol 2012;7:1567-673. [Crossref] [PubMed]
- Kim HK, Lee YJ, Han KN, et al. Pulmonary Function Changes Over 1 Year After Lobectomy in Lung Cancer. Respir Care 2016;61:376-82. [Crossref] [PubMed]
- Peng Z, Li H, Zhang C, et al. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One 2014;9:e90014. [Crossref] [PubMed]
- Moorjani N, Zhao F, Tian Y, et al. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg 2001;20:502-7. [Crossref] [PubMed]
- Chipko C, Ojwang J, Gharai LR, et al. Characterization of Chest Wall Toxicity During Long-term Follow Up After Thoracic Stereotactic Body Radiation Therapy. Pract Radiat Oncol 2019;9:e338-46. [Crossref] [PubMed]


