Changes in fibrinolytic activity and coagulation factors after epicardial left atrial appendage closure in patients with atrial fibrillation
Introduction
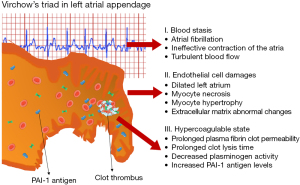
Nearly 15% of ischemic strokes are caused by atrial fibrillation (AF). AF is associated with a prothrombotic state that increases the risk of stroke fivefold compared with the general population. It is well known, that the increased risk of thromboembolism in AF is associated with a combination of pathophysiological mechanisms, a Virchow’s triad (1): (I) blood stasis; (II) abnormalities of the vessel wall; and (III) regional and systemic inflammation leading to a prothrombotic and hypercoagulable state (2). More than 90% of all intracardiac thrombi that form in patients with AF are observed in the left atrial appendage (LAA).
Elimination of the LAA from the circulatory system has become an alternative method for stroke prevention in patients with AF. The European Society of Cardiology and American College of Cardiology/American Heart Association guidelines recommend surgical LAA occlusion (LAAO) concomitant with cardiac surgery or thoracoscopic AF surgery as a Class 2B (3,4). Interventional percutaneous LAA ligation or occlusion procedures have a class 2B recommendation only for AF patients in whom oral anticoagulation (OAC) is contraindicated (3,4).
Several observational studies indicate the feasibility and safety of surgical or percutaneous LAAO or exclusion procedure (3-6). However, evaluation of the efficacy of these procedures is always based on clinical observations with stroke or other thromboembolic events as an endpoint. Unfortunately, these observations are not supported by the results of basic science or translational research studies based on a biomarker approach to assess thromboembolic risk after LAA elimination (3-6). Even, a large randomized trial such as left atrial appendage occlusion study (LAAOS) III, only evaluates the clinical outcomes of LAA elimination (7). Therefore, our goal was to conduct a translational study that supports existing observational studies of LAA elimination with basic research on the coagulation system and effect on prothrombotic status.
Aim
To evaluate the effects of epicardial LAA elimination from the cardiovascular system on the coagulation system and prothrombotic status in AF patients. We also analyzed the relationship between the level of hypercoagulability, fibrinolytic markers and clot lysis time (CLT) in relation to the presence of LAA. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1093/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Jagiellonian University Ethical Committee (27 September 2012, number KBET/282/B/2012). All patients signed a written informed consent form prior to inclusion.
Patients characteristic
A prospective study was performed in 22 consecutive patients with permanent nonvalvular AF of at least 6-months duration who were eligible for an epicardial LAAO procedure with Lariat or AtriClip device. All eligible patients had electrocardiographically confirmed long-term AF. These patients were at high risk of stroke and bleeding for continued long term systemic anticoagulation.
Data on demographics, cardiovascular risk factors, concomitant diseases and current treatment were collected from all patients using a standardized questionnaire. The diagnosis of stroke was based on World Health Organization criteria (8). Diabetes was defined as a history of diabetes regardless of duration of disease, need for hypoglycemic agents, or fasting glycemia greater than 7 mmol/L or 126 mg/dL. Coronary artery disease (CAD) was confirmed angiographically (>50% stenosis in at least one major epicardial artery).
LAA closure procedure and blood drawing
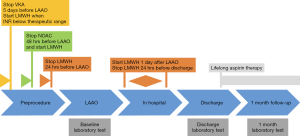
Patients receiving long-term vitamin K antagonist (VKA) or novel oral anticoagulation (NOAC) were eligible if their anticoagulation was stable within the last 3 months. Bridging therapy was administered before the procedure. VKAs were interrupted 5 days and NOACs were interrupted at least 2 days before the LAAO procedure and switched to low-molecular-weight heparin (LMWH) (Figure 1). All left LAAO were performed with epicardial devices: a Lariat device (SentreHEART Inc., Redwood, CA, USA) was used in 72.7% of cases and an AtriClip (AtriCure, Inc., West Chester, PA, USA) in 27.3% of cases.
In the LAAO procedure with the Lariat device, pericardial access was obtained via a telescoping-micropuncture technique at the beginning of the procedure, followed by femoral venous access. This method was described in detail in our previous study (9-11). The LAAO procedure with the AtriClip device was performed by a stand-alone, fully thoracoscopic elimination of LAA using a minimally invasive approach, that has also been described elsewhere (12).
One day after the procedure, all patients were continued on LMWH until hospital discharge. All patients were discharged on aspirin monotherapy (150 mg/day).
Echocardiography
To assess the presence of leaks after the procedure, transthoracic echocardiography was performed at discharge and 1 month later. Leaks were categorized into 3 categories: complete or <1 mm leak; <2 mm leak; <3 mm leak.
Laboratory investigations
Baseline blood samples were collected before LAAO on the day of the procedure. Control blood samples were collected at discharge and 1 month follow-up (Figure 1). The collection of blood samples was described in detail in our previous study (13). Tissue plasminogen activator (tPA) antigen, plasminogen activator inhibitor type 1 (PAI-1) antigen (R&D, Minneapolis, USA) thrombin activatable fibrinolysis inhibitor (TAFI) antigen, and plasminogen activity were determined by ELISA (both Hyphen BioMed, Neuville-Sur-Oise, France).
Fibrin clot analysis
Similar to our previous study, the plasma fibrin clot permeability was determined by the method described by Mills et al. (14). Technically, plasma fibrin clot permeability is a measure of the hydraulic conductivity of fibrin networks formed under standardized conditions; it represents the average pore size within plasma fibrin clots based on the volume of percolating buffer as a function of time. Low values for plasma fibrin clot permeability indicate a tightly packed fibrin structure. Mathematical descriptions of fluid flow in porous media are based on Darcy’s law. Plasma fibrin clot permeability was calculated using the following equation: Ks = Q × L × η/t × A × Δp, where Q is the flow rate in time t, L is the length of the fibrin gel, η is the viscosity of liquid (in poise), t is percolating time, A is the cross-sectional area (in cm2), and Δp is the differential pressure (in dyne/cm2). The normal value determined for healthy subjects (n=20) was 8.07 (7.22–8.63)×10−9 cm2.
CLT was measured as previously described in the study Pieters et al. (15). The normal value determined for healthy subjects (n=20) was 98 [89–103] min. The interassay coefficients of variation for the lysis variables were <8%.
Scanning electron microscopy (SEM) analysis
Fibrin clots prepared from plasma of 10 randomly-selected patients as described above were analyzed by SEM. Briefly, clots were fixed using 2.5% glutaraldehyde, washed with distilled water, dehydrated in graded water-ethanol solutions, dried by the critical point procedure, and sputter-coated with gold (14). Samples were scanned in 10 different areas (microscope JEOL JCM-6000; JEOL Ltd., Tokyo, Japan) at 5,000× and 10,000× magnification to determine a fibrin fiber diameter of at least 50 individual fibers per clot (n=50) within the fibrin network using ImageJ software (US National Institutes of Health, Bethesda, MD, USA). SEM images were analyzed manually by two independent investigators who were unaware of the origin of the samples.
Calibrated automated thrombogram
The kinetics of thrombin generation was measured using the Calibrated Automated Thrombogram (Thrombinoscope BV, Maastricht, The Netherlands) described in detail in our previous study (13).
Statistical analysis
Continuous variables were summarized as mean ± standard deviation (SD) if normally distributed; non-normal distributions were summarized as median and interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables [number (%)] were compared using Fisher’s exact test or Pearson’s χ2 test. For paired data the paired Wilcoxon signed-rank tests were used. Associations between nonparametric and parametric variables were assessed by Spearman’s and Pearson’s tests, respectively. P values of <0.05 were considered statistically significant. All statistical analyzes were performed using JMP® Version 13.1.0 and SAS 9.4 (SAS Institute Inc., North Carolina, USA).
Results
We recruited 22 patients with nonvalvular AF. Baseline characteristics are listed in Table 1. Patients were predominantly male (54.5%) and were at high risk for thromboembolic events (mean CHADS2 score of 2.8±1.1 and mean CHA2DS2-VASc score of 4.3±1.7) and at high risk for bleeding (mean HAS-BLED score 3.5±1.3).
Table 1
| Variable | Patients (n=22) |
|---|---|
| LAA epicardial device | |
| Lariat | 72.7% (n=16) |
| AtriClip | 27.3% (n=6) |
| Age, years (mean ± SD) | 68.2±8.5 |
| Female | 45.5% (n=10) |
| BMI, kg/m2 (mean ± SD) | 28.7±2.7 |
| LVEF (%) | 54.5±6.2 |
| CHADS2 score (mean ± SD) | 2.8±1.1 |
| CHA2DS2-VASc score (mean ± SD) | 4.3±1.7 |
| HAS-BLED score (mean ± SD) | 3.5±1.3 |
| Congestive heart failure | 9.1% (n=2) |
| Hypertension | 95% (n=21) |
| Hyperlipidemia | 59.1% (n=13) |
| Diabetes mellitus 2 | 31.8% (n=7) |
| Previous ischemic stroke/TIA | 72.7% (n=16) |
| Hemorrhagic stroke | 9.1% (n=2) |
| Myocardial infraction | 4.5% (n=1) |
| Vascular disease | 9.1% (n=2) |
| Alcoholism | 9.1% (n=2) |
| Laboratory parameters (mean ± SD) | |
| Platelet count (103) | 241.9±24 |
| PT-INR | 1.2±0.3 |
| PT sec | 13.1±2.5 |
| PT % | 81.3±21.5 |
| APTT (sek) | 35.9±7.8 |
| Anti-Xa activity (IU/mL) | 0.5±0.3 |
| C-reactive protein (mg/L) | 1.7±1.7 |
| Total cholesterol (mmol/L) | 4.2±1.0 |
| LDL cholesterol (mmol/L) | 2.5±1.0 |
| HDL cholesterol (mmol/L) | 1.3±0.4 |
| Triglyceride (mmol/L) | 1.7±1.1 |
| Glycated hemoglobin (HBA1C) (%) | 5.9±0.8 |
| Preoperative medications | |
| VKA | 18.2% (n=4) |
| Warfarin | 9.1% (n=2) |
| Acenocoumarol | 9.1% (n=2) |
| New oral anticoagulant | 13.6% (n=3) |
| Dabigatran | 4.5% (n=1) |
| Rivaroxaban | 9.1% (n=2) |
| LMWH | 9.1% (n=2) |
| Calcium channel blockers | 9.1% (n=2) |
| Aspirin | 18.2% (n=4) |
| Beta blocker | 81.8% (n=18) |
| Amiodarone | 27.3% (n=6) |
| Statin | 27.3% (n=6) |
| Diuretics | 36.4% (n=8) |
| Insulin | 9.1% (n=2) |
| Digoxin | 36.4% (n=8) |
| Angiotensin-converting enzyme inhibitors I | 45.4% (n=10) |
LAA, left atrial appendage; SD, standard deviation; BMI, body mass index; LVEF, left ventricle ejection fraction; CHADS2, congestive heart failure, hypertension, age =75 years, diabetes mellitus, stroke; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly; TIA, transient ischemic attack; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VKA, vitamin K antagonist; LMWH, low molecular weight heparin.
Complete epicardial closure of the LAA was confirmed by intraoperative transesophageal echocardiography. No significant leaks were detected on follow-up echocardiography performed at discharge and at 1-month follow-up. Plasma fibrinogen level before LAAO in peripheral blood was 2.23 (1.75–2.72) g/L and increased by 87% after the procedure (Table 2). However, after 1-month follow-up, fibrinogen concentration returned to baseline (Table 2). At baseline, AF patients had a 19.7% reduced permeability of clots prepared from LAA obtained blood compared with peripheral blood (Figure 2A). As expected, the LAAO procedure was associated with lower plasma fibrin clot permeability compared to baseline (Figure 2A). Interestingly, at 1-month follow-up, we observed a 39.3% improvement in plasma fibrin clot permeability in patients after LAAO compared with baseline plasma fibrin clot permeability measured in clots prepared from peripheral blood of AF patients procedure (Figure 2A), also after adjustment for fibrinogen (P=0.027).
Table 2
| Variable | Peripheral blood before LAAO (a) |
LAA | Peripheral blood after LAAO (b) |
P value (a vs. b) |
Peripheral blood after 1-month follow-up (c) | P value (a vs. c) |
Normal values (n=20) |
|---|---|---|---|---|---|---|---|
| Fibrinogen, g/L | 2.23 [1.75–2.72] | 2.42 [2.15–2.65] | 4.17 [3.69–4.86] | 0.0051 | 2.31 [2.06–2.63] | 0.028 | 2.49 [2.02–3.12] |
| ETP, nM × min | 1,480 [1,263–1,649] | 1,572 [1,238–2,122] | 1,791 [1,547–2,214] | 0.043 | 1,389 [1,273–1,460] | 0.69 | 1,207 [964–1,321] |
| Peak thrombin, nM | 215 [169–262] | 212 [152–273] | 243 [186–295] | 0.22 | 189 [146–256] | 0.50 | 173 [149–195] |
| tPA antigen, ng/mL | 9.48 [5.06–11.54] | 9.78 [4.32–12.02] | 9.50 [8.12–13.05] | 0.53 | 5.42 [3.43–7.46] | 0.15 | 6.67 [5.09–10.6] |
| PAI-1 antigen, ng/mL | 4.29 [1.76–6.44] | 3.82 [1.89–5.04] | 3.76 [2.83–4.39] | 0.81 | 2.06 [0.97–2.74] | 0.023 | 5.09 [2.31–8.45] |
| TAFI antigen, % | 81.0 [63.8–91.2] | 84.2 [67.3–95.1] | 100.1 [90.4–106.1] | 0.034 | 88.3 [75.6–99.5] | 0.075 | 87.7 [81.6–91.5] |
| Plasminogen, % | 90.6 [85.5–98.1] | 81.5 [70.6–89.4] | 101.3 [84.7–112.4] | 0.021 | 98.7 [91.2–113.4] | 0.0077 | 100.3 [93.2–104.1] |
Data are presented as median [Q1–Q3] and were compared using the Wilcoxon signed-rank test. LAAO, left atrial appendage occlusion; LAA, left atrial appendage; ETP, endogenous thrombin potential; tPA antigen, tissue plasminogen activator antigen; PAI-1 antigen, plasminogen activator inhibitor type 1 antigen; TAFI antigen, thrombin activatable fibrinolysis inhibitor antigen.
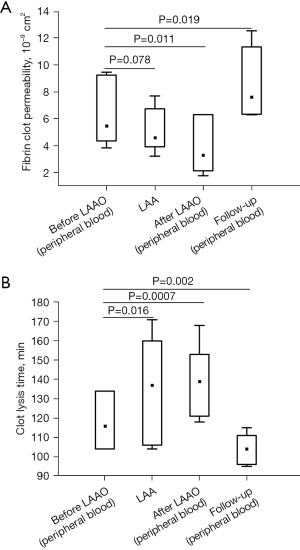
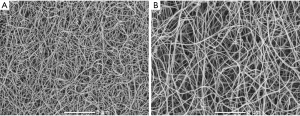
Similarly, we found a slightly shorter time for CLT in patients 1-month after LAAO procedure compared to baseline (Figure 2B). Of note, clots prepared from blood obtained from the LAA were characterized by 16.4% prolonged CLT compared with baseline (Figure 2B). The LAAO procedure was associated with a 19.8% prolonged CLT compared to baseline values (Figure 2B). Improvement in fibrin clot properties was supported by SEM analysis, which revealed 20.8% thicker fibrin fibers during follow-up compared to baseline {128 [107–133] vs. 106 [101–113] nm, P=0.002, Figure 3}. After 1-month of follow-up, higher Ks was associated with improved clot susceptibility to lysis (r=−0.67, P=0.013). There were no differences in thrombin generation parameters, reflected by endogenous thrombin potential (ETP) and peak thrombin generated one month after the procedure compared to baseline (Table 2). We found lower PAI-1 antigen levels along with slightly increased plasminogen activity 1 month after LAAO compared with baseline (Table 2). No differences were found in tPA or TAFI levels in AF patients before the LAA procedure and after 1-month follow-up (both P>0.05; Table 2). CLT before the LAAO and 1 month after the procedure was associated with PAI-1 (r=0.68, P=0.0026 and r=0.73, P=0.0018, respectively) and with the plasminogen activity (r=−0.38, P=0.048 and r=−0.36, P=0.044, respectively).
Discussion
Our hypothesis-generating study is the first to demonstrate the impact of epicardial LAAO in nonvalvular AF patients on fibrin clot characteristics and thrombin generation 1 month after the procedure. Our new finding is that LAAO improves fibrin clot permeability, clot morphology, and, at least in part, its susceptibility to fibrinolysis. Interestingly, the shortened CLT correlated with decreased PAI-1 antigen levels and increased plasminogen activity (Figure 4).
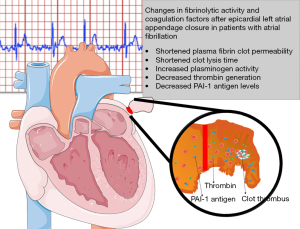
AF increases the risk of stroke by up to 20% (3). In nonvalvular AF patients, 90% of thrombi are located in the LAA, as confirmed by autopsy, transesophageal echocardiography, or direct inspection studies (16). In contrast to the anatomy of the other cardiac chambers, the LAA is a long, tubular structure with a narrow junction with the atrium (17). Increased risk of thromboembolism in AF is associated with a combination of pathophysiological mechanisms, a Virchow’s triad (1). The first factor is left atrial dysfunction with stasis observed on spontaneous echocardiographic contrast (18). The second factor is an abnormal change in the vessel that results in endothelial cell damage, as evidenced by an increase in soluble thrombomodulin (TM) (19,20). TM, an integral membrane protein expressed on the surface of endothelial cells, binds thrombin with high affinity and inhibits fibrinolysis by cleaving the thrombin-activatable fibrinolysis inhibitor into its active form (20,21). In addition, unfavorably-altered fibrin clot properties have also described (22). The third factor is a hypercoagulable or prothrombotic state, reflected by increased levels of prothrombin fragments 1+2, increased markers of thrombin generation, plasma fibrinogen and hypofibrinolysis in association with increased levels of PAI-1 and plasmin-α2-antiplasmin (20,23). In our previous study, we showed that in patients with AF, the LAA chamber has reduced fibrin clot permeability and prolonged lysis time suggestive of a state (13). In addition, it has been shown that CLT predicts stroke during anticoagulant therapy in patients with AF and that patients with chronic AF and previous stroke are characterized by prolonged CLT and higher TAFI antigen than those without stroke (20). Therefore, the mechanisms underlying LAA thrombus formation in patients with AF are complex (Figure 5).
However, the CHA2DS2-VASc score, the clinically-applicable model for stratifying stroke risk in AF patients, does not account for biomarkers (3,4). Interestingly, CLT, PAI-1 antigen, and TAFI activity were positively associated with CHA2DS2-VASc score, reflecting stroke risk in AF (20).
In recent decades, LAA has become a therapeutic target for stroke prevention in patients at high risk of stroke and contraindications to long-term OAC (European Society of Cardiology guidelines Class IIb, Level of Evidence B) (24). Several observational studies indicate the feasibility and safety of surgical or percutaneous LAAO or exclusion procedure (3-6), even in high risk patients at increased risk of thromboembolism (10,11,25). In epicardial LAAO, the LAA is completely ligated the LAA, causing necrosis and fibrosis and permanently eliminating the LAA (26).
Our study supports the concept of LAA elimination from the circulatory system based on a biomarker approach in the assessment of thromboembolic risk in patients with AF. The LAAO procedure improves fibrin clot permeability and susceptibility to fibrinolysis. Importantly, this effect lasts is long-lasting, as shown by the shortened CLT that correlates with decreased PAI-1 antigen levels and increased plasminogen activity measured 1 month after the LAAO procedure. Importantly, this effect was also obtained in patients not receiving OAC.
Therefore, our study confirms two important practical aspects. First, that LAA plays a key role in thrombogenesis. Second, that the LAAO procedure reduces the risk of thromboembolism not only by eliminating the local source of thrombus but also by the improving the fibrin clot permeability and susceptibility to fibrinolysis in the peripheral blood. To our knowledge, the present prospective cohort study is unique in this regard.
Our study is based on biomarker concepts for thromboembolic risk in AF and may explain the efficacy and successful outcomes in reducing strokes or other thromboembolic episodes in observational studies evaluating the percutaneous LAAO or exclusion procedure (27,28).
For cardiac surgery, the obtained results are particularly important as they support the idea of performing concomitant surgical LAA closure in patients with AF/flutter undergoing routine cardiac surgery (29). However, it should be noted, that in our study all LAAO were electively performed with Lariat or AtriClip (epicardial devices). There were no leaks or thrombi at 1 month transthoracic echocardiography (TTE) follow-up. It should be noted, that we did not investigate the effects of LAA amputation and closure technique approved in the LAAO III trial (7).
Study limitations
The number of patients enrolled in the study was limited. However, we would like to emphasize, that the number of patients enrolled in our study is comparable to the average number of patients win which this type of blood collection has been published so far. Based on our results, larger studies are needed to substantiate our observations. Since AF patients received LMWH prior to LAA closure (with an average anti-FXa level of 0.5 U/mL), which may be a potential confounder for the assessment of fibrin clot properties, exogenous human thrombin was used as the clotting activator for the two measurements of fibrin clots. According to a recent study, the positive effect of LMWH therapy on fibrin clot properties was achieved at an anti-FXa activity >0.6 U/mL (30) when fibrin clots were prepared using TF as the clotting activator, which is more sensitive to anticoagulation than thrombin (31). LMWH is known to impair the ability to produce thrombin, and residual anti-FXa activity has been observed in most patients undergoing invasive procedures requiring interruption of OAC on the day of the intervention (32). Therefore, the results of thrombin generation assay should be interpreted with caution and larger studies are needed to substantiate our observations.
Conclusions
Our results show that the LAA plays a key role in thrombogenesis. Our study suggests that LAA elimination from the circulatory system not only eliminates the local source of thrombus, but also improves fibrin clot permeability and susceptibility to fibrinolysis in the peripheral blood. The result of our study is based on the concept of biomarkers of thromboembolic risk in AF and supports the results of observation of surgical or percutaneous LAAO or exclusion. Further studies are needed to validate our observations.
Acknowledgments
Funding: This study is the results of the research grant No. UMO-2015/17/B/NZ5/00125 and No. UMO-2019/33/B/NZ5/02395 funded by the Polish National Science Centre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1093/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1093/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1093/coif). KB and his institutions Jagiellonian University Medical College and John Paul II Hospital in Krakow Poland were recipients of the research grant No. UMO-2015/17/B/NZ5/00125 and No. UMO-2019/33/B/NZ5/02395 funded by the Polish National Science Centre. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Jagiellonian University Ethical Committee (27 September 2012, number KBET/282/B/2012). All patients signed a written informed consent form prior to inclusion.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tóth NK, Csanádi Z, Hajas O, et al. Intracardiac Hemostasis and Fibrinolysis Parameters in Patients with Atrial Fibrillation. Biomed Res Int 2017;2017:3678017. [Crossref] [PubMed]
- Cove CL, Albert CM, Andreotti F, et al. Female sex as an independent risk factor for stroke in atrial fibrillation: possible mechanisms. Thromb Haemost 2014;111:385-91. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-51. [Crossref] [PubMed]
- Litwinowicz R, Bartus M, Malec-Litwinowicz M, et al. Left Atrial Appendage Occlusion for Secondary Stroke Prevention in Patients with Atrial Fibrillation: Long-Term Results. Cerebrovasc Dis 2019;47:188-95. [Crossref] [PubMed]
- Bajaj NS, Parashar A, Agarwal S, et al. Percutaneous left atrial appendage occlusion for stroke prophylaxis in nonvalvular atrial fibrillation: a systematic review and analysis of observational studies. JACC Cardiovasc Interv 2014;7:296-304. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- The World Health Organization MONICA Project. (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol 1988;41:105-14. [Crossref] [PubMed]
- Litwinowicz R, Bartus M, Burysz M, et al. Long term outcomes after left atrial appendage closure with the LARIAT device-Stroke risk reduction over five years follow-up. PLoS One 2018;13:e0208710. [Crossref] [PubMed]
- Litwinowicz R, Bartus M, Ceranowicz P, et al. Left atrial appendage occlusion for stroke prevention in diabetes mellitus patients with atrial fibrillation: Long-term results. J Diabetes 2019;11:75-82. [Crossref] [PubMed]
- Litwinowicz R, Bartuś M, Ceranowicz P, et al. Stroke risk reduction after left atrial appendage occlusion in elderly patients with atrial fibrillation: long-term results. Pol Arch Intern Med 2018;128:327-9. [PubMed]
- Suwalski P, Witkowska A, Drobiński D, et al. Stand-alone totally thoracoscopic left atrial appendage exclusion using a novel clipping system in patients with high risk of stroke - initial experience and literature review. Kardiochir Torakochirurgia Pol 2015;12:298-303. [Crossref] [PubMed]
- Bartus K, Litwinowicz R, Natorska J, et al. Coagulation factors and fibrinolytic activity in the left atrial appendage and other heart chambers in patients with atrial fibrillation: is there a local intracardiac prothrombotic state? (HEART-CLOT study). Int J Cardiol 2020;301:103-7. [Crossref] [PubMed]
- Mills JD, Ariëns RA, Mansfield MW, et al. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation 2002;106:1938-42. [Crossref] [PubMed]
- Pieters M, Philippou H, Undas A, et al. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost 2018;16:1007-12. [Crossref] [PubMed]
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9. [Crossref] [PubMed]
- Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531-8. [Crossref] [PubMed]
- Mitusch R, Siemens HJ, Garbe M, et al. Detection of a hypercoagulable state in nonvalvular atrial fibrillation and the effect of anticoagulant therapy. Thromb Haemost 1996;75:219-23. [Crossref] [PubMed]
- Califano F, Giovanniello T, Pantone P, et al. Clinical importance of thrombomodulin serum levels. Eur Rev Med Pharmacol Sci 2000;4:59-66. [PubMed]
- Ząbczyk M, Majewski J, Lelakowski J. Thromboembolic events are associated with prolonged clot lysis time in patients with permanent atrial fibrillation. Pol Arch Med Wewn 2011;121:400-7. [Crossref] [PubMed]
- Adams TE, Huntington JA. Thrombin-cofactor interactions: structural insights into regulatory mechanisms. Arterioscler Thromb Vasc Biol 2006;26:1738-45. [Crossref] [PubMed]
- Drabik L, Wołkow P, Undas A. Denser plasma clot formation and impaired fibrinolysis in paroxysmal and persistent atrial fibrillation while on sinus rhythm: association with thrombin generation, endothelial injury and platelet activation. Thromb Res 2015;136:408-14. [Crossref] [PubMed]
- Roldán V, Marín F, Marco P, et al. Hypofibrinolysis in atrial fibrillation. Am Heart J 1998;136:956-60. [Crossref] [PubMed]
- Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. [Crossref] [PubMed]
- Wiebe J, Franke J, Lehn K, et al. Percutaneous Left Atrial Appendage Closure With the Watchman Device: Long-Term Results Up to 5 Years. JACC Cardiovasc Interv 2015;8:1915-21. [Crossref] [PubMed]
- Bartus K, Morelli RL, Szczepanski W, et al. Anatomic analysis of the left atrial appendage after closure with the LARIAT device. Circ Arrhythm Electrophysiol 2014;7:764-7. [Crossref] [PubMed]
- Litwinowicz R, Bartus M, Kapelak B, et al. Reduction in risk of stroke and bleeding after left atrial appendage closure with LARIAT device in patients with increased risk of stroke and bleeding: Long term results. Catheter Cardiovasc Interv 2019;94:837-42. [Crossref] [PubMed]
- Burysz M, Litwinowicz R, Bryndza M, et al. Percutaneous left atrial appendage closure using the LAmbre device. First clinical results in Poland. Postepy Kardiol Interwencyjnej 2019;15:251-4. [Crossref] [PubMed]
- Whitlock R, Healey J, Vincent J, et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg 2014;3:45-54. [PubMed]
- Ząbczyk M, Natorska J, Malinowski KP, et al. Effect of enoxaparin on plasma fibrin clot properties and fibrin structure in patients with acute pulmonary embolism. Vascul Pharmacol 2020;133-134:106783. [Crossref] [PubMed]
- Ząbczyk M, Blombäck M, Majewski J, et al. Assays of fibrin network properties altered by VKAs in atrial fibrillation - importance of using an appropriate coagulation trigger. Thromb Haemost 2015;113:851-61. [Crossref] [PubMed]
- Eijgenraam P, Ten Cate H, Henskens Y, et al. Effects of peri-operative bridging with low molecular weight heparins on coagulation during interruption of vitamin K antagonists: A mechanistic study. Thromb Res 2016;140:59-65. [Crossref] [PubMed]