
Regional anesthesia for thoracic surgery: a narrative review of indications and clinical considerations
Introduction
Postoperative pain is common and sometimes severe after thoracic surgery. Inadequate postoperative analgesia has been associated with insufficient mobility, more atelectasis, prolonged hospital stays, and increased costs for the healthcare system (1-3). High-dose opioid use is not ideal due to undesirable side effects such as nausea, vomiting, constipation, urinary difficulty, respiratory depression, sedation, possible persistent postsurgical pain, and concerns relating to opioid use dependency (4,5). Therefore, balanced multi-modal analgesia techniques have been used in recent years and regional anesthesia techniques have become one of the indispensable cornerstones (6).
Until recently, regional techniques were limited to the thoracic epidural, thoracic paravertebral blocks (TPVB), and intercostal blocks (7,8). However, with the introduction of ultrasound into clinical practice of regional anesthesia in the last decade, nerve blocks have become safer and more successful (9,10). Different approaches to previously defined blocks, as well as novel blocks like fascial plan blocks have been developed and introduced into clinical practice.
This review article summarizes current research and addresses popular regional anesthetic techniques for patients having thoracic surgery, including thoracotomy, video-assisted thoracoscopy, and rib resection. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-599/rc).
Methods
For this review, the authors first conducted an extensive literature search. For this, a computer-based search was performed in English databases, including PubMed, Web of Science, Embase, and Cochrane Library. The date range for the search was set from January 1985 to March 2022. Among the search terms were the names of all the blocks included in our study (Table 1). Care was taken to ensure that the studies were in a prospective randomized controlled design to be included in this review. After reading the titles and abstracts, the records obtained from the database were pre-selected by the authors. Afterward, it was tried to reach the full texts of the studies planned to be included in the review. Those whose full text could not be accessed or which, after reading the full text, were not thought to be random and would not contribute to the review in terms of content were excluded. Since our study was designed as an article in the category of narrative review, standard methodological methods and statistical analyzes were not used in meta-analyses.
Table 1
| Items | Specification |
|---|---|
| Date of search | From 1 February 2021 to 15 March 2021 |
| Databases and other sources searched | PubMed, Web of Science, Embase and Cochrane Library |
| Search terms used | Refer to Table S1 |
| Timeframe | From 1 January 1985 to 30 March 2022 |
| Inclusion and exclusion criteria | Mainly prospective randomized controlled trials and meta-analyses were included in the study |
| Those whose full text could not be reached or who would not contribute to the review in terms of content were excluded from the study | |
| Selection process | All authors performed the search strategy independently. Two researchers (GS, YT) then re-performed a secondary access of eligible studies and evaluated potentially relevant articles against the selection criteria above |
Chest wall anatomy and innervation
Typical intercostal nerves and atypical intercostal nerves are the two most common types of intercostal nerves. T3 through T6 are typical intercostal nerves, while T1 through T2 and T8 through T11 are atypical intercostal nerves. The fundamental difference between the two groups is that typical intercostal nerves remain limited to their own intercostal spaces, whereas atypical intercostal nerves pass through the thoracic wall and partially or completely serve other areas (11).
The typical intercostal nerve enters the intercostal space between the parietal pleura and the intercostal membrane, running laterally behind the sympathetic trunk. It flows along the intercostal vessels and in front of the internal thoracic artery while in the costal groove. The rami communicantes, muscle branches, collateral branch, lateral cutaneous branch, and anterior cutaneous branch are the primary branches of the typical intercostal nerves. Gray and white rami innervate the corresponding thoracic ganglion via the rami communication branches. The intercostal muscles, parietal pleura, and rib periosteum are all innervated via the collateral branch. The muscles are crossed by the lateral cutaneous branch.
The anterior cutaneous branch, which separates into medial and lateral branches to supply the skin of the anterior thoracic wall, is the terminal branch of the typical intercostal nerves (12).
Along with the ventral ramus of C8, the first intercostal nerve contributes to the lower trunk of the brachial plexus. The rest of the first intercostal nerve is devoid of both the lateral and anterior cutaneous branches that are found in conventional intercostal nerves. The intercostobrachial nerve is a branch of the second intercostal nerve that provides cutaneous information from the axilla floor and superior region of the upper extremity. This nerve might be responsible for the pathognomonic pain that patients having coronary artery disease describe on the medial side of the arm (13).
Chest tubes in thoracic surgery are frequent causes of pain that are difficult to address. Thoracostomy tube pain potentially can emanate from a variety of sources. The parietal pleura receives somatic afferent (sensory) innervation from two different sources; the intercostal nerves (T1-T11) and the phrenic nerve (C3-C5). The intercostal nerves (T1-T11) provide innervation to the costal pleura and peripheral diaphragmatic pleura. The mediastinal pleura and the central parietal pleura are innervated by the phrenic nerve (C3-C5) (14). This innervation pattern is likely why chest wall blocks that potentially penetrate the paravertebral space seem to be more effective in thoracic surgery (with thoracostomy tubes) than others.
Intercostal nerve block (ICNB)
Understanding chest wall anatomy and innervation is important when considering clinical indications for and the application of ICNB. Intercostal nerves are ventral continuations of spinal nerves T1-11. In the intervertebral foramen, each nerve exits the pleura and endothoracic fascia and then pierces the posterior intercostal membrane (posterior continuation of the innermost intercostal muscle) to flow between the innermost and internal intercostal muscles within the subcostal groove (15) (Figure 1). Along their course, the intercostal nerves have lateral cutaneo us branches at the level of the midaxillary line and finally continue anteriorly towards the midline to give rise to anterior cutaneous branches (15). T1 usually does not have lateral or anterior cutaneous branches. The intercostal nerves provide segmental innervation with an overlap between the adjacent nerves, requiring blockade of 1–2 nerves above and below the level of injury/incision to provide effective analgesia. The ICNB can be performed anywhere along the nerve course dorsal to the midaxillary line (before the lateral cutaneous branches take off).
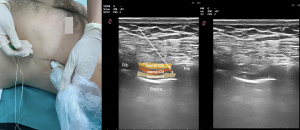
Any procedure involving the chest wall (thoracic surgeries), as well as rib fractures, could be an indication for ICNB (16). Indications outside the operating room include postsurgical analgesia after thoracostomy and postherpetic neuralgia (17). Thoracic epidural analgesia (TEA) has been considered the gold standard for postoperative pain control after thoracic surgery and was an integral part of early Enhanced Recovery After Surgery (ERAS) protocols for many years. However, adverse events and complications such as dural puncture, postoperative radicular pain, subarachnoid block, spinal cord-root injury leading to permanent motor deficits, TEA-associated cardiovascular/respiratory collapse, peripheral nerve lesions, and epidural hematomas or infection (18) associated with it have led clinicians to seek alternative regional methods with similar efficacy and lower risk of adverse events, especially for minimally invasive thoracoscopic surgeries (19-21). Both, the ERAS and the European Society of Thoracic Surgeons (ESTS), recommend either TPVB or ICNB for thoracic surgery as part of ERAS protocols (22,23). Contraindications for ICNB include clinically significant coagulopathy, local infection, or patient refusal (24). When used in thoracic surgery, ICNB is rarely performed via transcutaneous injection (ultrasound or traditional landmark-based technique), and more commonly via transparietal injection from within the pleural cavity or direct injection through surgical or port incision by the surgeon (25,26). Evidence suggests that ICNB is superior to systemic analgesia alone in thoracic surgery (27). However, it is inconclusive when comparing ICNB with other techniques such as paravertebral block (PVB) or TEA, as also concluded by two systematic reviews and meta-analyses (3,21,28,29). A recent meta-analysis compared TEA with ICNB and reported that single-injection ICNB was not clinically inferior to TEA in terms of pain reduction within the first 24 h after thoracic surgery. In this article, it was emphasized that ICNB analgesia has opioid sparing effects, but the decrease in postoperative morphine milligram equivalents (MMEs) is not greater than that of TEA. The authors therefore reported that ICNB may be most useful when TEA is not indicated (28).
Comparisons of ICNB with some of the novel facial plane blocks suggest similar efficacy, but the evidence is limited (30). Unlike other regional techniques, ICNB can also be performed with cryoanalgesia, which involves cooling the nerve and reversible inhibition of function lasting weeks to months (31). Although studies show the effectiveness of intercostal nerve cryoanalgesia in the immediate postoperative period, concerns have been raised about its contribution to post-thoracotomy chronic neuropathic pain. Further investigation is needed to evaluate this modality in thoracic surgery (32,33). The main complications of ICNB are infection, bleeding, pneumothorax or intraneural injection. Although local anesthetic systemic toxicity (LAST) is a rare event, local anesthetic (LA) uptake from this region is high. Therefore, providers should be alert to LAST. A few case reports of spinal block after ICNB have been reported (34). This is thought to occur after injection of a LA spreading medially through the dura or into a dural sac protruding laterally from the vertebral foramen. Aspiration must be performed beforehand to avoid intravascular, intrapleural or intrathecal injection (24).
Thoracic paravertebral block
TPVB is a nerve block administered by injecting LA into the thoracic paravertebral space (TPVS) (Figure 2). TPVB aims at spinal and sympathetic nerves to create an ipsilateral segmental somatic and sympathetic block. The TPVS is a cuneiform chamber that lies on the lateral side of the vertebral column between the heads and necks of the upper and lower ribs. The largest dimension of the TPVS is its medial side. The TPVB is performed to obtain regional anesthesia and analgesia for thoracic surgery as an alternative to thoracic epidural surgery patients (35-37).
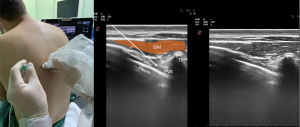
The block-level should be as close to the location of the dermatome for the incision or injury as possible. Blockage of three to five adjacent intercostal nerves may be required to achieve a complete block of one dermatome owing to the interconnections between spinal nerves and overlap in the innervation of dermatomes. This can be achieved by multiple blocks or a single injection of a larger volume of LA. More medial access for placement of a TPVB may decrease the risk of pleural puncture. However, more medial placement may raise the risk of neuraxial complications such as excessive epidural spread, accidental dural puncture (with or without spinal block), as well as increased the chance of anterior catheter migration into the mediastinum. Furthermore, the application of high volumes of LA and high pressure during injection increases the likelihood of epidural spread (38).
TPVS communicates medially with the epidural space, laterally with the intercostal space, and cranially with the cervical paravertebral space. There is controversy over the caudal extent of the TPVS below T12. Some cadaveric studies have found low dye diffusion from injections below the arcuate ligament into the TPVS, while others have found no spread at all (39-41). There are cavities in the superior costotransverse ligament, which form most of the dorsal border of the TPVS, which may allow LA to diffuse into the paravertebral space from more superficial injections [e.g., erector spinae plane (ESP) blocks].
A recent meta-analysis compared TPVB and TEA in patients having thoracoscopic surgery and reported minor, but clinically unimportant short-term benefits of TPVB over TEA. Furthermore, TPVB can be safely used in patients with coagulation dysfunction, spinal deformity, infection or in patients with contraindications against TEA like high risk of hypotension (42).
Epidural block
Thoracic epidural anesthesia (TEA) is a form of neuraxial anesthesia in which LA, often combined with adjunct medication, is injected into the epidural space at the levels of the thoracic vertebrae. TEA is an effective and versatile technique frequently used for perioperative analgesia in patients requiring thoracic surgery. If required for patients in whom a general anesthetic is contraindicated, TEA can also be used as a regional anesthetic strategy in awake or sedated patients.
The epidural space is a potential space that exists between the dura mater and ligamentum flavum. This space can be accessed with a needle to inject the medication, or, more commonly, a catheter can be threaded through the needle and left in the space to infuse medication that bathes the spinal roots as they emerge from the spinal column. Typically, a thoracic epidural placed above the T5 vertebrae is denoted as a high thoracic epidural, while access between T5 and T12 denotes a lower thoracic epidural (43).
The main indications for epidural blocks are thoracic surgery, including thoracotomy, video-assisted thoracoscopy, rib resection (44), as well as trauma patients with rib and chest wall injury (45).
For some complex patients, such as those suffering from chronic pain, opiate sensitivity, obstructive sleep apnea, respiratory depression, and pulmonary disease, TEA can be an ideal perioperative analgesic modality (46). TEA’s potential to significantly reduce the dose of opiate analgesia required can be a key benefit for patients recovering from thoracotomy. This also includes lower rates of postoperative ileus (18). With the advent of ultrasound, never regional techniques depending on fascial planes have begun to supplement and, in some cases replace the TEA as the standard analgesic technique for patients undergoing thoracic surgery. Initial retrospective studies suggest potential noninferiority of fascial plane blocks to TEA (47). However, this study included video-assisted thoracoscopic surgery (VATS) or thoracotomy for wedge resection, lobectomy, esophagectomy, or pectus repair causing varying levels of postoperative pain. In addition, the number of cases included in the study was relatively small.
Also, unlike VATS, ESRA recommends TEA containing LA and opioid for 2–3 days or continuous TPVB with LA for pain management after thoracotomy. If these regional techniques are not feasible or are contraindicated, ESRA recommends systemic opioid and non-opioid analgesic with or without patient-controlled analgesia (48).
Ultrasound is also being used to place TEAs, though most recent randomized controlled trials do not show any significant advantages to ultrasound-assisted placement versus the traditional palpation technique (49).
Importantly, TEA can cause sympatholysis and subsequent drops in systemic vascular resistance. In patients with limited cardiovascular reserves, this can precipitate cardiovascular collapse. In patients with increased intracranial pressure (ICP), instilling the medication in the epidural space can compound ICP and cause herniation (50). While a functioning TEA provides effective analgesia for many surgical procedures, there are drawbacks during placement. Thoracic epidural placement can be technically challenging and stressful for awake patients. The most common adverse event following TEA placement is a nonfunctioning block (up to 30%) (51). Catheter migration or displacement can leave patients with unilateral or patchy blocks. During the sequence, vascular injury, paresthesia, and neural injury are serious complications, more common in patients with challenging anatomy.
Ultrasound-guided interfascial plane blocks
Most of the ultrasound-guided peripheral blocks for thoracic surgery are fascial plane blocks. Since the nerves involved are small and difficult to discern by imaging, the basic principle is to inject a significant volume of LAs (20–30 mL for adult, or 0.2–0.4 mL/kg for children) between the fascial planes to reach the nerves traveling between and adjacent to the fascial planes. The injected LAs may also spread to other compartments, such as the paravertebral area. The pattern and extent of spread are variables between individuals, resulting in variability of the amount of LA reaching and acting on the nerves. Therefore, sensory and motor blocks are always balanced, but individual variation can be expected (52).
Since the effectiveness of the fascial plane blocks depends on the extent of spread and LA volume, there is always a risk of LA toxicity due to systemic absorption. Therefore, maximum dose limits should be respected. As these blocks are primarily used for analgesic purposes, diluted LA concentrations can be used (bupivacaine 0.125%, 0.25%, ropivacaine 0.2%). If using a catheter for continuous infiltration, a loading dose of 20 mL or 0.2 mL/kg of dilute LA concentration (0.125% bupivacaine vs. 0.2% ropivacaine), followed by either 8–10 mL/h continuous infusion or intermittent doses of 10–15 mL every 1–3 h are recommended.
Infusion techniques used in chest wall blocks can be continuous infusion of LA or intermittent boluses of LA. It is recommended not to keep the catheters for longer than 48 h due to the risk of infection. In a study conducted in patients who underwent TPVB for postoperative analgesia after VATS, continuous infusion and programmed intermittent bolus infusion were compared, and postoperative pain and opioid use were found to be similar in both groups (53).
Fascial plane blocks are:
- Pectoral nerve blocks (PECS type I and II);
- Serratus plane block;
- Pecto-intercostal block;
- Parasternal block;
- And erector spine plane (ESP) block.
Interfacial plane blocks of the chest wall can be used alone or in combination for surgical anesthesia and postoperative analgesia as an alternative to more invasive paravertebral and epidural blocks, depending on the surgical procedure and patient preference and co-morbidities (25,26,54). One of the problems with these blocks is the lack of sufficiently robust randomized, controlled trials comparing them to the gold standard epidural blocks or TPVB. Another problem is catheter positioning, as catheters can easily become dislodged by the movement of muscles and extremities.
Pectoral nerve blocks
The first description of a pectoral nerve block type I (PECS I) in breast surgery was published in 2011 (10). To achieve a PEC I block, a LA is injected into the tissue plane between the pectoralis major and minor muscles, thereby targeting the medial and lateral nerves arising from the brachial plexus (Figure 3).
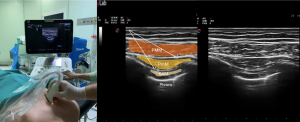
PECS I block has a limited list of indications. Still, it is particularly useful for procedures that require dissection of the pectoralis major and minor muscles, such as lumpectomy, breast prosthesis, and sub-pectoral implantation (55).
There is an ongoing debate, if pectorals nerves are actually sensory nerves, rather than being proprioceptive nerves instead. In a randomized controlled study by Desroches et al., there was a significant difference in adductor strength between volunteers who received placebo and LA in PECS I block, but no difference was found in dermatomal skin sensation tests (56).
However, nowadays, this block is mostly applied together with the PECS II block rather than alone. Apart from this, it can also be used with other chest wall blocks such as PECS I block, serratus anterior plane (SAP) block, transversus transthoracic muscle block, and ESP block for surgery involving the chest wall.
PECS II block includes LA injection into the plane between the pectoralis minor and serratus anterior muscles (Figure 2). The medial and lateral pectoral nerves, the lateral branches of the thoracic 3–6 intercostal nerves, and the long thoracic and thoracodorsal nerves are all targeted (26). Blocking the long thoracic and thoracodorsal nerves has a greater effect on the deep tissues of the chest. Indications for the PECS II block include: pectoral muscle dissection, thoracotomy, chest wall trauma, and minimally invasive procedures such as VATS, port catheter insertion or removal, and pacemaker placement (57,58) (Table 2).
Table 2
| Blocks type of the chest wall | Indications | Potential complications |
|---|---|---|
| Intercostal nerve block | Thoracotomy Video-assisted thoracic surgery Rib fractures Postherpetic neuralgia |
Pneumothorax Local anesthetic systemic toxicity |
| Pectoral nerve block I | Lumpectomy Sub-pectoral paresis Sub-pectoral implantation |
Pneumothorax Vascular injury (cephalic vein/thoracoacromial artery) |
| Pectoral nerves block II | Anterior thoracotomies Pectoral muscle dissection Sternotomy Minimally invasive cardiac surgeries Port catheter insertion or removal Pacemaker placement Chest wall pain caused by Herpes Zoster |
Pneumothorax Vascular injury (cephalic vein/thoracoacromial artery) Inadequate or failed block Temporary scapula of the wings |
| Serratus plane block | Video-assisted thoracic surgery | Pneumothorax Vascular damage Bleeding |
| Rib fractures, rib contusions | Hematoma | |
| Minimally invasive cardiac surgery | Temporary wing scapula | |
| Parasternal block | Median sternotomy Cardiac surgery |
Pneumothorax Vascular damage Bleeding Hematoma Sternal wound infection |
| Erector spinae plane block | Thoracotomy Video-assisted thoracic surgery Cardiac surgery |
Pneumothorax Sensory blockade |
| Pecto-intercostal fascial block | Thymectomy Sternotomy Cardiac surgery Thoracotomy Video-assisted thoracic surgery |
Pneumothorax Intravenous injection |
| Interpleural block | Thoracotomy Video-assisted thoracic surgery Multiple rib fractures Cancer pain Post-herpetic neuralgia Complex regional pain syndromes |
Pneumothorax Hemidiaphragm paralysis Horner syndrome Hemothorax Catheter migration Pleural effusion Bronchial trauma Bronchopleural fistula formation |
| Thoracic paravertebral block | Thoracotomy Video-assisted thoracic surgery Rib fractures |
Pneumothorax Epidural block, spinal block, or intrathecal spread Neuraxial complications mild hypotension |
| Epidural block | Thoracotomy | Post puncture headache Transient neurological syndrome (symmetrical back pain, radiated to the buttocks and legs, without sensitive or motor component) Nerve injury with possible neuropathy—paresis is extremely rare Epidural hematoma |
| Video-assisted thoracic surgery | Epidural abscess | |
| Cardiac surgery | Meningitis | |
| Rib fractures | Accidental intrathecal injection with total spinal anesthesia |
PECS I and II blocks do not provide sensory blockage of the skin and subcutaneous tissues in the parasternal areas, as both do not involve the anterior branches of the intercostal nerves. The PECS II block may be used in conjunction with other interfascial chest wall blocks. Published reports describe its use in conjunction with an SAP block in the awake VATS (59). According to a recent meta-analysis, PECS II block and TPVB resulted in similar postoperative opioid consumption and similar postoperative pain scores after the first measurement (57).
Compared with other regional anesthesia techniques, the ability to perform this procedure in the supine position is a critical advantage. In a recently published study, PECS II block was used in VATS. It was reported that the block provides improved analgesia and reduces opioid consumption to an extent equivalent to TPVB. However, the fact that the postoperative rescue analgesic requirement was significantly higher in the PECS group may also have contributed to this result. In addition, the authors found that PECS II block provides better intraoperative hemodynamic stability compared to TPVB (60).
Serratus plane block
SAP block is performed by injecting a LA into a fascial plane above the serratus anterior muscles (Figure 4).
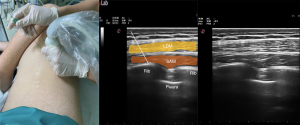
A LA injection targets the intercostal nerves’ lateral cutaneous branches and muscular branches (9). It aims to provide analgesia to the anterolateral and posterior sides of the chest wall as an alternative to epidural block and TPVB. If this block is performed above the serratus anterior muscle, it numbs the anterolateral branch of the thoracic intercostal nerves between the serratus anterior and the latissimus dorsi, the serratus anterior nerve, and the thoracodorsal nerve. If the block is performed below the serratus anterior muscle, the lateral and anterior cutaneous branches of the thoracic intercostal nerves are blocked.
The superficial SAP block is anatomically a variation of PECS II, whereby the needle is placed more caudally and posteriorly (9). The evidence of whether a deep serratus plane block is more effective than the superficial one in analgesia of deep muscle and bone structures is unclear (19). However, the long thoracic and thoracodorsal nerves run in the fascial plane on the surface of the serratus anterior muscle and can be inadvertently blocked with a superficial SAP block. This condition can cause temporary paralysis of the long thoracic nerve (LTN) and result in the winged scapula.
A study conducted in healthy volunteers determined that sensory paresthesia occurred in dermatomes between T2-9. A difference was found in the duration of analgesic effect between the superficial and the deep SAP blocks; if the LA was injected superficially into the SA muscle, the effective time was doubled.
Indications for SAP block include pain control after VATS and thoracotomy for lung resection (61-65) (Table 2).
Although SAP does not directly target the pectoral nerves, it has an effect similar to PECS II block and therefore provides sufficient analgesia in thoracic surgery (66). Effect can be topped by simultaneously blocking pectoral and intercostal nerves. Recently, a technique combining PECS II and SAP block has been described. Some randomized controlled studies reported, that the SAP block is as effective as the PVB.
Additionally, some studies including patients having pneumonectomy, lobectomy and wedge resection with thoracotomy and thoracoscopy reported that hemodynamics remained more stable in the SAP block group compared to patients receiving paravertebral and thoracic epidural blocks (64,67) (Table 3).
Table 3
| References | Procedure | Block type | Comparator intervention | Primary outcome | Result |
|---|---|---|---|---|---|
| Semyonov et al., 2019, (61) | Thoracic surgery | SAPB | No block | Pain scores | Lower pain scores in SAP group in the first 8 h postoperatively, no difference after the 9th hour |
| Park et al., 2018, (62) | VATS | SAPB | No block | Opioid use | Less opioid use in SAP group in the first 24 h |
| Finnerty et al., 2020, (68) | Minimally invasive thoracic surgery | ESPB | SAPB | Quality of patient recovery | ESP provides superior quality of recovery at the 24th hour |
| Yildirim et al., 2022, (60) | VATS | PECS II | TPVB | Opioid use | No difference |
| Baldinelli et al., 2021, (63) | VATS | SAPB | ICNB | Pain scores | Lower pain scores in SAP group while coughing in 6, 12, 24 h postoperatively, no difference before the first 5 h and after 24 h |
| Chu et al., 2020, (35) | VATS | TPVB | No block | Pain scores | Pain scores at rest at the 4th and 24th hours, on cough at the 4th hour were lower in PVB group |
| Li et al., 2018, (36) | Thoracotomy | TPVB | No block | Pain scores | Lower acute pain scores, no difference chronic post-thoracotomy pain incidence |
| Kadomatsu et al., 2018, (27) | VATS | ICNB | TPVB | Pain scores | Lower pain scores in ICNB group at the 48th h postoperatively, no differences before the 48th hour |
| Hanley et al., 2020, (64) | VATS | SAPB | TPVB | Opioid use | Non-inferior opioid use in SAP group in the first 48 h |
| Vilvanathan et al., 2020, (21) | Thoracotomy | ICNB | TEA | Pain scores | Lower pain scores in epidural group in 12 h postoperatively |
| Dikici et al., 2022, (65) | VATS | SAPB | Infiltration block | Pain scores | Lower pain scores in SAP group in 12 h postoperatively |
SAPB, serratus anterior plane block; SAP, serratus anterior plane; ESPB, erector spinae plane block; PECS II, pectoral nerve block II; TPVB, thoracic paravertebral block; TEA, thoracic epidural analgesia; ICNB, intercostal nerve block; VATS, video-assisted thoracic surgery.
Additional advantages over thoracic epidural block and TPVB are the ability to perform them on supine patients, including in situations involving head and spine injuries and in the presence of coagulopathy. A disadvantage is the short duration of action, but this problem can be overcome by inserting a catheter for continuous or intermittent further application of LA.
The European Society of Regional Anesthesia (ESRA) recommends a PVB or an erector spina plane block as the first choice in the management of analgesia after VATS.
The SAP block can be considered as a second option, but TEA is not recommended in patients having VATS, but can be considered in patients having thoracotomy (69).
ESP block
ESP block was first described in 2016 as a treatment for chronic pain (70). Today, its application has expanded to help with postoperative pain control after surgery to the thorax. ESP block is performed by administering a LA to the fascial plane between the erector spinae muscle and the transverse process, and targets the dorsal and ventral nerves (Figure 5). The dorsal rami of the spinal nerves pass here and can be effectively blocked.
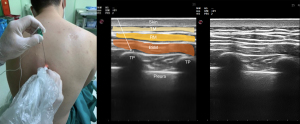
Blockage of the ventral rami and other branches can develop due to the anterior spread of LA to the paravertebral and epidural areas. Cadaveric and imaging studies have shown that LAs also spread to the paravertebral and epidural areas. The vertebral level to which the block will be applied should be determined based on the center of the area where analgesia will be provided (71).
After a single injection of LA, the spread can cover at least 3 and up to 6–8 vertebral levels. For this reason, it is often appropriate to apply a block at the T4 or T5 level (71,72). ESP blocks are used for thoracic (68) procedures when applied at T3-4 level (Table 2). A single shot ESP block provides analgesia for a limited time, but catheter insertion allows for continuous or repeat dosing and can provide prolonged analgesia.
The most significant advantage of the ESP block over the SAP and PECS blocks is that the dorsal rami and branches of the spinal nerves are blocked and it therefore also covers the posterior and lateral thorax. Sympathetic chain blockade is considered a component of ensuing visceral analgesia, although this has not been conclusively proven. A meta-analysis of 140 studies reported that ESP provides adequate analgesia in various surgical procedures (73). However, prospective randomized controlled clinical studies are needed to define the optimal volume of LA used, the mode and frequency of LA administration, such as continuous infusion, intermittent bolus, single shot, and the subsequent number of dermatomes spread (74).
Pecto-intercostal fascial (PIF) block
PIF block injects LA between the pectoralis major and internal intercostal muscles (Figure 6). The anterior cutaneous branches of the intercostal nerves located in the fascial plane between these muscles are targeted (75).
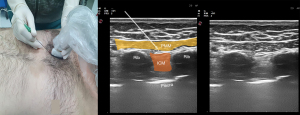
This block was first described to treat postoperative pain in breast surgery. Subsequently, it was also used for various surgeries such as thymectomy and sternotomy, and the comparative results showed that this block could provide analgesia equivalent to a transversus thoracis muscle plane (TTP) block (76).
It has also been reported to produce an analgesic effect covering the T1-T6 dermatomes after sternotomy (77). It can provide adequate analgesia for four days with bilateral catheter placement and repeat or continuous application of LAs after thymectomy (78).
Although PIF block is a new development that requires additional research, it may offer the potential for a safer approach to regional anesthesia, particularly for medial parts of the anterior chest wall (79,80).
Interpleural block
Interpleural blockade was first published by Strömskag et al. to treat acute postoperative pain (81). It is a technique performed by LA injection into the intrapleural space between the visceral and parietal pleura to create the ipsilateral somatic block of thoracic dermatomes. In this technique, it is believed that the LA administered from a single intrapleural injection site reaches the intercostal nerves retrogradely (82). Interpleural blockade can treat unilateral surgical or nonsurgical pain originating in the chest or upper abdomen in both acute and chronic settings. It has been shown to provide analgesia for thoracotomy, multiple rib fractures, post-herpetic neuralgia, and complex regional pain syndromes (82-84).
Specific adverse events for interpleural blocks are paralysis of a hemidiaphragm and the full picture of Horner syndrome. Both are based on the proximity of the phrenic nerve and the upper thoracic sympathetic ganglia.
Limitations
This narrative review summarizes indications and clinical considerations of regional anesthetic techniques for patients having thoracic surgery. Findings of this review are only eligible to adult patients and cannot be extrapolated to special patient populations such as pediatric, obese and geriatric patients. The authors of this narrative review focused on the most commonly used regional anesthetic techniques for patients having thoracic surgery in their institutions, and did not discuss any further regional techniques used for other kind of surgeries. Furthermore, we limited our summary to widely used medications, and did not address newly introduced LA agents. Especially with the last, more research is needed to provide a better cost-benefit assessment.
Conclusions
In summary, sonoanatomy and the use of ultrasound in regional anesthesia have made it possible to safely and successfully perform interfascial plane blocks in patients undergoing thoracic surgery. Since fascial plan blocks are more superficial, they are easier to apply, which is one of the reasons for their increasing use and importance, often performed instead of thoracic epidural block and TPVB. Conversely, these epidural block and PVB for patients undergoing thoracic surgery are well established with proven effectiveness and a solid evidence base supporting their use. Therefore, to evolve clinical practice, high-quality studies comparing the novel blocks to the established standards are needed.
Acknowledgments
The authors want to thank Dr. Yaser Pektaş and Dr. Yasemin Çelik from University of Health Science, Bakırköy and Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey for providing the ultrasound photos.
Funding: This study was supported solely by institutional and/or departmental sources.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-599/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-599/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-599/coif). KR serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2021 to July 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [Crossref] [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. Erratum in: Br J Anaesth 2007 Nov;99(5):768. [Crossref] [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [Crossref] [PubMed]
- Anwar S, O' Brien B. The Impact of Remifentanil Infusion During Cardiac Surgery on the Prevalence of Persistent Postsurgical Pain. J Cardiothorac Vasc Anesth 2021;35:467-9. [Crossref] [PubMed]
- Anwar S, Herath B, O'Brien B. Adding Insult to Injury-Are We Fueling the Opioid Crisis During the Perioperative Period? J Cardiothorac Vasc Anesth 2021;35:1712-4. [Crossref] [PubMed]
- Wildgaard K, Petersen RH, Hansen HJ, et al. Multimodal analgesic treatment in video-assisted thoracic surgery lobectomy using an intraoperative intercostal catheter. Eur J Cardiothorac Surg 2012;41:1072-7. [Crossref] [PubMed]
- Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121. [Crossref] [PubMed]
- Kelava M, Alfirevic A, Bustamante S, et al. Regional Anesthesia in Cardiac Surgery: An Overview of Fascial Plane Chest Wall Blocks. Anesth Analg 2020;131:127-35. [Crossref] [PubMed]
- Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107-13. [Crossref] [PubMed]
- Blanco R. The 'pecs block': a novel technique for providing analgesia after breast surgery. Anaesthesia 2011;66:847-8. [Crossref] [PubMed]
- Haam S, Kim D, Hwang J, et al. An anatomical study of the relationship between the sympathetic trunk and intercostal veins of the third and fourth intercostal spaces during thoracoscopy. Clin Anat 2010;23:702-6. [Crossref] [PubMed]
- Miyawaki M. Constancy and characteristics of the anterior cutaneous branch of the first intercostal nerve: correcting the descriptions in human anatomy texts. Anat Sci Int 2006;81:225-41. [Crossref] [PubMed]
- Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat 2005;18:346-9. [Crossref] [PubMed]
- Finley DJ, Rusch VW. Anatomy of the pleura. Thorac Surg Clin 2011;21:157-63. vii. [Crossref] [PubMed]
- Rendina EA, Ciccone AM. The intercostal space. Thorac Surg Clin 2007;17:491-501. [Crossref] [PubMed]
- Urits I, Ostling PS, Novitch MB, et al. Truncal regional nerve blocks in clinical anesthesia practice. Best Pract Res Clin Anaesthesiol 2019;33:559-71. [Crossref] [PubMed]
- Lee HJ, Park HS, Moon HI, et al. Effect of Ultrasound-Guided Intercostal Nerve Block Versus Fluoroscopy-Guided Epidural Nerve Block in Patients With Thoracic Herpes Zoster: A Comparative Study. J Ultrasound Med 2019;38:725-31. [Crossref] [PubMed]
- Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology 2011;115:181-8. [Crossref] [PubMed]
- Cook TM, Counsell D, Wildsmith JA, et al. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102:179-90. [Crossref] [PubMed]
- Bolotin G, Lazarovici H, Uretzky G, et al. The efficacy of intraoperative internal intercostal nerve block during video-assisted thoracic surgery on postoperative pain. Ann Thorac Surg 2000;70:1872-5. [Crossref] [PubMed]
- Vilvanathan S, Kuppuswamy B, Sahajanandan R. A randomized control trial to compare thoracic epidural with intercostal block plus intravenous morphine infusion for postoperative analgesia in patients undergoing elective thoracotomy. Ann Card Anaesth 2020;23:127-33. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Luketich JD, Land SR, Sullivan EA, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg 2005;79:1845-9; discussion 1849-50. [Crossref] [PubMed]
- Baxter CS, Singh A, Ajib FA, et al. Intercostal Nerve Block. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Temes RT, Won RS, Kessler RM, et al. Thoracoscopic intercostal nerve blocks. Ann Thorac Surg 1995;59:787-8. [Crossref] [PubMed]
- Elkhashab Y, Wang D. A Review of Techniques of Intercostal Nerve Blocks. Curr Pain Headache Rep 2021;25:67. Erratum in: Curr Pain Headache Rep 2021;25:74. [Crossref] [PubMed]
- Kadomatsu Y, Mori S, Ueno H, et al. Comparison of the analgesic effects of modified continuous intercostal block and paravertebral block under surgeon's direct vision after video-assisted thoracic surgery: a randomized clinical trial. Gen Thorac Cardiovasc Surg 2018;66:425-31. [Crossref] [PubMed]
- Guerra-Londono CE, Privorotskiy A, Cozowicz C, et al. Assessment of Intercostal Nerve Block Analgesia for Thoracic Surgery: A Systematic Review and Meta-analysis. JAMA Netw Open 2021;4:e2133394. [Crossref] [PubMed]
- Meierhenrich R, Hock D, Kühn S, et al. Analgesia and pulmonary function after lung surgery: is a single intercostal nerve block plus patient-controlled intravenous morphine as effective as patient-controlled epidural anaesthesia? A randomized non-inferiority clinical trial. Br J Anaesth 2011;106:580-9. [Crossref] [PubMed]
- Lee J, Lee DH, Kim S. Serratus anterior plane block versus intercostal nerve block for postoperative analgesic effect after video-assisted thoracoscopic lobectomy: A randomized prospective study. Medicine (Baltimore) 2020;99:e22102. [Crossref] [PubMed]
- Park R, Coomber M, Gilron I, et al. Cryoanalgesia for postsurgical pain relief in adults: A systematic review and meta-analysis. Ann Med Surg (Lond) 2021;69:102689. [Crossref] [PubMed]
- Ba YF, Li XD, Zhang X, et al. Comparison of the analgesic effects of cryoanalgesia vs. parecoxib for lung cancer patients after lobectomy. Surg Today 2015;45:1250-4. [Crossref] [PubMed]
- Graves CE, Moyer J, Zobel MJ, et al. Intraoperative intercostal nerve cryoablation During the Nuss procedure reduces length of stay and opioid requirement: A randomized clinical trial. J Pediatr Surg 2019;54:2250-6. [Crossref] [PubMed]
- Chaudhri BB, Macfie A, Kirk AJ. Inadvertent total spinal anesthesia after intercostal nerve block placement during lung resection. Ann Thorac Surg 2009;88:283-4. [Crossref] [PubMed]
- Chu H, Dong H, Wang Y, et al. Effects of ultrasound-guided paravertebral block on MMP-9 and postoperative pain in patients undergoing VATS lobectomy: a randomized, controlled clinical trial. BMC Anesthesiol 2020;20:59. [Crossref] [PubMed]
- Li XL, Zhang Y, Dai T, et al. The effects of preoperative single-dose thoracic paravertebral block on acute and chronic pain after thoracotomy: A randomized, controlled, double-blind trial. Medicine (Baltimore) 2018;97:e11181. [Crossref] [PubMed]
- Li XL, Zhang J, Wan L, et al. Efficacy of Single-shot Thoracic Paravertebral Block Combined with Intravenous Analgesia Versus Continuous Thoracic Epidural Analgesia for Chronic Pain After Thoracotomy. Pain Physician 2021;24:E753-9.
- Krediet AC, Moayeri N, van Geffen GJ, et al. Different Approaches to Ultrasound-guided Thoracic Paravertebral Block: An Illustrated Review. Anesthesiology 2015;123:459-74. [Crossref] [PubMed]
- Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: Executive Summary. Reg Anesth Pain Med 2016;41:181-94. [Crossref] [PubMed]
- Patnaik R, Chhabra A, Subramaniam R, et al. Comparison of Paravertebral Block by Anatomic Landmark Technique to Ultrasound-Guided Paravertebral Block for Breast Surgery Anesthesia: A Randomized Controlled Trial. Reg Anesth Pain Med 2018;43:385-90. [Crossref] [PubMed]
- Uppal V, Sondekoppam RV, Sodhi P, et al. Single-Injection Versus Multiple-Injection Technique of Ultrasound-Guided Paravertebral Blocks: A Randomized Controlled Study Comparing Dermatomal Spread. Reg Anesth Pain Med 2017;42:575-81. [Crossref] [PubMed]
- Liang XL, An R, Chen Q, et al. The Analgesic Effects of Thoracic Paravertebral Block versus Thoracic Epidural Anesthesia After Thoracoscopic Surgery: A Meta-Analysis. J Pain Res 2021;14:815-25. [Crossref] [PubMed]
- New York School of Regional Anesthesia. Epidural Anesthesia and Analgesia - NYSORA February 22, 2022. Available online: https://www.nysora.com/topics/regional-anesthesia-for-specific-surgical-procedures/abdomen/epidural-anesthesia-analgesia/
- Waurick R, Van Aken H. Update in thoracic epidural anaesthesia. Best Pract Res Clin Anaesthesiol 2005;19:201-13. [Crossref] [PubMed]
- Golic DA, Svraka D, Keleman N, et al. Epidural Analgesia With Surgical Stabilization of Flail Chest Following Blunt Thoracic Trauma in Patients With Multiple Trauma. Front Med (Lausanne) 2018;5:280. [Crossref] [PubMed]
- Hausman MS Jr, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg 2015;120:1405-12. [Crossref] [PubMed]
- Kukreja P, Herberg TJ, Johnson BM, et al. Retrospective Case Series Comparing the Efficacy of Thoracic Epidural With Continuous Paravertebral and Erector Spinae Plane Blocks for Postoperative Analgesia After Thoracic Surgery. Cureus 2021;13:e18533. [Crossref] [PubMed]
- Available online: https://esraeurope.org/prospect/procedures/thoracotomy-2015/summary-recommendations-8/
- Chin KJ. Recent developments in ultrasound imaging for neuraxial blockade. Curr Opin Anaesthesiol 2018;31:608-13. [Crossref] [PubMed]
- Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth 2017;37:82-5. [Crossref] [PubMed]
- Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth 2012;109:144-54. [Crossref] [PubMed]
- Chin KJ, Adhikary SD, Forero M. Understanding ESP and Fascial Plane Blocks: A Challenge to Omniscience. Reg Anesth Pain Med 2018;43:807-8. [Crossref] [PubMed]
- Taketa Y, Irisawa Y, Fujitani T. Programmed intermittent bolus infusion versus continuous infusion of 0.2% levobupivacaine after ultrasound-guided thoracic paravertebral block for video-assisted thoracoscopic surgery: A randomised controlled trial. Eur J Anaesthesiol 2019;36:272-8. [Crossref] [PubMed]
- Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim 2012;59:470-5. [Crossref] [PubMed]
- Yamak Altinpulluk E, Turan A. Future in regional anesthesia: new techniques and technological advancements. Minerva Anestesiol 2021;87:85-100. [Crossref] [PubMed]
- Desroches J, Belliveau M, Bilodeau C, et al. Pectoral nerves I block is associated with a significant motor blockade with no dermatomal sensory changes: a prospective volunteer randomized-controlled double-blind study. Can J Anaesth 2018;65:806-12. [Crossref] [PubMed]
- Versyck B, van Geffen GJ, Chin KJ. Analgesic efficacy of the Pecs II block: a systematic review and meta-analysis. Anaesthesia 2019;74:663-73. [Crossref] [PubMed]
- Chin KJ. Thoracic wall blocks: From paravertebral to retrolaminar to serratus to erector spinae and back again - A review of evidence. Best Pract Res Clin Anaesthesiol 2019;33:67-77. [Crossref] [PubMed]
- Corso RM, Maitan S, Russotto V, et al. Type I and II pectoral nerve blocks with serratus plane block for awake video-assisted thoracic surgery. Anaesth Intensive Care 2016;44:643-4.
- Yildirim K, Sertcakacilar G, Hergunsel GO. Comparison of the Results of Ultrasound-Guided Thoracic Paravertebral Block and Modified Pectoral Nerve Block for Postoperative Analgesia in Video-Assisted Thoracoscopic Surgery: A Prospective, Randomized Controlled Study. J Cardiothorac Vasc Anesth 2022;36:489-96. [Crossref] [PubMed]
- Semyonov M, Fedorina E, Grinshpun J, et al. Ultrasound-guided serratus anterior plane block for analgesia after thoracic surgery. J Pain Res 2019;12:953-60. [Crossref] [PubMed]
- Park MH, Kim JA, Ahn HJ, et al. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia 2018;73:1260-4. [Crossref] [PubMed]
- Baldinelli F, Capozzoli G, Pedrazzoli R, et al. Are Thoracic Wall Blocks Efficient After Video-Assisted Thoracoscopy Surgery-Lobectomy Pain? A Comparison Between Serratus Anterior Plane Block and Intercostal Nerve Block. J Cardiothorac Vasc Anesth 2021;35:2297-302. [Crossref] [PubMed]
- Hanley C, Wall T, Bukowska I, et al. Ultrasound-guided continuous deep serratus anterior plane block versus continuous thoracic paravertebral block for perioperative analgesia in videoscopic-assisted thoracic surgery. Eur J Pain 2020;24:828-38. [Crossref] [PubMed]
- Dikici M, Akesen S, Yavaşcaoğlu B, et al. Comparison of intraoperative and post-operative effects of serratus anterior plane block performed with ultrasound and infiltration block in patients undergoing video-assisted thoracoscopic surgery. Agri 2022;34:23-32. [Crossref] [PubMed]
- Madabushi R, Tewari S, Gautam SK, et al. Serratus anterior plane block: a new analgesic technique for post-thoracotomy pain. Pain Physician 2015;18:E421-4.
- Khalil AE, Abdallah NM, Bashandy GM, et al. Ultrasound-Guided Serratus Anterior Plane Block Versus Thoracic Epidural Analgesia for Thoracotomy Pain. J Cardiothorac Vasc Anesth 2017;31:152-8. [Crossref] [PubMed]
- Finnerty DT, McMahon A, McNamara JR, et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth 2020;125:802-10. [Crossref] [PubMed]
- Feray S, Lubach J, Joshi GP, et al. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2022;77:311-25. [Crossref] [PubMed]
- Forero M, Adhikary SD, Lopez H, et al. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med 2016;41:621-7. [Crossref] [PubMed]
- Adhikary SD, Bernard S, Lopez H, et al. Erector Spinae Plane Block Versus Retrolaminar Block: A Magnetic Resonance Imaging and Anatomical Study. Reg Anesth Pain Med 2018;43:756-62. [Crossref] [PubMed]
- Yang HM, Choi YJ, Kwon HJ, et al. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia 2018;73:1244-50. [Crossref] [PubMed]
- De Cassai A, Bonvicini D, Correale C, et al. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol 2019;85:308-19. [Crossref] [PubMed]
- Athar M, Parveen S, Yadav M, et al. A Randomized Double-Blind Controlled Trial to Assess the Efficacy of Ultrasound-Guided Erector Spinae Plane Block in Cardiac Surgery. J Cardiothorac Vasc Anesth 2021;35:3574-80. [Crossref] [PubMed]
- Hong B, Yoon SH, Youn AM, et al. Thoracic interfascial nerve block for breast surgery in a pregnant woman: a case report. Korean J Anesthesiol 2017;70:209-12. [Crossref] [PubMed]
- Kaya C, Dost B, Dokmeci O, et al. Comparison of Ultrasound-Guided Pecto-intercostal Fascial Block and Transversus Thoracic Muscle Plane Block for Acute Poststernotomy Pain Management After Cardiac Surgery: A Prospective, Randomized, Double-Blind Pilot Study. J Cardiothorac Vasc Anesth 2022;36:2313-21. [Crossref] [PubMed]
- Liu V, Mariano ER, Prabhakar C. Pecto-intercostal Fascial Block for Acute Poststernotomy Pain: A Case Report. A A Pract 2018;10:319-22. [Crossref] [PubMed]
- Jones J, Murin PJ, Tsui JH. Opioid free postoperatively using Pecto-Intercostal Fascial Block (PIFB) with multimodal Analgesia (MMA) in a patient with myasthenia gravis underwent thymectomy via sternotomy. J Clin Anesth 2020;59:32-3. [Crossref] [PubMed]
- Khera T, Murugappan KR, Leibowitz A, et al. Ultrasound-Guided Pecto-Intercostal Fascial Block for Postoperative Pain Management in Cardiac Surgery: A Prospective, Randomized, Placebo-Controlled Trial. J Cardiothorac Vasc Anesth 2021;35:896-903. [Crossref] [PubMed]
- Kumar AK, Chauhan S, Bhoi D, et al. Pectointercostal Fascial Block (PIFB) as a Novel Technique for Postoperative Pain Management in Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth 2021;35:116-22. [Crossref] [PubMed]
- Strömskag KE, Reiestad F, Holmqvist EL, et al. Intrapleural administration of 0.25%, 0.375%, and 0.5% bupivacaine with epinephrine after cholecystectomy. Anesth Analg 1988;67:430-4.
- Strømskag KE, Hauge O, Steen PA. Distribution of local anesthetics injected into the interpleural space, studied by computerized tomography. Acta Anaesthesiol Scand 1990;34:323-6. [Crossref] [PubMed]
- Murphy DF. Interpleural analgesia. Br J Anaesth 1993;71:426-34. [Crossref] [PubMed]
- VadeBoncouer TR. A randomized, double-blind comparison of the effects of interpleural bupivacaine and saline on morphine requirements and pulmonary function after cholecystectomy. Anesthesiology 1989;71:339-43. [Crossref] [PubMed]


