Multiple factors affecting perioperative antibiotherapy in minimally invasive lung surgery: a retrospective case-control study
Introduction
Antimicrobial resistance has become an important public health problem in the world. The prevention and control measures of antimicrobial resistance should be implemented to avoid deaths caused by drug-resistant bacteria. In China, guidelines stipulate that antibiotic use for inpatients in tertiary hospitals should not exceed 40 defined daily doses (DDD) per 100 patient-days. Additionally, among inpatients, the pathogen detection rate should not be below 50% before the use of restricted antibiotics and 80% for antibiotics for special use (1).
As we know, the safety and effectiveness of minimally invasive surgeries have been well recognized in thoracic surgery. However, escalation of empiric antibiotherapy is not uncommon, leading to longer postoperative hospital stay and higher medical expenses during hospitalization. Reducing postoperative empirical escalation of antibiotics can delay bacterial resistance and promote rational drug use.
However, reducing antibiotic use in the perioperative period remains challenging due to a variety of factors, including multiple surgical options, complicated preoperative health status of patients, different prescribing habits of doctors, pathogen detection reports with hysteresis, and nonspecific clinical manifestations of infections. In particular, there are quite a few infection cases with negative pathogen detection reports, and empiric antibiotic escalation therapy after surgery is common. Therefore, this paper is a retrospective case-control study, aims to investigate the factors affecting the use of empiric antibiotic escalation therapy after minimally invasive lung surgery during hospitalization , improve perioperative patient management and promote the rational use of antibiotics. Herein, we present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-982/rc).
Methods
Data source
This was a retrospective case-control study. Cases were collected from the hospital information system (HIS) of a tertiary hospital. The clinical data of all patients who received minimally invasive surgery [including video-assisted thoracic surgery (VATS) or da Vinci robotic-assisted thoracic surgery (RATS)] for pulmonary diseases from January 2019 to December 2020 were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics board of Shanghai Chest Hospital (No. IS22071). Individual consent for this retrospective analysis was waived.
Subjects
The inclusion criteria included: (I) with complete medical records, (II) aged 18 years or older but younger than 90 years, (III) with normal liver and kidney function, (IV) the thoracic surgeon had performed minimally invasive lung surgery for more than 5 years, and (V) perioperative prophylactic use of antibiotics in compliance with requirements.
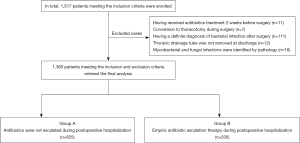
The exclusion criteria were: (I) treated with antibiotics 2 weeks before surgery due to syphilis, positive microbial documentation of sputum culture, etc.; (II) converted to thoracotomy during surgery; (III) had a definite diagnosis of bacterial infection after surgery, or diagnosed as surgical site infections (SSI); (IV) thoracic drainage tube was not removed at the time of discharge; and (V) mycobacterial and fungal infections were identified by pathology. Based on the above criteria, a total of 1,360 patients were enrolled in this study. These patients were divided into 2 groups: group A (n=825), in which antibiotics were not escalated after surgery; and group B (n=535), in which patients received empiric antibiotic escalation therapy after surgery (Figure 1).
All patients underwent perioperative prophylaxis consisting of intravenous administration of 1.0–2.0 g cefazolin sodium pentahydrate or injection, or 1.5 g cefuroxime 30–60 minutes before the surgical incision, with repeat doses every 12 hours for 48 hours. Patients with documented β-lactam allergy received clindamycin (0.6 g). If the operative time was more than 3 hours or intraoperative blood loss was over 1,500 mL, an additional dose of antibiotics was added.
The definition of empiric antibiotic escalation therapy was as follows: patients received empiric antibiotherapy and were treated with restricted antibiotics and/or antibiotics for special use during hospitalization for suspected potential infection after minimally invasive lung surgery, but pathogen detection showed negative results. A catalogue of hierarchical management of clinically used antibiotics at our center are listed in Table 1.
Table 1
| Antibiotics categories | For unrestricted use | For restricted use | For special use |
|---|---|---|---|
| First-generation cephalosporins | Cefazolin | ||
| Second-generation cephalosporins | Cefuroxime | Cefotiam | |
| Cefmetazole | |||
| Third-generation cephalosporins | Ceftriaxone | ||
| Fourth-generation cephalosporins | Cefepime | ||
| Carbapenems | Imipenem/cilastatin | ||
| Meropenem | |||
| Macrolides | Azithromycin (for injection) | ||
| Fluoroquinolones | Levofloxacin | ||
| Moxifloxacin | |||
| Lincomycins | Clindamycin |
Note: (I) This catalog was formulated by the Hospital Pharmacy Management Committee in accordance with the guiding principles and the relevant regulations in Hu Wei Yao Zheng [2021] No. 3. (II) The excerpt covers the drugs involved in this study, which have not been published in other journals. Note: (I) Drugs “for unrestricted use” (i.e., preferred drugs/first-line drugs) are antibacterial drugs with good efficacy, mild side effects, low bacterial resistance, and low price. They can be prescribed by clinicians at all levels when appropriate. (II) Drugs “for restricted use” (i.e., the second-choice drugs/second-line drugs) are antibiotics with good efficacy but high price or with limitations in toxic/side effects and bacterial resistance. Use of these drugs must be justified and approved by an attending (or senior) physician (with a signature). (III) Drugs “for special use” (i.e., third-line drugs) are antibiotics with good efficacy but high price. There are few clinical data on the efficacy or safety of these newly-marketed drugs in treating drug-resistant bacteria. Clinically, special measures should be taken to prevent bacteria from developing resistance to these drugs too quickly. The use of these antibiotics must be based on strict indications or conclusive evidence and must involve consultations with relevant experts or be approved by the director of the department. The prescription must be signed by the deputy director and chief physician.
Study variables
Perioperative information was collected. The independent variables included demographics, perioperative examination indicators, surgical anesthesia, postoperative complications, pathological reports, and drug prescriptions. Among them, the demographic variables included age, gender, body mass index (BMI), past medical history, smoking history, and allergy history. The perioperative examination indicators included body temperature (T), routine blood tests, biochemical indicators, pulmonary function, urine test, coagulation indicators, and pathogen detection results. Surgical anesthesia-related information included the surgeon, American Society of Anesthesiologists (ASA) score, surgical approach, surgical site, operation time, resection method, number of dissected lymph node stations, and intraoperative blood loss. The pathological reports included benign/malignant lesion and lesion size, etc. The drug prescription-related information included the drug name, dose, frequency, course of treatment, and administration time. The covariate was whether the patients received empiric antibiotic escalation therapy during postoperative hospitalization. Missing data were removed before the data analysis was conducted. All the collected data were double-checked.
Statistical analysis
Values were assigned to the variables. Univariate analysis was performed first. Measurement data that were normally distributed are presented as mean ± standard deviation and compared with t test. The count data are presented as cases and percentages and compared with chi square test or Fisher exact test. Variables with a P value of ≤0.1 were included in the logistic regression model (categorical: indicator = first). The ‘enter’ method was used for multivariate analysis. All variables that were to be included in the regression analysis were used in the imputation process. All statistical tests of the hypothesis were two-sided and performed at the 0.05 level of significance. The Hosmer-Lemeshow global goodness-of-fit statistic for logistic regression models was applied. All analyses were performed using SPSS 26.0 software.
Results
Sample characteristics
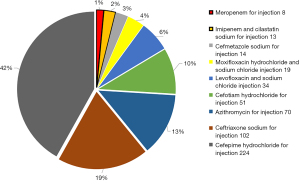
Of the 1,360 cases, there were 575 males (average age, 60.33±9.81 years) and 785 females (average age, 57.68±10.71 years). During hospitalization, antibiotics were not escalated in 825 patients (group A), accounting for 60.66% of all cases, and were escalated in 535 patients (group B), accounting for 39.34%. In group B, 514 patients received restricted antibiotics and 21 patients received antibiotics for special use. Compared with group A, group B had significantly longer postoperative hospital stay (5.05±2.78 vs. 4.49±2.24 days, P<0.001) and slightly higher total hospitalization costs (74,080.85±23,796.51 vs. 71,798.09±21,307.26 yuan, P=0.067). The empiric antibiotic escalation therapy involved 9 antibiotics, including cefotiam, cefmetazole, ceftriaxone, cefepime, azithromycin, levofloxacin, moxifloxacin, imipenem/cilastatin, and meropenem (Figure 2).
Univariate analysis
Univariate analysis revealed 6 statistically significant factors, including history of secondary lung surgery (P=0.01), preoperative hemoglobin A1c (HbA1c) ≥6.5% (P=0.004), preoperative urine red blood cell (RBC) count >10/µL and/or white blood cell (WBC) count >12/µL (P=0.005), postoperative fever of unknown origin (T >38 ℃; P=0.004), air leakage (≥5 days; P=0.006), and postoperative hypoalbuminemia (intravenous albumin administration for ≥2 days; P=0.001). Variables that showed no statistical significance included age, gender, BMI, history of allergy to β-lactam antibiotics, history of radiotherapy and/or chemotherapy, history of chronic obstructive pulmonary disease (COPD), history of diabetes, history of smoking, preoperative fever of unknown origin (T ≥38 °C), hemoglobin (HGB; male <120 g/L, female <110 g/L), albumin (<35 g/L), D-dimer (>0.55), ASA score, surgical approach, surgical site, resection method, number of dissected lymph node stations, operation time, intraoperative blood loss, bleeding/hemoptysis, dysuria (patient complaint), chylothorax, pathological type, lesion size, and pleural invasion (Table 2).
Table 2
| Variable name and description | Variable assignment | Group A, n=825 (60.7%) | Group B, n=535 (39.3%) | P value | ||
|---|---|---|---|---|---|---|
| MRFP | ||||||
| Total cost of hospitalization (yuan) | 71,798.09±21,307.26 | 74,080.85±23,796.51 | 0.067 | |||
| Postoperative hospital stay (days) | 4.49±2.24 | 5.05±2.78 | 0.000 | |||
| Demography | ||||||
| Age | 0.841 | |||||
| 18–44 years (young people) | 1 | 83 (10.1) | 54 (10.1) | |||
| 45–59 years (middle-aged people) | 2 | 312 (37.8) | 215 (40.2) | |||
| 60–74 years (elderly people) | 3 | 377 (45.7) | 233 (43.6) | |||
| 75–89 (seniors) | 4 | 53 (6.4) | 33 (6.2) | |||
| Gender | 0.753 | |||||
| Female | 0 | 479 (58.1) | 306 (57.2) | |||
| Male | 1 | 346 (41.9) | 229 (42.8) | |||
| BMI (kg/m2) | 0.298 | |||||
| <18 underweight | 1 | 494 (59.9) | 316 (59.1) | |||
| 18–23.9 normal | 0 | 18 (2.2) | 18 (3.4) | |||
| 24–27.9 overweight | 2 | 253 (30.7) | 172 (32.1) | |||
| ≥28 obese | 3 | 60 (7.3) | 29 (5.4) | |||
| History of secondary lung surgery | 0.010 | |||||
| No | 0 | 818 (99.2) | 521 (97.4) | |||
| Yes | 1 | 7 (0.8) | 14 (2.6) | |||
| History of radiotherapy/chemotherapy | 0.228 | |||||
| No | 0 | 815 (98.8) | 532 (99.4) | |||
| Yes | 1 | 10 (1.2) | 3 (0.6) | |||
| History of allergy to β-lactam antibiotics | 0.788 | |||||
| No | 0 | 803 (97.3) | 522 (97.6) | |||
| Yes | 1 | 22 (2.7) | 13 (2.4) | |||
| History of COPD | 0.338 | |||||
| No | 0 | 768 (93.1) | 505 (94.4) | |||
| Yes | 1 | 57 (6.9) | 30 (5.6) | |||
| History of diabetes | 0.999 | |||||
| No | 0 | 788 (95.5) | 511 (95.5) | |||
| Yes | 1 | 37 (4.5) | 24 (4.5) | |||
| History of smoking | 0.813 | |||||
| Never | 0 | 685 (83.0) | 447 (83.6) | |||
| Light | 1 | 44 (5.3) | 31 (5.8) | |||
| Heavy | 2 | 96 (11.6) | 57 (10.7) | |||
| Preoperative physical examination indicators | ||||||
| Fever of unknown origin (T ≥38 ℃) | 0.976 | |||||
| No | 0 | 822 (99.6) | 533 (99.6) | |||
| Yes | 1 | 3 (0.4) | 2 (0.4) | |||
| FEV1 <80 | 0.163 | |||||
| No | 0 | 768 (93.1) | 508 (95.0) | |||
| Yes | 1 | 57 (6.9) | 27 (5.0) | |||
| HbA1c ≥6.5% | 0.004 | |||||
| No | 0 | 746 (90.4) | 456 (85.2) | |||
| Yes | 1 | 79 (9.6) | 79 (14.8) | |||
| HGB (<120 g/L for males and <110 g/L for females) | 0.378 | |||||
| No | 0 | 734 (89.0) | 484 (90.5) | |||
| Yes | 1 | 91 (11.0) | 51 (9.5) | |||
| Serum albumin level <35 g/L | 0.318 | |||||
| No | 0 | 821 (99.5) | 530 (99.1) | |||
| Yes | 1 | 4 (0.5) | 5 (0.9) | |||
| D-dimer | 0.486 | |||||
| ≤0.55 | 0 | 690 (83.6) | 455 (85.0) | |||
| >0.55 | 1 | 135 (16.4) | 80 (15.0) | |||
| Routine urine test: abnormal (preoperative urine RBC count >10/μL and/or WBC count >12/μL) | 0.005 | |||||
| No | 0 | 819 (99.3) | 521 (97.4) | |||
| Yes | 1 | 6 (0.7) | 14 (2.6) | |||
| Surgical anesthesia-related information | ||||||
| ASA score | 0.919 | |||||
| I | 1 | 23 (2.8) | 16 (3.0) | |||
| II | 2 | 677 (82.1) | 445 (83.2) | |||
| III | 3 | 123 (14.9) | 73 (13.6) | |||
| IV | 4 | 2 (0.2) | 1 (0.2) | |||
| Surgical approach | 0.164 | |||||
| VAT | 0 | 544 (65.9) | 333 (62.2) | |||
| RAT | 1 | 281 (34.1) | 202 (37.8) | |||
| Surgical site | 0.290 | |||||
| Right side | 0 | 561 (68.0) | 349 (65.2) | |||
| Left side | 1 | 264 (32.0) | 186 (34.8) | |||
| Resection method | 0.539 | |||||
| Wedge resection | 1 | 33 (4.0) | 28 (5.2) | |||
| Segmentectomy | 2 | 34 (4.1) | 17 (3.2) | |||
| Lobectomy | 3 | 755 (91.5) | 487 (91.0) | |||
| Sleeve resection | 4 | 3 (0.4) | 3 (0.6) | |||
| Number of dissected lymph node stations | 0.493 | |||||
| No dissection | 0 | 19 (2.3) | 18 (3.4) | |||
| 1 station (N1/N2) | 1 | 44 (5.3) | 27 (5.0) | |||
| 2 stations (N1 + N2) | 2 | 762 (92.4) | 490 (91.6) | |||
| Operation time (>3 hours) | 0.377 | |||||
| No | 0 | 804 (97.5) | 517 (96.6) | |||
| Yes | 1 | 21 (2.5) | 18 (3.4) | |||
| Intraoperative blood loss (>100 mL) | 0.369 | |||||
| No | 0 | 798 (96.7) | 522 (97.6) | |||
| Yes | 1 | 27 (3.3) | 13 (2.4) | |||
| Postoperative complications and chief complaints | ||||||
| Fever of unknown origin (T ≥38 ℃) | 0.004 | |||||
| No | 0 | 808 (97.9) | 509 (95.1) | |||
| Yes | 1 | 17 (2.1) | 26 (4.9) | |||
| Difficulty urinating (chief complaint) | 0.569 | |||||
| No | 0 | 799 (96.8) | 521 (97.4) | |||
| Yes | 1 | 26 (3.2) | 14 (2.6) | |||
| Bleeding (hemostatic medication for >2 days) | 0.121 | |||||
| No | 0 | 806 (97.7) | 515 (96.3) | |||
| Yes | 1 | 19 (2.3) | 20 (3.7) | |||
| Air leakage (≥5 days) | 0.006 | |||||
| No | 0 | 816 (98.9) | 518 (96.8) | |||
| Yes | 1 | 9 (1.1) | 17 (3.2) | |||
| Serum albumin level (<35 g/L and intravenous albumin administration for ≥2 days) | 0.001 | |||||
| No | 0 | 824 (99.9) | 526 (98.3) | |||
| Yes | 1 | 1 (0.1) | 9 (1.7) | |||
| Chylothorax | 0.565 | |||||
| No | 0 | 824 (99.9) | 533 (99.6) | |||
| Yes | 1 | 1 (0.1) | 2 (0.4) | |||
| Post-operative pathology | ||||||
| Histological type | 0.453 | |||||
| Benign | 0 | 759 (92.0) | 486 (90.8) | |||
| Malignant | 1 | 66 (8.0) | 49 (9.2) | |||
| Lesion size (cm) | 0.160 | |||||
| ≤1 | 0 | 711 (86.2) | 454 (84.8) | |||
| 1–3 | 1 | 108 (13.1) | 71 (13.3) | |||
| 3–5 | 2 | 6 (0.7) | 10 (1.9) | |||
| Pleural invasion (elastic layer-positive) | 0.725 | |||||
| No | 0 | 721 (87.4) | 471 (88.0) | |||
| Yes | 1 | 104 (12.6) | 64 (12.0) | |||
Variables of MRFP are presented as mean ± standard deviation and compared with t test. Other variables are presented as cases and percentages and compared with chi square test or Fisher exact test. MRFP, medical record front page; BMI, body mass index; COPD, chronic obstructive pulmonary disease; T, temperature; FEV1, forced expiratory volume in 1 second; HbA1c, hemoglobin A1c; HGB, hemoglobin; RBC, red blood cell; WBC, white blood cell; ASA, American Society of Anesthesiologists; VAT, video-assisted thoracic; RAT, robotic-assisted thoracic.
Multivariate analysis
Six variables were involved in the logistic model for multivariate analysis, including history of secondary lung surgery, preoperative HbA1c ≥6.5%, preoperative urine RBC count >10/µL and/or WBC count >12/µL, postoperative fever of unknown origin (T >38 ℃), air leakage (≥5 days), and postoperative hypoalbuminemia (intravenous albumin administration for ≥2 days). The results showed that the statistically significant factors included history of secondary lung surgery [odds ratio (OR): 3.267; 95% confidence interval (CI): 1.305–8.178; P=0.011], preoperative HbA1c ≥6.5% (OR: 1.603; 95% CI: 1.143–2.249; P=0.006), postoperative fever (T >38 ℃; OR: 2.494; 95% CI: 1.321–4.708; P=0.005), and postoperative hypoalbuminemia (intravenous albumin administration for ≥2 days; OR: 14.125; 95% CI: 1.777–112.282; P=0.012). Preoperative urine RBC count >10/µL and/or WBC count >12/µL and air leakage (≥5 days) showed no statistical significance. The P value in the Hosmer and Lemeshow test was 0.558, indicating that the information in the current data was fully extracted, and the model had a high goodness of fit. Percentage accuracy in classification was 62.9% (Table 3).
Table 3
| Variables | B | OR (95% CI) | P value |
|---|---|---|---|
| Pulmonary reoperation | 1.184 | 3.267 (1.305–8.178) | 0.011 |
| Preoperative HbA1c (≥6.5%) | 0.472 | 1.603 (1.143–2.249) | 0.006 |
| Postoperative fever of unknown origin (T >38 ℃) | 0.914 | 2.494 (1.321–4.708) | 0.005 |
| Postoperative serum albumin (<35 g/L and intravenous albumin administration for ≥2 days) | 2.648 | 14.125 (1.777–112.282) | 0.012 |
| Preoperative urine RBC count >10/μL and/or WBC count >12/μL | 1.402 | 4.064 (0.587–28.146) | 0.156 |
| Air leakage (≥5 days) | −0.039 | 0.96 (0.179–5.173) | 0.963 |
B, regression coefficient; OR, odds ratio; CI, confidence interval; HbA1c, hemoglobin A1c; T, temperature; RBC, red blood cell; WBC, white blood cell.
Discussion
In China, guidelines stipulate that inpatient antibiotic use in tertiary hospitals should not exceed 40 DDDs per 100 patient-days, and the rate of pathogen detection should not be below 50% before the use of restricted antibiotics and 80% before antibiotics for special use (1). In the past 2 years, the incidence of nosocomial infection at our hospital was about 3%, 40% of which occurred in the department of thoracic surgery. Notably, the rate of pathogen detection was close to 100% before antibiotics for special use. Normally, for patients with mild or local infection, nonrestrictive antibiotics are the first choice. For patients suspected to have moderate or severe bacterial infection, empiric antibiotic escalation therapy may be considered based on the patient’s characteristics, and the severity and location of the infection. Specimens should be collected before treatment and sent for pathogen detection and antibiotic susceptibility tests in a timely manner, and the antibiotic prescription made by the surgeon in charge of the patient should be based on local environment and patterns of resistance. After the pathogen is identified, appropriate antibacterial drugs should be selected based on the drug’s characteristics and by referring to the results of drug susceptibility tests. However, the pathogen detection results may not be available immediately, and there are many cases of infection with no detection of pathogen. As a result, empiric antibiotic escalation therapy after surgery is common. Unfortunately, few studies have investigated these issues. As shown in our current retrospective analysis, history of secondary lung surgery, preoperative HbA1c ≥6.5%, postoperative fever of unknown origin (T >38 ℃), and postoperative hypoalbuminemia (<35 g/L and intravenous albumin administration ≥2 days) were the 4 independent risk factors for empiric antibiotic escalation therapy after minimally invasive lung surgery.
With the surge in the incidence of lung cancer, more patients diagnosed with multiple primary lung cancers (MPLC) or second primary lung cancer (SPLC) choose to receive repeated lung surgery. According to the literatures (2-6), patients undergoing a secondary lung surgery typically have poor pulmonary function, along with the risk of thoracic adhesions for ipsilateral lesions, which increases the difficulty of surgery and the incidence of postoperative complications. Age >70 years and tumors >2 cm are independent risk factors for poor prognosis (2-5). In our study, 21 patients underwent secondary lung surgery, all on different sides. The secondary pathological diagnosis was lung cancer. Among them, 3 patients also had COPD, and 5 patients had preoperative forced expiratory volume in 1 second (FEV1) between 50% and 70%. The median age was 60 years (range, 32–74 years), and the median tumor size was 1.7 cm (range, 0.6–3.3 cm). No postoperative complications were noted. Fourteen patients received empiric antibiotic escalation therapy after surgery, and the selected antibiotics included cefepime, ceftriaxone, cefotiam, and azithromycin. The median duration of antibiotic treatment was 3 days (range, 3–21 days). At present, the patients have been urged to take lung-protecting measures, including quitting smoking for at least 2 weeks before surgery, climbing stairs, cough and expectoration training, and atomizing inhalation used for dilating the trachea to promote expectoration. In the current analysis, history of COPD and preoperative FEV1 <70% did not show statistical significance. Therefore, for patients undergoing lung reoperation, further analysis is needed to determine whether it is necessary to receive empiric antibiotic escalation therapy.
Several studies have found that poor glycemic control affects overall survival (OS) or progression-free survival (PFS) in patients after lung cancer surgery. However, preoperative diabetes mellitus (DM) does not affect postoperative recovery in the acute phase (6-9). Our results showed that high preoperative blood glucose level (HbA1c ≥6.5%) was a risk factor for the escalation of postoperative antibiotic use. In the current study, there were 61 patients with DM and 159 with poor glycemic control (HbA1c ≥6.5%) before surgery, among whom 79 patients had their antibiotics escalated after surgery. The antibiotics used included cefepime, azithromycin, levofloxacin, moxifloxacin, imipenem/cilastatin, and meropenem, and the median duration of treatment was 4 days (range, 3–23 days). At present, all patients in our hospital are tested for HbA1c before surgery. For patients with poor blood sugar control, a less strict criterion is used, that is, fasting plasma glucose (FPG) below 10 mmol/L for at least 3 consecutive days. Some research has shown that glycated serum protein (GSP) is an indicator of the average blood glucose concentration of the past 1–3 weeks and is believed to better reflect short-term blood sugar changes and the efficacy of various sugar-lowering therapy (SLT) options. The mean blood glucose concentration reflected by perioperative GSP is closer to the blood glucose level at the time of surgery and is closely associated with adverse outcomes such as infection and death. In contrast, HbA1c has no such role. Preoperative and postoperative monitoring of GSP can also help to understand the overall perioperative blood glucose level and accurately identify stress-induced hyperglycemia (10,11). Therefore, whether perioperative GSP monitoring helps to reduce the escalation of empiric antibiotic use after lung surgery requires further investigation.
Fever is a physiological response of the human body to a pyrogenic stimulus and is common after a lung surgery. Many patients have fever at different times and of varying degrees after surgery. More severe surgical trauma often leads to longer duration of postoperative fever. Nevertheless, body T will return to normal within a week after surgery in the vast majority of patients. Even if there is a small amount of pleural effusion to be absorbed, body T rarely exceeds 38 ℃. In the current study, 27 patients experienced fever of unknown etiology (T >38 ℃), and no obvious abnormality was found in the tests and examinations. Among them, 26 patients had their antibiotics escalated after the operation. The drugs used included cefepime, ceftriaxone, azithromycin, levofloxacin, moxifloxacin, imipenem/cilastatin, and meropenem, and the median treatment course was 4 days (range, 2–20 days). Fever can be caused by a variety of health conditions and lacks characteristic clinical manifestations or positive laboratory findings, and thus its management remains challenging. When postoperative body T is repeatedly or persistently higher than 38 ℃, some possible comorbidities need to be considered, including pleural effusion, pneumonia, incision infection, deep venous thrombosis of the lower extremity, urinary tract infection, and common cold (12). For patients with potential infection, dynamic monitoring of procalcitonin (PCT) may help to reduce the use of restricted antibiotics or antibiotics for special use (13).
Recent studies have shown that minimally invasive surgery can reduce tissue damage and effectively prevent postoperative hypoalbuminemia. Low preoperative serum albumin level (<35 g/L) is an independent risk factor for postoperative pulmonary complications and also an important risk factor for postoperative 30-day mortality (14). At present, nutritional support, treatment of anemia, and treatment of electrolyte imbalances before lung surgery have become important components of ERAS. Postoperative hypoalbuminemia is mainly caused by inadequate intake, excessive consumption, and insufficient synthesis. Clinically, there is no systematic and effective treatment for postoperative hypoalbuminemia, with controlling biochemical indicators as the main targets (15). For patients who have undergone a minimally invasive lung surgery, increased protein intake and an adjusted diet is effective. In the current study, postoperative serum albumin <35 g/L and albumin treatment for more than 2 days were independent risk factors for postoperative empiric antibiotic escalation therapy. Nine of 10 patients were administered antibiotics, including cefepime, imipenem/cilastatin, and meropenem, and the median treatment course was 5 days (range, 4–23 days). These patients also had excessive pleural effusion, and some of them had hypovolemic lower extremity edema. For patients at high risk of infection, dynamic monitoring of PCT may guide the rational use of antibiotics (13).
Multivariate analysis showed that history of secondary lung surgery, preoperative HbA1c ≥6.5%, postoperative fever of unknown origin (T >38 ℃), and postoperative hypoalbuminemia (<35 g/L and intravenous albumin administration ≥2 days) were the 4 independent risk factors for empiric antibiotic escalation therapy after minimally invasive lung surgery. For preoperative risk factors, further cohort studies should be conducted to explore better intervention indicators or measures to improve the management of patients in the perioperative period and promote the rapid recovery after lung surgery. Postoperative risk factors may be associated with surgeons’ prescription habits, which were not systematically analyzed in the current study. However, for difficult problems (e.g., fever) or for some susceptible high-risk groups, dynamic monitoring of PCT can guide the rational use of antibiotics after surgery. In particular, it may help to reduce the use of restricted antibiotics or antibiotics for special use, lower the risk of drug resistance, shorten the length of hospital stay, and decrease the costs of hospitalization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-982/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-982/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-982/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics board of Shanghai Chest Hospital (No. IS22071). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guiding Principles for Clinical Application of antibiotics, 2015 edition. People's Medical Publishing House, 2015;154.
- Wang G, Zeng Y, Zheng H, et al. Prognostic analysis and clinical characteristics of dual primary lung cancer: a population study based on surveillance, epidemiology, and end results (SEER) database. Gen Thorac Cardiovasc Surg 2022;70:740-9. [Crossref] [PubMed]
- Bae MK, Byun CS, Lee CY, et al. The role of surgical treatment in second primary lung cancer. Ann Thorac Surg 2011;92:256-62. [Crossref] [PubMed]
- Leroy T, Monnet E, Guerzider S, et al. Let us not underestimate the long-term risk of SPLC after surgical resection of NSCLC. Lung Cancer 2019;137:23-30. [Crossref] [PubMed]
- Song N, Yang J, He W, et al. Surgical efficacy and prognostic factors of stage IA secondary primary lung cancer. Chinese J Thorac Cardiovasc Surg 2016;32:4.
- Komatsu T, Chen-Yoshikawa TF, Ikeda M, et al. Impact of diabetes mellitus on postoperative outcomes in individuals with non-small-cell lung cancer: A retrospective cohort study. PLoS One 2020;15:e0241930. [Crossref] [PubMed]
- Deng HY, Zheng X, Zha P, et al. Diabetes mellitus and survival of non-small cell lung cancer patients after surgery: A comprehensive systematic review and meta-analysis. Thorac Cancer 2019;10:571-8. [Crossref] [PubMed]
- Motoishi M, Sawai S, Hori T, et al. The preoperative HbA1c level is an independent prognostic factor for the postoperative survival after resection of non-small cell lung cancer in elderly patients. Surg Today 2018;48:517-24. [Crossref] [PubMed]
- Gao Y, Xu C. Expert consensus on perioperative blood glucose monitoring in adults. Chinese J Diabetes 2021;29:5.
- Lee EY, Lee BW, Kim D, et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol 2011;48:167-72. [Crossref] [PubMed]
- Mendes N, Tavares Ribeiro R, Serrano F. Beyond self-monitored plasma glucose and HbA1c: the role of non-traditional glycaemic markers in gestational diabetes mellitus. J Obstet Gynaecol 2018;38:762-9. [Crossref] [PubMed]
- Kelkar KV. Post-operative pulmonary complications after non-cardiothoracic surgery. Indian J Anaesth 2015;59:599-605. [Crossref] [PubMed]
- Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotics stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med 2019;57:1308-18. [Crossref] [PubMed]
- Wang L, Sun G. Risk factors related to pulmonary complications after abdominal surgery. J Clin Pulmonol 2010;612-4.
- Jiang S, Zhang J. Recent progress in etiology and treatment of hypoproteinemia after surgical stress. Chinese Critical Care Emergency Medicine 2017;29:5.
(English Language Editor: A. Muylwyk)