
A clinical risk model for assessing the survival of patients with stage IA–IIA non-small cell lung cancer after surgery
Introduction
Lung cancer is one of the most common carcinomas globally, accounting for 11.4% of all cancer diagnoses, and it is the leading cause of cancer-related death worldwide (approximately 18.0%) (1,2). According to pathological types, lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), of which NSCLC accounts for about 80–85% (2,3). For patients with early-stage NSCLC, the main treatment strategy is radical resection of the primary tumor, which has a 5-year overall survival (OS) rate of 73–90% (3-5). However, there is still a risk of tumor recurrence and metastasis after surgery in a portion of these patients, leading to a decrease in survival after surgical removal of the primary tumor (6,7). This risk is the main reason some patients with early-stage carcinoma require more frequent follow-up management and adjuvant therapy after surgery, which can improve the survival of patients to a certain extent (8,9). Administration of adjuvant therapy is strongly recommended for all patients with advanced NSCLC to improve their prognosis (10), but for patients with early-stage lung cancer, rigorous selection is required. The update of the eighth edition of the American Joint Committee on Cancer (AJCC) Staging Manual has proposed some high-risk factors related to poor survival in early-stage patients (11-13). Many studies have also confirmed that tumor sizes larger than 4 cm and visceral pleura invasion (VPI) could aggravate the prognosis of patients with early-stage lung cancer (14-17).
In the past, clinicians usually evaluated the prognosis of patients through the tumor-node-metastasis (TNM) staging system and used it to guide some options of clinical treatment. Nonetheless, even with NSCLC of the same stage, individuals have significant heterogeneity in survival, so using the TNM staging system alone may not accurately predict survival (18,19). Currently, many tumor-related prognostic models have been established, mainly in the form of nomograms (20-22). A nomogram contains the important prognosis factors and can provide individual risk scores for each person; therefore, it is considered a reliable way to predict the survival of patients (20,22,23). In addition, several studies have suggested that nomograms provide a more accurate survival prediction of various malignancies than traditional TNM staging systems (2,21,24,25). We obtained patient data from the Surveillance Epidemiology and End Results (SEER) database. We aimed to assess the risk factors that could affect the survival of postoperative patients with early-stage NSCLC. A prognostic nomogram was then created to calculate the risk scores of patients. According to the risk scores, patients were classified into high-risk and low-risk groups. The nomogram might provide information for clinicians to evaluate the prognosis of early-stage patients and may guide them in decision-making. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-890/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. K22-209). The requirement for informed consent was waived since the SEER database is publicly available and anonymous. Our study cohort consisted of 9,914 patients from the SEER database who underwent surgery between January 2004 and December 2015 and 270 patients from Shanghai Pulmonary Hospital who underwent surgery between 2010 and 2013. Patients who met all of the following selection criteria were enrolled in this retrospective study: (I) histopathologic diagnosis confirmed as NSCLC; (II) no metastasis to the lymph node or other organs; (III) known resection type; (IV) exact differentiation and location of the tumor; (V) the had presence of 1 primary tumor only; (VI) tumor size was between 0 and 5 cm; and (VII) age was within the range of 18 to 80 years. Patients who met any of the following conditions were excluded from this study: (I) a past or current history of another malignancy; (II) invasion of the parietal pleura, vessels, or ribs; and/or (III) they had received neoadjuvant and/or adjuvant chemotherapy. The baseline information of patients we collected covered sex, race, age at diagnosis, surgical approach, tumor grade, histologic type, number of examined lymph nodes, location of the tumor, tumor size, and VPI. Patients from the SEER database were classified into a training cohort (SEER-A) and a validation cohort (SEER-B) by R version 4.1.1 (https://www.r-project.org/; The R Foundation for Statistical Computing, Vienna, Austria) to make the baseline data comparable between the 2 groups. The patients from Shanghai Pulmonary Hospital were regarded as an external testing group. The SEER-A cohort and SEER-B cohort each consisted of 4,957 patients, and there was no difference in the baseline data. According to the patients’ records, we translated their pathological staging into types set out in the eighth edition of AJCC.
Follow-up and outcome
After the surgical resection of the primary tumor, the patients were followed up. In the SEER database, follow-up duration ranged from 2.0 to 83.0 months, with an average of 59.7 months. Among the patients from Shanghai Pulmonary Hospital, follow-up duration ranged from 3.0 to 91.0 months, with an average of 68.7 months. The OS was calculated from the date of surgery to the date of death or the last day of follow-up.
Surgery
According to the records in the SEER and Shanghai Pulmonary Hospital databases, the extent of the surgery included sublobar resection, lobectomy, and pneumonectomy. Sublobar resection was implemented when the extent of excision or resection was less than a lobe and included bronchial sleeve resection only, wedge resection, and segmental resection. In the SEER database, the median number of lymph nodes examined was 8.0. In the Shanghai Pulmonary Hospital database, the median number of lymph nodes was 10.0.
Variable declaration
The types of histology were classified as adenocarcinoma (ADC), squamous cell carcinoma (SCC), large cell carcinoma (LCC), acinar cell carcinoma (ACC), adenosquamous carcinoma (ASC), bronchial alveolar carcinoma (BAC), and others. The locations of the primary tumor included the right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), left lower lobe (LLL), and others. The grades ranged from I to IV and represented well differentiated, moderately differentiated, poorly differentiated, and undifferentiated, respectively.
Statistical analysis
The proportions of categorical outcomes were assessed with the Pearson chi-squared test and Fisher’s exact test. OS of patients was analyzed using the Kaplan-Meier method, and the difference in survival between the 2 groups was compared using a log-rank test. Univariable and multivariable Cox proportional hazards models were adopted to identify the independent prognostic predictors. Predictors (P<0.05) in the univariable analysis and known factors affecting prognosis were brought into a multivariable analysis. Results of the univariable and multivariable analyses were presented as hazard ratio (HR) and 95% confidence interval (CI), respectively, and the 2-sided statistical significance level was considered P<0.05. Statistical analyses were conducted using SPSS 23.0 (IBM Corp., Armonk, NY, USA). The nomogram was constructed with R version 4.1.1 based on the risk factors concluded from the multivariable analysis. The concordance index (C-index) of the model was measured by comparing the predicted survival with the observed survival probability: the larger the C-index, the more accurate the prognostic stratification. The cut-off values for total risk points were assessed using the X-tile software (Copyright: Camp/Rimm; Yale University, New Haven, CT, USA), and patients were divided into high-risk and low-risk groups based on the cut-off value of their risk score.
Results
Patients and characteristics
After selection, 9,914 patients during 2004–2015 were finally included in this study from the SEER database. Patients selected were randomly divided into the SEER-A and SEER-B groups. Among the 9,914 patients, 4,353 (43.9%) were men and 5,561 (56.1%) were women. There were 1,016 (10.2%) patients with VPI, and the others did not have VPI. Patients’ ages ranged from 19 to 80 years (median, 65 years; Table 1). The primary tumors were located in RUL (35.8%), RML (5.5%), RLL (18.1%), LUL (25.7%), and LLL (14.0%), and the remaining portion of tumors were in other locations (n=92, 0.9%) including the main bronchi and multiple positions. The main histologic types were ADC (n=6,240, 63.0%) and SCC (n=2,334, 23.5%). A total of 4,827 (48.7%) patients had less than or equal to 7.0 lymph nodes removed, and 5,087 (51.3%) patients had more than 7.0 lymph nodes removed during the surgery. Regarding the grade of tumor differentiation, the tumors in 2,356 (23.8%) patients were well differentiated, 4,864 (49.1%) were moderately differentiated, 2,600 (26.2%) were poorly differentiated, and 94 (0.9%) were undifferentiated. In the training cohort, the 1-, 3-, and 5-year OS rates were 95.0%, 84.5%, and 74.3%, respectively. In addition, in the validation cohort, the 1-, 3-, and 5-year OS rates were 94.9%, 84.2%, and 74.9%, respectively. Clinical characteristics of patients in Shanghai Pulmonary Hospital are listed in Table 2, and their 1-, 3-, and 5-year OS rates were 94.0%, 82.8%, and 69.7%, respectively.
Table 1
| Variables | All patients (n=9,914) | Training cohort (SEER-A; n=4,957) | Validation cohort (SEER-B; n=4,957) | P value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 4,353 (43.9) | 2,180 (44.0) | 2,173 (43.8) | 0.887 |
| Female | 5,561 (56.1) | 2,777 (56.0) | 2,784 (56.2) | |
| Age at diagnosis (years), n (%) | ||||
| ≤65 | 4,202 (42.4) | 2,133 (43.0) | 2,069 (41.7) | 0.193 |
| >65 | 5,712 (57.6) | 2,824 (57.0) | 2,888 (58.3) | |
| Location of tumor, n (%) | ||||
| RUL | 3,548 (35.8) | 1,803 (36.4) | 1,745 (35.2) | 0.815 |
| RML | 545 (5.5) | 270 (5.4) | 275 (5.5) | |
| RLL | 1,795 (18.1) | 874 (17.7) | 921 (18.6) | |
| LUL | 2,544 (25.7) | 1,271 (25.6) | 1,273 (25.7) | |
| LLL | 1,390 (14.0) | 693 (14.0) | 697 (14.1) | |
| Others | 92 (0.9) | 46 (0.9) | 46 (0.9) | |
| Tumor size (cm), n (%) | ||||
| ≤1 | 821 (8.3) | 415 (8.4) | 406 (8.2) | 0.883 |
| 1–2 | 4,173 (42.1) | 2,084 (42.0) | 2,089 (42.1) | |
| 2–3 | 3,071 (31.0) | 1,539 (31.1) | 1,532 (30.9) | |
| 3–4 | 1,347 (13.6) | 660 (13.3) | 687 (13.9) | |
| 4–5 | 502 (5.0) | 259 (5.2) | 243 (4.9) | |
| Histologic type, n (%) | ||||
| ADC | 6,240 (63.0) | 3,089 (62.3) | 3,151 (63.6) | 0.049 |
| SCC | 2,334 (23.5) | 1,213 (24.5) | 1,121 (22.6) | |
| ASC | 196 (2.0) | 110 (2.2) | 86 (1.7) | |
| ACC | 568 (5.7) | 279 (5.6) | 289 (5.8) | |
| BAC | 131 (1.3) | 67 (1.4) | 64 (1.3) | |
| LCC | 151 (1.5) | 70 (1.4) | 81 (1.7) | |
| Others | 294 (3.0) | 129 (2.6) | 165 (3.3) | |
| Tumor grade, n (%) | ||||
| I | 2,356 (23.8) | 1,194 (24.1) | 1,162 (23.4) | 0.658 |
| II | 4,864 (49.1) | 2,429 (49.0) | 2,435 (49.2) | |
| III | 2,600 (26.2) | 1,292 (26.1) | 1,308 (26.4) | |
| IV | 94 (0.9) | 42 (0.8) | 52 (1.0) | |
| VPI, n (%) | ||||
| No | 8,898 (89.8) | 4,435 (89.5) | 4,463 (90.0) | 0.354 |
| Yes | 1,016 (10.2) | 522 (10.5) | 494 (10.0) | |
| Surgical approach, n (%) | ||||
| Sublobar resection | 1,403 (14.2) | 698 (14.1) | 705 (14.2) | 0.586 |
| Lobectomy | 8,411 (84.8) | 4,214 (85.0) | 4,197 (84.7) | |
| Pneumonectomy | 100 (1.0) | 45 (0.9) | 55 (1.1) | |
| Examined lymph nodes, n (%) | ||||
| ≥8 | 5,087 (51.3) | 2,514 (50.7) | 2,573 (51.9) | 0.236 |
| ≤7 | 4,827 (48.7) | 2,443 (49.3) | 2,384 (48.1) | |
| Race, n (%) | ||||
| White | 8,129 (82.0) | 4,072 (82.1) | 4,057 (81.8) | 0.104 |
| Black | 912 (9.2) | 429 (8.7) | 483 (9.8) | |
| Others | 832 (8.4) | 438 (8.8) | 394 (7.9) | |
| Unknown | 41 (0.4) | 18 (0.4) | 23 (0.5) |
P value was calculated by χ2 test. SEER, Surveillance Epidemiology and End Results; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; VPI, visceral pleural invasion; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ACC, acinar cell carcinoma; ASC, adeno-squamous carcinoma; BAC, bronchial alveolar carcinoma.
Table 2
| Variables | All patients (n=270) |
|---|---|
| Sex, n (%) | |
| Male | 168 (62.2) |
| Female | 102 (37.8) |
| Age at diagnosis (years), n (%) | |
| ≤65 | 170 (63.1) |
| >65 | 100 (36.9) |
| Location of tumor, n (%) | |
| RUL | 77 (28.5) |
| RML | 20 (7.4) |
| RLL | 48 (17.8) |
| LUL | 84 (31.1) |
| LLL | 25 (9.3) |
| Others | 16 (5.9) |
| Tumor size (cm), n (%) | |
| ≤1 | 33 (12.2) |
| 1–2 | 92 (34.1) |
| 2–3 | 82 (30.4) |
| 3–4 | 42 (15.5) |
| 4–5 | 21 (7.8) |
| Histologic type, n (%) | |
| ADC | 189 (70.0) |
| SCC | 81 (30.0) |
| Tumor grade, n (%) | |
| I | 54 (20.0) |
| II | 172 (63.7) |
| III | 44 (16.3) |
| IV | 0 (0) |
| VPI, n (%) | |
| No | 228 (84.4) |
| Yes | 42 (15.6) |
| Surgical approach, n (%) | |
| Sublobar resection | 69 (25.6) |
| Lobectomy | 188 (69.6) |
| Pneumonectomy | 13 (4.8) |
| Examined lymph nodes, n (%) | |
| ≤9 | 170 (63.1) |
| ≥10 | 100 (36.9) |
RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; VPI, visceral pleural invasion; ADC, adenocarcinoma; SCC, squamous cell carcinoma.
Univariable and multivariable analyses
To determine the independent prognostic factors for the survival of patients after surgery, we analyzed the OS using a Cox regression model. The results of the univariable and multivariable analyses are shown in Table 3. The multivariable analysis confirmed the following 6 characteristics as independent prognostic factors: sex (HR 0.706; 95% CI: 0.619–0.806; P<0.001), age at diagnosis (HR 1.676; 95% CI: 1.453–1.933; P<0.001), tumor size (2–3 cm: HR 1.394; 95% CI: 1.037–1.875; P=0.028. 3–4 cm: HR 1.528; 95% CI: 1.110–2.104; P=0.009. 4–5 cm: HR 1.772; 95% CI: 1.238–2.535; P=0.002), tumor grade (II: HR 2.333; 95% CI: 1.855–2.934; P<0.001. III: HR 2.563; 95% CI: 2.004–3.280; P<0.001. IV: HR 2.769; 95% CI: 1.440–5.321; P=0.002), the number of examined lymph nodes (HR 0.867; 95% CI: 0.761–0.988; P=0.032), and VPI (HR 1.349; 95% CI: 1.120–1.624; P=0.002).
Table 3
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex | |||||||
| Male vs. female | 0.623 | 0.547–0.709 | <0.001 | 0.706 | 0.619–0.806 | <0.001 | |
| Age at diagnosis (years) | |||||||
| ≤65 vs. >65 | 1.037 | 1.028–1.045 | <0.001 | 1.676 | 1.453–1.933 | <0.001 | |
| Location of tumor | 0.397 | ||||||
| RUL | 1.275 | 0.603–2.695 | 0.525 | ||||
| RML | 0.961 | 0.431–2.141 | 0.922 | ||||
| RLL | 1.265 | 0.594–2.695 | 0.543 | ||||
| LUL | 1.288 | 0.608–2.731 | 0.509 | ||||
| LLL | 1.108 | 0.517–2.375 | 0.792 | ||||
| Others | 1 | Reference | |||||
| Tumor size (cm) | <0.001 | <0.001 | |||||
| 4–5 | 2.563 | 1.799–3.651 | <0.001 | 1.772 | 1.238–2.535 | 0.002 | |
| 3–4 | 1.987 | 1.448–2.727 | <0.001 | 1.528 | 1.110–2.104 | 0.009 | |
| 2–3 | 1.715 | 1.277–2.303 | <0.001 | 1.394 | 1.037–1.875 | 0.028 | |
| 1–2 | 1.131 | 0.841–1.522 | 0.415 | 1.030 | 0.765–1.387 | 0.847 | |
| ≤1 | 1 | Reference | 1 | Reference | |||
| Histologic type | <0.001 | 0.005 | |||||
| ADC | 1.256 | 0.763–2.066 | 0.371 | 1.507 | 0.963–2.360 | 0.955 | |
| SCC | 2.299 | 1.392–3.797 | 0.001 | 1.722 | 1.094–2.710 | 0.437 | |
| ASC | 2.097 | 1.143–3.848 | 0.017 | 1.489 | 0.839–2.643 | 0.687 | |
| ACC | 1.170 | 0.635–2.155 | 0.614 | 1.292 | 0.738–2.259 | 0.590 | |
| BAC | 0.506 | 0.198–1.294 | 0.155 | 1.291 | 0.564–2.956 | 0.474 | |
| LCC | 3.690 | 2.011–6.770 | <0.001 | 2.069 | 1.201–3.566 | 0.310 | |
| Others | 1 | Reference | 1 | Reference | |||
| Tumor grade | <0.001 | <0.001 | |||||
| IV | 4.503 | 2.521–8.042 | <0.001 | 2.769 | 1.440–5.321 | 0.002 | |
| III | 3.552 | 2.825–4.467 | <0.001 | 2.563 | 2.004–3.280 | <0.001 | |
| II | 2.755 | 2.208–3.437 | <0.001 | 2.333 | 1.855–2.934 | <0.001 | |
| I | 1 | Reference | 1 | Reference | |||
| VPI | |||||||
| No vs. yes | 1.595 | 1.328–1.916 | <0.001 | 1.349 | 1.120–1.624 | 0.002 | |
| Surgical approach | 0.029 | ||||||
| Sublobar resection | 0.569 | 0.334–0.972 | 0.039 | ||||
| Lobectomy | 0.518 | 0.311–0.864 | 0.012 | ||||
| Pneumonectomy | 1 | Reference | |||||
| Examined lymph nodes | |||||||
| ≤7 vs. ≥8 | 1.117 | 0.981–1.272 | 0.096 | 0.867 | 0.761–0.988 | 0.032 | |
| Race | 0.017 | ||||||
| White | 2.781 | 0.391–19.766 | 0.307 | ||||
| Black | 2.462 | 0.342–17.706 | 0.371 | ||||
| Others | 1.858 | 0.257–13.421 | 0.539 | ||||
| Unknown | 1 | Reference | |||||
HR, hazard ratio; CI, confidence interval; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; VPI, visceral pleural invasion; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ACC, acinar cell carcinoma; ASC, adenosquamous carcinoma; BAC, bronchial alveolar carcinoma.
Construction and validation of the prognostic risk model
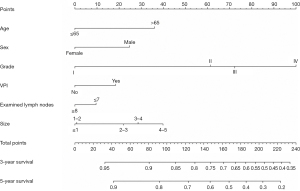
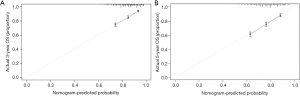
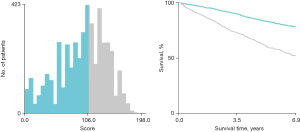
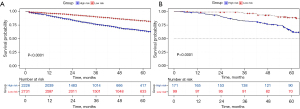
First, we built a nomogram for OS that included the independent prognostic factors mentioned above (Figure 1). The nomogram showed that tumor grade made the largest contribution to prognosis. We could easily draw a straight line down to determine the estimated probability of survival at each time point through the nomogram. The calibration curves showed a good agreement between the nomogram-predicted probability and the actual observation for 3- and 5-year OS (Figure 2A,2B). The C-index of the nomogram for predicting OS was 0.671 (95% CI: 0.653–0.689). We counted the scores of all patients and confirmed the optimal cut-off value of the scores by X-tile software. Cases were classified into high-risk and low-risk groups according to the optimal cut-off value (Figure 3). Then, the OS of those 2 groups in the validation cohort and Shanghai Pulmonary Hospital cohort were compared using the Kaplan-Meier method. Patients in the low-risk subgroups showed statistically better survival than those in the high-risk subgroups (P<0.001; Figure 4A,4B).
Discussion
In this study, based on the results of univariable and multivariable analyses, factors such as age at diagnosis, sex, tumor grade, the number of examined lymph nodes, tumor size, and VPI were found to influence the prognosis in NSCLC patients in stages IA to IIA. We constructed a nomogram to improve the prediction of survival. The calibration plots showed optimal agreement between the prediction and actual observation. The C-indices of the training cohort (0.671), validation cohort (0.668), and testing cohort (0.707) also demonstrated the reliability of the established nomogram. Further, patients were categorized into high-risk and low-risk subgroups with significant differences in OS by the cut-off value. It is well known that tumor size is an important independent prognostic factor; it has now been introduced into the TNM staging system (26). In this study, tumor size was significantly associated with the survival of patients (P<0.001). Moreover, poorly differentiated malignancies are always more invasive than well-differentiated malignancies, and the grade of the tumor is well known to be an important independent prognostic factor (27). In this study, tumor grade was significantly associated with OS of patients with IA–IIA NSCLC (P<0.001).
Currently, the total morbidity and mortality of NSCLC remain high in China (28). With the widespread application of low-dose computed tomography (LDCT) (1,29,30), the diagnostic rate of the early-stage disease has increased, but the follow-up management and the determination of whether to use adjuvant therapy remain controversial (31). Thus, it is necessary to analyze the prognosis of early-stage patients and identify high-risk patients, then to provide them with different recommendations for postoperative management and adjuvant treatment. Currently, the primary treatment for patients with IA–IIIB remains complete surgical resection of the tumor; however, postoperative patients still have the possibility of tumor recurrence and distant metastasis, which causes heterogeneity in the survival of individuals (31). Therefore, the decision to administer adjuvant therapy to the patients after surgery is always worth considering.
On the one hand, some studies suggest that adjuvant therapy might be an important strategy to reduce recurrence, prolong survival, and improve the quality of life in patients with early-stage NSCLC after the complete removal of tumors (32). On the other hand, other studies have indicated that the administration of adjuvant therapy to patients with stage IA–IIA NSCLC is not always effective and might harm some patients after surgery (33). Guidelines have already indicated that patients with IIB–IIIB NSCLC need to accept adjuvant therapy routinely after surgery, and survival benefits have been supported (34). For postoperative patients with IB–IIA NSCLC, adjuvant therapy is only recommended among patients with risk factors, such as a tumor size larger than 4 cm, VPI, and poor differentiation (35). However, some risk factors can also greatly influence long-term survival, even in patients with IA NSCLC (36,37). Thus, it is necessary to identify the patients with poor prognosis in stage IA–IIA diseases and provide additional therapy or intensive follow-up. The nomogram in this study can successfully identify high-risk patients in a cohort of stage IA–IIA.
Recent years have seen an increasing number of researchers participating in this field, but most previous studies established Cox regression models to analyze the prognosis of patients (38). Those models could not be used in clinical practice due to their low predictive ability and limited extrapolation (39). Several studies have suggested that nomograms provide a more accurate survival prediction of various malignancies than traditional TNM staging systems (24,25). There have been some nomograms created for early-stage NSCLC, yet previous models could not focus on predicting the survival in patients with a tumor size less than or equal to 4 cm, or these earlier models only included a small sample of patients with stage I NSCLC (3,12). Our study incorporated a large sample to build a predictive model for patients with IA–IIA NSCLC. Even though the data from the SEER database may have regional limits, the model was validated by the external testing data from Shanghai Pulmonary Hospital, implying its potential broad applicability.
Despite the precision of the model in our study, there were still some limitations. First, the study was retrospective in nature and failed to incorporate some baseline information of patients from the SEER database, such as the history of smoking, preoperative and postoperative complications, and physical conditions. Second, the prognostic model constructed in this study was based on the data from the SEER database. It is not easy to find a large-scale external validation cohort because of diverse standards in different regions. Third, some pathological factors affecting survival were not included in the SEER database, such as percentages of micropapillary, solid pattern, and the presence of spread through air spaces. Some molecular factors which are potentially important factors affecting the survival of patients with IA–IIA NSCLC were also vacant, such as the mutations of epidermal growth factor receptor and Kirsten rat sarcoma viral oncogene, and the fusion of echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (40-42). Further efforts on prospective data collection and the incorporation of more potential risk factors mentioned above will improve the model.
Conclusions
The nomogram established in this study could predict the prognosis of patients with stage IA–IIA NSCLC after surgical resection and help clinicians to identify high-risk populations.
Acknowledgments
Funding: This study was supported by Shanghai Health Commission (No. 2019SY072), Shanghai ShenKang Hospital Development Center (No. SHDC22020218), Outstanding Young Medical Talent of Rising Star in Medical Garden of Shanghai Municipal Health Commission “Dong Xie”, and Shanghai Pulmonary Hospital Research Fund (Nos. FK18001 & FKGG1805). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The funding bodies played a role in the interpretation of data, in writing, and in reviewing the manuscript.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-890/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-890/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-890/coif). All authors report funding from Shanghai Health Commission (No. 2019SY072), Shanghai ShenKang Hospital Development Centre (No. SHDC22020218), Outstanding Young Medical Talent of Rising Star in Medical Garden of Shanghai Municipal Health Commission “Dong Xie”, and Shanghai Pulmonary Hospital Research Fund (Nos. FK18001 & FKGG1805). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (NO. K22-209). The requirement for informed consent was waived since the SEER database is publicly available and anonymous.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oudkerk M, Liu S, Heuvelmans MA, et al. Lung cancer LDCT screening and mortality reduction - evidence, pitfalls and future perspectives. Nat Rev Clin Oncol 2021;18:135-51. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cao X, Zheng YZ, Liao HY, et al. A clinical nomogram and heat map for assessing survival in patients with stage I non-small cell lung cancer after complete resection. Ther Adv Med Oncol 2020;12:1758835920970063. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Chen S, Ye T, Yang S, et al. Prognostic implication of tumor spread through air spaces in patients with pathologic N0 lung adenocarcinoma. Lung Cancer 2022;164:33-8. [Crossref] [PubMed]
- Cai JS, Wang X, Yang F, et al. Lymphovascular invasion: A non-sized T descriptor for stage IA non-small cell lung cancer. Thorac Cancer 2022;13:2413-20. [Crossref] [PubMed]
- Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol 2021;18:547-57. [Crossref] [PubMed]
- Pathak R, Goldberg SB, Canavan M, et al. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol 2020;6:1741-50. [Crossref] [PubMed]
- Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol 2019;16:341-55. [Crossref] [PubMed]
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 2017;35:2960-74. [Crossref] [PubMed]
- Wu LL, Liu X, Jiang WM, et al. Stratification of Patients With Stage IB NSCLC Based on the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging Manual. Front Oncol 2020;10:571.
- Sakakura N, Mizuno T, Kuroda H, et al. The eighth TNM classification system for lung cancer: A consideration based on the degree of pleural invasion and involved neighboring structures. Lung Cancer 2018;118:134-8. [Crossref] [PubMed]
- Cho BC. Prognostic Factors in Early-stage NSCLC: Analysis of the Placebo Group in the MAGRIT Study. Anticancer Res 2019;39:1403-9. [Crossref] [PubMed]
- Iwano S, Umakoshi H, Kamiya S, et al. Postoperative recurrence of clinical early-stage non-small cell lung cancers: a comparison between solid and subsolid nodules. Cancer Imaging 2019;19:33. [Crossref] [PubMed]
- Li X, Zhang C, Sun Z, et al. Propensity-matched analysis of adjuvant chemotherapy for completely resected Stage IB non-small-cell lung cancer patients. Lung Cancer 2019;133:75-82. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Sculier JP, Chansky K, Crowley JJ, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol 2008;3:457-66.
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 2011;29:3163-72. [Crossref] [PubMed]
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834-40. [Crossref] [PubMed]
- Yap WK, Shih MC, Kuo C, et al. Development and Validation of a Nomogram for Assessing Survival in Patients With Metastatic Lung Cancer Referred for Radiotherapy for Bone Metastases. JAMA Netw Open 2018;1:e183242. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Zuo Z, Zhang G, Song P, et al. Survival Nomogram for Stage IB Non-Small-Cell Lung Cancer Patients, Based on the SEER Database and an External Validation Cohort. Ann Surg Oncol 2021;28:3941-50. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Yang D, Liu Y, Bai C, et al. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett 2020;468:82-7. [Crossref] [PubMed]
- Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019;30:1162-9. [Crossref] [PubMed]
- Beaty BT, Weiner AA. Alternatives to Surgery for Early-Stage Non-Small Cell Lung Cancer: Stereotactic Radiotherapy. Clin Chest Med 2020;41:185-95. [Crossref] [PubMed]
- Padda SK, Burt BM, Trakul N, et al. Early-stage non-small cell lung cancer: surgery, stereotactic radiosurgery, and individualized adjuvant therapy. Semin Oncol 2014;41:40-56. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther 2018;18:63-70. [Crossref] [PubMed]
- Wang C, Wu Y, Shao J, et al. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer 2020;20:150. [Crossref] [PubMed]
- Schuchert MJ, Normolle DP, Awais O, et al. Factors influencing recurrence following anatomic lung resection for clinical stage I non-small cell lung cancer. Lung Cancer 2019;128:145-51. [Crossref] [PubMed]
- Ding N, Mao Y, Gao S, et al. Predictors of lymph node metastasis and possible selective lymph node dissection in clinical stage IA non-small cell lung cancer. J Thorac Dis 2018;10:4061-8. [Crossref] [PubMed]
- Qi Y, Wu S, Tao L, et al. Development of Nomograms for Predicting Lymph Node Metastasis and Distant Metastasis in Newly Diagnosed T1-2 Non-Small Cell Lung Cancer: A Population-Based Analysis. Front Oncol 2021;11:683282. [Crossref] [PubMed]
- Suda K, Mitsudomi T, Shintani Y, et al. Clinical Impacts of EGFR Mutation Status: Analysis of 5780 Surgically Resected Lung Cancer Cases. Ann Thorac Surg 2021;111:269-76. [Crossref] [PubMed]
- Finn SP, Addeo A, Dafni U, et al. Prognostic Impact of KRAS G12C Mutation in Patients With NSCLC: Results From the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol 2021;16:990-1002. [Crossref] [PubMed]
- Li J, Zhang B, Zhang Y, et al. Concomitant mutation status of ALK-rearranged non-small cell lung cancers and its prognostic impact on patients treated with crizotinib. Transl Lung Cancer Res 2021;10:1525-35. [Crossref] [PubMed]
(English Language Editors: C. Mullens and J. Jones)


