Effects of preoperative Staphylococcus aureus screening and targeted decolonization bundle protocols in cardiac surgery: a nine-year review of a regional cardiovascular center in China
Introduction
Nosocomial infection (NI) tends to prolong hospital stay and heighten mortality among patients who undergoing cardiovascular surgery (1,2). Although hand hygiene and evidence-based strategies have been applied to prevent respiratory, bloodstream, urinary tract, and surgical site infections (SSI), NI remains a great challenge for the field of cardiovascular surgery (3,4).
Previous research has demonstrated the relationship between Staphylococcus aureus (SA) colonization and SA-related infection. Allen et al. reported that SA colonization was detected by swab culture in 18.3% (701 of 3,832) of patients undergoing elective cardiovascular surgery, and that the risk of postoperative SA-related infection was threefold higher in SA-colonized patients (5). Further analysis indicated that preoperative SA colonization was an independent predictor of postoperative SA-related bacteremia and SSI (6). In Maillet’s study, patients with SA colonization (22.4%) were twice as likely to develop sternal wound infection after cardiovascular surgery compared to patients without SA colonization (7). The rate of methicillin-resistant Staphylococcus aureus (MRSA) colonization was low (1.4–1.6%) in the work from Allen et al. (5), but was also associated with MRSA-related infection, which corresponded to a 2.4-fold higher mortality rate compared to that of methicillin-sensitive SA (MSSA) infection (5). Nevertheless, few studies have described the prevalence of SA colonization in the Chinese population. Zhou et al. reported a nasal SA colonization rate of 25.2% among 161 healthy participants, where MRSA was detected in 4.3% of the group (8). Until now, research on SA carriage among Chinese cardiac patients is largely absent.
Decolonization protocols (e.g., chlorhexidine and mupirocin) have been applied to reduce SA carriage, the incidence of NI, and the cost of cardiac surgery (9-11). Using chlorhexidine gluconate to cleanse the mouth, nose, and body can decrease total NI content by one fourth (10), and preoperative chlorhexidine mouthwash has been found to nearly halve the risk of postoperative pneumonia (12). Intranasal mupirocin treatment is effective in lessening SA carriage and the total incidence of SSI, but the evidence remains controversial for MRSA-related SSI (13-15).
MRSA carriers are at high risk for MRSA-related infection and transmission. Researchers have found that targeted MRSA decolonization, which includes contact isolation and glycopeptide antibiotic prophylaxis for the MRSA carrier, decreases SSI caused by MRSA and other gram-positive bacteria (11,16,17). However, few Chinese cardiovascular centers have implemented preoperative screening and decolonization, and no study has been published on the topic.
Our center is the adult cardiovascular surgery department of an academic hospital in Shanghai, China, and we have around four hundred operations per year. Since 2014, we have implemented preoperative SA screening and targeted decolonization bundle protocols into our surgical procedures. Ultimately, the purpose of this retrospective cohort study was to clarify: (I) the prevalence of SA/MRSA nasal colonization among adult Chinese patients undergoing cardiac surgery; (II) the effectiveness of SA/MRSA decolonization; and (III) the role of decolonization bundle therapy in reducing postoperative SA/MRSA-related infection. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-591/rc) (18).
Methods
Patient population and data
We constructed a population-based cohort by reviewing the electronic medical records and hospital infection surveillance records of adult patients who had received cardiovascular surgeries at our department between January 2012 and December 2020. We began using SA screening and decolonization bundle protocols in January 2014 and fully implemented them in April 2014, so patients treated during this period were excluded. With this in mind, we divided patients into two groups: the baseline group (treated between January 2012 and December 2013) and the intervention group (treated between May 2014 and December 2020).
During the study period, our hospital had enhanced NI surveillance and control measures. Control measures included hand hygiene, disinfection of environment and subjects, and other protocols. These practices likely impacted the incidence of NI. Our hospital has a southern campus, which is located 50 kilometers southwest of the central campus. This location has a team consisting of five cardiovascular surgeons and has seen around 100 operations per year since 2017. The standard NI control measures at the southern campus were the same as those at the central campus, but we did not implement SA protocols at the southern campus until 2022. Therefore, we collected the medical records of patients who were treated at this campus between 2017 and 2020 to serve as a control.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine (No. 2021KY050) and individual consent for this retrospective analysis was waived.
Interventions for SA screening and decolonization in a cardiovascular surgery department
We formulated our perioperative SA screening and targeted decolonization bundle protocols, which referenced the research of Schweizer ML and Saraswat MK (9,17) and were modified to fit our situation (see Table S1). Our procedure is listed as follows:
- Bilateral nostril swabs were performed by nurses to screen SA upon admission to the ward. Standard bacterial culturing and subsequent antimicrobial susceptibility testing were performed by a microbiological laboratory. If the patient tested positive for SA, we used nasal swabs again upon admission to the intensive care unit (ICU) after operation to assess the effectiveness of decolonization.
- All patients were instructed to bathe with chlorhexidine gluconate shower gel (Petel, Li-Kang Sterilization Co. LTD, Shanghai, China) on the day before operation. All patients rinsed their mouths and scrubbed their nares using cotton swabs with chlorhexidine gluconate solution (Jin-Kou-Xin, Jiangsu Chenpai Bond Pharmaceutical Co. LTD) three times on the day before operation and on the morning of the operation day. If the patient was unable to bathe, body wipes containing chlorhexidine gluconate (Petel, Li-Kang Sterilization Co. LTD, Shanghai, China) were used as an alternative. After operation, all patients were cleaned with chlorhexidine gluconate body wipes once a day and subjected to oral hygiene care using chlorhexidine gluconate solution three times a day.
- Mupirocin ointment (Bactroban; GlaxoSmithKline, Brentford, UK) was applied intranasally twice a day to patients who screened positive for either MRSA or MSSA.
- Cefuroxime served as the perioperative antibiotic prophylaxis for noncarriers and MSSA carriers, and was administrated 30 minutes before incision and again if the operation exceeded more than four hours. For MRSA carriers, the combination of vancomycin and cefuroxime was prescribed on the operation day and the day after.
- MRSA carriers were subjected to contact isolation during their entire hospital stays.
Definitions
Standard bacterial culturing was performed by the microbiological laboratory at our hospital. Briefly, the swab sample was inoculated in blood agar medium and incubated at 35 ℃ for 24–48 hours. The laboratorian observed the bacterial colonies, selected SA colonies, separated and purified them, and performed drug sensitivity testing using the disk diffusion method. Patients were defined as having SA/MRSA colonization if they had a positive microbiologic SA/MRSA nasal swab sample, and they were SA/MRSA carrier. If the nasal swab culture of SA/MRSA carrier was negative upon ICU admission, we defined the SA/MRSA as decolonized. The diagnosis of NI was made according to the diagnostic criteria of NI from the Chinese Ministry of Health (19). We recorded all NIs that occurred during each patient’s hospital stay, and did not include any follow-up issues. If the patient underwent more than one surgery, only the first surgery was recorded.
Statistics analysis
We used SPSS 24.0 statistical software (IBM Corp., Armonk, NY, USA) to perform all statistical analyses. Categorical data are presented as numbers and percentages, and were compared using the chi-square test or Fisher’s exact test. Continuous variables with a normal distribution are presented as mean ± standard deviation. Continuous variables with a skewed distribution are presented as median with 25th and 75th percentiles. The unpaired t-test was used to compare continuous data with a normal distribution, whereas the nonparametric Mann-Whitney U-test was used for data with a skewed distribution. Since variables were collected through manual review of the medical records, missing values were unavoidable. Our dataset was highly complete with most variables having missing rates ranging 0–2.7%. We handled missing values using the multiple imputation method. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Characteristics of the study population
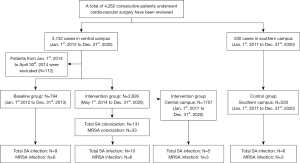
A total of 4,262 patients underwent cardiovascular surgery during our study period. There were 794 cases in the baseline group (January 2012 to December 2013) and 2,826 in the intervention group (May 2014 to December 2020) at our central campus. Using the period from January 2017 to December 2020, we assigned 1,757 patients to the central group (intervention group) and 530 patients to the southern group (control group) (Figure 1).
Compared to the baseline group, patients in the intervention group were older and more likely to be male. Although ejection fraction was similar between the two groups, the intervention group had more patients with New York Heart Function Classification (NYHF) III and less with NYHF IV. Surgical category significantly differed between the two groups. In the intervention group, the rates of aortic surgery, including open surgery and endovascular surgery, urgent surgery, and emergent surgery, were significantly higher. The rates of other surgeries, included heart tumor excision, maze procedure for atrial fibrillation, heart rupture repair, and so on, were also increased. The rates of conventional surgeries, such as coronary artery bypass grafting (CABG), valve surgery, and congenital heart surgery, were relatively lower. The rate of cardiopulmonary bypass was lower in the intervention group because of the increased rates of off-pump CABG and endovascular surgery in this group (Table 1).
Table 1
| Characteristics | Baseline, 2012.1–2013.12, N=794 | Intervention, 2014.5–2020.12, N=2,826 | P value |
|---|---|---|---|
| Age, years | 58.6 (14.4) | 59.7 (14.3) | 0.060 |
| >60 years | 401 (50.5) | 1,560 (55.2) | 0.019 |
| Male, N (%) | 468 (58.9) | 1,839 (65.1) | 0.005 |
| Body mass index, kg/m2 | 25.5 (3.9) | 25.2 (3.5) | 0.182 |
| Diabetes mellitus, N (%) | 150 (18.9) | 554 (19.6) | 0.685 |
| Hypertension, N (%) | 368 (46.3) | 1,232 (43.6) | 0.168 |
| Atrial fibrillation, N (%) | 151 (19.0) | 525 (18.6) | 0.794 |
| Stroke, N (%) | 73 (9.2) | 240 (8.5) | 0.521 |
| Preoperative renal insufficiency, N (%) | 61 (7.7) | 243 (8.6) | 0.469 |
| Preoperative LVEF, N (%) | 57.6 (13.0) | 57.9 (5.6) | 0.103 |
| New York Heart Function Classification, N (%) | <0.001 | ||
| II | 115 (14.5) | 487 (17.2) | |
| III | 463 (58.3) | 1,802 (63.8) | |
| IV | 216 (27.2) | 537 (19.0) | |
| Surgical procedures, N (%) | <0.001 | ||
| CABG | 289 (36.4) | 624 (25.7) | |
| Valve | 289 (36.4) | 714 (29.4) | |
| Congenital | 78 (9.8) | 168 (6.9) | |
| Aortic | 74 (9.3) | 550 (22.7) | |
| Others | 6 (7.7) | 370 (15.3) | |
| Urgent and emergency, N (%) | 8 (1.0) | 107 (3.8) | <0.001 |
| CPB, N (%) | 706 (88.9) | 2,227 (78.8) | <0.001 |
| Mortality, N (%) | 9 (1.1) | 40 (1.4) | 0.785 |
The variables are presented as n (%) or mean (standard deviation). LVEF, left ventricular eject fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass.
Prevalence of SA colonization and decolonization effect
During the intervention period, 2,719 patients were subjected to preoperative SA screening, where 131 cases were SA/MRSA positive. There were a total of 107 emergency operations. Screening and decolonization for these patients was performed after surgery, and none were SA/MRSA positive. Overall, 98 (3.5%) cases of MSSA and 33 (1.2%) cases of MRSA were detected (Table 2).
Table 2
| Cases | 2014.5–2016.12, N=1,069 | 2017.1–2018.12, N=891 | 2019.1–2020.12, N=866 | Total, N=2,826 |
|---|---|---|---|---|
| Total SA colonization, N (%) | 48 (4.5) | 47 (5.3) | 36 (4.2) | 131 (4.6) |
| Decolonization, N (%) | 45 (93.7) | 44 (93.6) | 36 (100.0) | 125 (95.4) |
| MSSA colonization, N (%) | 38 (3.6) | 35 (3.9) | 25 (2.9) | 98 (3.5) |
| Decolonization, N (%) | 35 (92.1) | 33 (94.3) | 25 (100.0) | 93 (94.9) |
| MRSA colonization, N (%) | 10 (0.9) | 12 (1.3) | 11 (1.3) | 33 (1.2) |
| Decolonization, N (%) | 10 (100.0) | 11 (91.7) | 11 (100.0) | 32 (97.0) |
The variables are presented as n (%). SA, Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Nasal swab sampling was performed again upon ICU admission for patients with SA colonization. In mostly all cases, SA was not detected [93 (94.9%) of MSSA carriers, 32 (97.0%) of MRSA carriers]. These results indicate that our methods were effective in achieving decolonization (Table 2).
SA/MRSA-related infection at the central campus
The incidence of NI caused by SA was significantly lower in the intervention group (0.354%) compared to the baseline group (1.133%) [P=0.021, risk ratio (RR): 0.312, 95% confidence interval (CI): 0.127–0.766]. For MRSA-related infection, the incidence was also lower in the intervention group (0.212%) compared to the baseline group (0.756%) (P=0.030, RR: 0.281, 95% CI: 0.091–0.869). To prevent one case of postoperative SA-related infection through screening and decolonization, the number needed to treat (NNT) was 143 patients. To prevent one MRSA-related infection, the NNT was 167 patients (Table 3).
Table 3
| SA/MRSA-related infection | Baseline, 2012.1–2013.12, N=794 | Intervention, 2014.5–2020.12, N=2,826 | RR (95% CI) | P value | NNT |
|---|---|---|---|---|---|
| Total SA-related infection, N (%) | 9 (1.133) | 10 (0.354) | 0.312 (0.127–0.766) | 0.021 | 143 |
| Total MRSA-related infection, N (%) | 6 (0.756) | 6 (0.212) | 0.281 (0.091–0.869) | 0.030 | 167 |
| Lower respiratory tract infection, N (%) | |||||
| SA | 6 (0.756) | 4 (0.142) | 0.187 (0.053–0.662) | 0.011 | 143 |
| MRSA | 4 (0.503) | 2 (0.071) | 0.140 (0.026–0.766) | 0.031 | 250 |
| Blood stream infection/catheter related infection, N (%) | |||||
| SA | 2 (0.252) | 4 (0.142) | 0.561 (0.103–3.062) | 0.856 | – |
| MRSA | 2 (0.252) | 4 (0.142) | 0.561 (0.103–3.062) | 0.856 | – |
| Surgical site infection, N (%) | |||||
| SA | 1 (0.126) | 2 (0.071) | 0.562 (0.051–6.189) | 1.000 | – |
| MRSA | 0 (0) | 0 (0) | – | – | – |
The variables are presented as n (%). SA, Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; RR; risk ratio; CI, confidence interval; NNT, number needed to treat.
Our results indicate that screening and decolonization bundle treatment significantly decrease the incidence of lower respiratory tract infection (LRTI) caused by SA/MRSA (SA: 0.756% vs. 0.142%, P=0.011, RR: 0.187, 95% CI: 0.053–0.662; MRSA: 0.503% vs. 0.071%, P=0.031, RR: 0.140, 95% CI: 0.026–0.766). Our protocol also resulted in lower incidences of blood stream /catheter related infection (all MRSA: 0.252% vs. 0.142%), and SSI (all MSSA: 0.126% vs. 0.071%) (Table 3). However, these results were not significant.
One patient with MSSA colonization suffered from LRTI with MSSA. No other patients with SA/MRSA colonization were infected by this pathogen.
SA/MRSA-related infection at the southern campus
Since the southern group followed the same NI control measures as the central group except for SA intervention bundle therapy, we used data from this group to validate the treatment effect. The clinical characteristics of the patients who underwent cardiovascular surgery at the southern campus were similar to those of the central group, especially in terms of age and surgical procedures (Table 4).
Table 4
| Characteristics | Central group, 2017.1–2020.12, N=1,757 | Southern group, 2017.1–2020.12, N=530 | P value |
|---|---|---|---|
| Age, years | 59.6 (14.5) | 59.9 (18.5) | 0.727 |
| >60 years, N (%) | 970 (55.2) | 310 (58.5) | 0.182 |
| Male, N (%) | 1,145 (65.2) | 333 (62.8) | 0.324 |
| Body mass index, kg/m2 | 25.3 (3.5) | 25.5 (2.2) | 0.110 |
| Smoking, N (%) | 197 (11.2) | 38 (7.2) | 0.007 |
| Diabetes mellitus, N (%) | 341 (19.4) | 100 (18.9) | 0.782 |
| Hypertension, N (%) | 755 (43.0) | 284 (53.6) | 0.000 |
| Atrial fibrillation, N (%) | 321 (18.3) | 101 (19.1) | 0.682 |
| Stroke, N (%) | 152 (8.7) | 44 (8.3) | 0.801 |
| Preoperative renal insufficiency, N (%) | 148 (8.4) | 41 (7.8) | 0.614 |
| Preoperative LVEF, N (%) | 57.6 (5.8) | 57.1 (6.4) | 0.104 |
| New York Heart Function Classification, N (%) | 0.748 | ||
| II | 298 (17.0) | 96 (18.1) | |
| III | 1,128 (64.2) | 340 (64.1) | |
| IV | 331 (18.8) | 94 (17.8) | |
| Operation, N (%) | 0.225 | ||
| CABG | 476 (27.1) | 147 (27.7) | |
| Valve | 517 (29.4) | 163 (30.8) | |
| Congenital | 138 (7.9) | 50 (9.4) | |
| Aortic | 368 (20.9) | 112 (21.1) | |
| Other | 258 (14.7) | 58 (11.0) | |
| Urgent and emergency, N (%) | 95 (5.4) | 32 (6.0) | 0.578 |
| CPB, N (%) | 1,388 (79.0) | 421 (79.4) | 0.829 |
| Mortality, N (%) | 44 (2.5) | 19 (3.6) | 0.183 |
| Total SA-related infection, N (%) | 5 (0.284) | 6 (1.132) | 0.035 |
| Total MRSA-related infection, N (%) | 3 (0.171) | 3 (0.566) | 0.282 |
The variables are presented as n (%) or mean (standard deviation). SA, Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass.
The rate of SA-related infection in the southern group was significantly higher than that of the central group (1.132% vs. 0.284%, respectively; P=0.035; RR: 0.251; 95% CI: 0.077–0.820), and relatively similar to that of the baseline group. Accordingly, the incidence of MRSA-related infection was higher in the southern group compared to the central group, but this difference was not significant (0.566% vs. 0.171%, respectively; P=0.282) (Table 4).
Adherence to SA intervention
All patients were able to tolerate the swabbing and decolonization protocols. Two patients felt uncomfortable being flagged for contact isolation. After providing a full explanation to these patients, they understood and complied with our protocols.
Discussion
Preoperative SA/MRSA colonization is a leading risk factor for subsequent infection among cardiac surgery patients (5,6). In this study, we found that the incidences of total SA, MSSA, and MRSA colonization in the anterior nares of our patient cohort were 4.6%, 3.5%, and 1.2%, respectively. Decolonization bundle treatment decreased SA/MRSA colonization by approximately 95%, and decreased total SA/MRSA-related infection to one third that of the baseline group. In case of other nosocomial stewardship or the Hawthorne effect, we compared patients treated at our central campus to those treated at our southern campus during the same period. Our results confirmed that screening and decolonization bundle treatment reduced total SA-related infection.
The prevalence of SA colonization varies by country, medical center, sampling method, and detection method. Reddy et al. found that MRSA was colonized at admission among 1.5% of their cardiovascular patient cohort in the UK (20). Segers et al. determined that more than 30% of their group of Netherlandish cardiovascular patients carried MSSA in their nares—in this same study, the prevalence of MRSA was only 0.2% (10). In Schweizer’s multicenter trial on cardiovascular and orthopedic surgery, it was found that most American centers use chromogenic agar culture, which is more sensitive in detecting SA/MRSA. Accordingly, this study reported a MSSA colonization rate of 11.1% and MRSA colonization rate of 2.8% among preoperative patients (9). Real-time polymerase chain reaction (PCR) testing for MRSA is quick (less than two hours) with high sensitivity and specificity (21). In Harbarth ’s study, this method was used to determine that 5.1% of the study participants were MRSA positive (15). Anterior nasal swabbing is the most effective and convenient screening method, and some studies even combine throat, axillae, and perineal swabbing methods, revealing even higher levels of SA colonization. In Maillet’s study, MSSA and MRSA were detected in the nares of 18.5% and 1.0% of cardiac surgery patients at admission, respectively. The combinition of sampling from the axillae and perineal regions raised these rates to 20.5% and 1.4%, respectively (7). At our center, the rates of SA and MRSA colonization (4.6% and 1.2%, respectively) were lower than those observed in studies from the USA. We speculate that this is due to our use of the standard bacterial culture method, which is less sensitive than chromogenic agar culturing and PCR testing.
Several decolonization strategies have been explored, but none have been fully accepted. Universal decolonization refers to the treatment of all patients regardless of colonization state (11), while targeted decolonization is defined as treatment for only SA or MRSA carriers, thus protecting non-carriers by avoiding SA transmission (11,17). Universal decolonization includes: (I) intranasal mupirocin or chlorhexidine gluconate gel for nasal application (10,12); and (II) chlorhexidine gluconate body wash and oral rinse (22). Universal measures are easier to implement than targeted methods, considering targeted methods require a wait to check screening results (11). However, the wide application of mupirocin would promote the emergence of mupirocin-resistant SA (23). Targeted decolonization involves: (I) patients with SA carriage receiving intranasal mupirocin decolonization (II) contact isolation and perioperative prescription of glycopeptide prophylaxis for MRSA carriers; (III) PCR testing and chromogenic agar culturing to quickly detect MRSA; and (IV) chlorhexidine gluconate bath and oral rinse for all patients before surgery regardless of the colonization result. In Schweizer’s multicenter prospective study on patients undergoing orthopedic and cardiac surgery, bundled SA screening and targeted decolonization halved the rate of complex SA SSI (9). Several retrospective studies have shown that targeted decolonization is associated with decreased surgical site MRSA infection (17,24). Recently, Nicolas’s study revealed that good compliance and detectable intranasal mupirocin concentrations are crucial for SA decolonization (25). After carefully reviewing these studies, we developed our SA screening and decolonization bundle procedure and implemented it in 2014. This study demonstrates that these protocols were operable with only minor side effects, and that they may have resulted in effective decolonization and significantly decreased total SA-related infection and MRSA-related infection.
In this study, we found that SA/MRSA-related infection was relatively low in the whole study period, which is consistent with other Chinese research reporting on SA-related NI in cardiac surgery (3,26,27). The SA screening and targeted decolonization bundle treatment reduced total SA/MRSA-related NI to about one third that of the baseline group. LRTI is the most common type of NI following cardiovascular surgery (28), and the reduction was most prominent for SA/MRSA-related LRTI. However, the reductions in blood stream/catheter-related infection and SSI were not statistically significant—this may have been because of the limited number of cases in our study. The southern campus group served as a comparable background in terms of hospital setting and patient characteristics. The SA/MRSA-related NI data from the southern group further supported that the perioperative SA decolonization bundle treatment may enable cardiac surgery patients to avoid SA-related infection.
To the best of our knowledge, this is the first study to assess the prevalence of SA/MRSA nasal colonization among Chinese adult cardiac patients, as well as the effectiveness of targeted SA/MRSA decolonization bundle therapy in preventing postoperative SA/MRSA-related infection. However, there are several limitations to our study. First, considering its retrospective design, we gathered data from medical records and were therefore unable to avoid bias. Second, the incidence of SA/MRSA-related infection was lower than expected. Although we verified our results using a parallel control study, this still raises concern for bias.
In conclusion, we found that the incidence of SA/MRSA colonization among Chinese patients undergoing cardiovascular surgery is relatively low. Targeted decolonization bundle therapy was associated with decolonization and reduced incidence of SA/MRSA-related infection.
Acknowledgments
Funding: This work was sponsored by the Clinical Research Plan of SHDC (No. SHDC2020CR3100B) and Scientific Funding of Shanghai Jiao Tong University School of Medicine (No. jyh 1411).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-591/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-591/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-591/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine (No. 2021KY050) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kollef MH, Sharpless L, Vlasnik J, et al. The impact of nosocomial infections on patient outcomes following cardiac surgery. Chest 1997;112:666-75. [Crossref] [PubMed]
- Mazzeffi M, Gammie J, Taylor B, et al. Healthcare-Associated Infections in Cardiac Surgery Patients With Prolonged Intensive Care Unit Stay. Ann Thorac Surg 2017;103:1165-70. [Crossref] [PubMed]
- Jiang WL, Hu XP, Hu ZP, et al. Morbidity and Mortality of Nosocomial Infection after Cardiovascular Surgery: A Report of 1606 Cases. Curr Med Sci 2018;38:329-35. [Crossref] [PubMed]
- Greco G, Shi W, Michler RE, et al. Costs associated with health care-associated infections in cardiac surgery. J Am Coll Cardiol 2015;65:15-23. [Crossref] [PubMed]
- Allen KB, Fowler VG Jr, Gammie JS, et al. Staphylococcus aureus Infections After Elective Cardiothoracic Surgery: Observations From an International Randomized Placebo-Controlled Trial of an Investigational S aureus Vaccine. Open Forum Infect Dis 2014;1:ofu071. [Crossref] [PubMed]
- Paling FP, Olsen K, Ohneberg K, et al. Risk prediction for Staphylococcus aureus surgical site infection following cardiothoracic surgery; A secondary analysis of the V710-P003 trial. PLoS One 2018;13:e0193445. [Crossref] [PubMed]
- Maillet JM, Oghina G, Le Besnerais P, et al. Preoperative carriage and postoperative same-species sternal wound infection after cardiac surgery. Interact Cardiovasc Thorac Surg 2011;13:381-5. [Crossref] [PubMed]
- Zhou K, Sun F, Xu XL, et al. Prevalences and characteristics of cultivable nasal bacteria isolated from preclinical medical students. J Int Med Res 2020;48:300060520961716. [Crossref] [PubMed]
- Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162-71. [Crossref] [PubMed]
- Segers P, Speekenbrink RG, Ubbink DT, et al. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006;296:2460-6. [Crossref] [PubMed]
- Hong JC, Saraswat MK, Ellison TA, et al. Staphylococcus Aureus Prevention Strategies in Cardiac Surgery: A Cost-Effectiveness Analysis. Ann Thorac Surg 2018;105:47-53. [Crossref] [PubMed]
- Bardia A, Blitz D, Dai F, et al. Preoperative chlorhexidine mouthwash to reduce pneumonia after cardiac surgery: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2019;158:1094-100. [Crossref] [PubMed]
- Wang L, Ji Q, Hu X. Role of targeted and universal mupirocin-based decolonization for preventing surgical-site infections in patients undergoing cardiothoracic surgery: A systematic review and meta-analysis. Exp Ther Med 2021;21:416. [Crossref] [PubMed]
- Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9-17. [Crossref] [PubMed]
- Harbarth S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 2008;299:1149-57. [Crossref] [PubMed]
- Schweizer M, Perencevich E, McDanel J, et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ 2013;346:f2743. [Crossref] [PubMed]
- Saraswat MK, Magruder JT, Crawford TC, et al. Preoperative Staphylococcus Aureus Screening and Targeted Decolonization in Cardiac Surgery. Ann Thorac Surg 2017;104:1349-56. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Chinese-ministry-of-health. Diagnostic Criteria of Nosocomial Infection. Chinese Medical Journal (Chinese) 2001;81:314-20.
- Reddy SL, Grayson AD, Smith G, et al. Methicillin resistant Staphylococcus aureus infections following cardiac surgery: incidence, impact and identifying adverse outcome traits. Eur J Cardiothorac Surg 2007;32:113-7. [Crossref] [PubMed]
- Tom TS, Kruse MW, Reichman RT. Update: Methicillin-resistant Staphylococcus aureus screening and decolonization in cardiac surgery. Ann Thorac Surg 2009;88:695-702. [Crossref] [PubMed]
- Cimochowski GE, Harostock MD, Brown R, et al. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann Thorac Surg 2001;71:1572-8; discussion 1578-9. [Crossref] [PubMed]
- Boyce JM. Preventing staphylococcal infections by eradicating nasal carriage of Staphylococcus aureus: proceeding with caution. Infect Control Hosp Epidemiol 1996;17:775-9. [Crossref] [PubMed]
- Walsh EE, Greene L, Kirshner R. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med 2011;171:68-73. [Crossref] [PubMed]
- Nicolas R, Carricajo A, Morel J, et al. Evaluation of effectiveness and compliance with the mupirocin nasal ointment part of Staphylococcus aureus decolonization in real life using UPLC-MS/MS mupirocin quantification. J Antimicrob Chemother 2020;75:1623-30. [Crossref] [PubMed]
- Pan L, Mo R, Zhou Q, et al. Deep sternal wound infection after cardiac surgery in the Chinese population: a single-centre 15-year retrospective study. J Thorac Dis 2017;9:3031-7. [Crossref] [PubMed]
- Wang DS, Huang XF, Wang HF, et al. Clinical risk score for postoperative pneumonia following heart valve surgery. Chin Med J (Engl) 2021;134:2447-56. [Crossref] [PubMed]
- Michalopoulos A, Geroulanos S, Rosmarakis ES, et al. Frequency, characteristics, and predictors of microbiologically documented nosocomial infections after cardiac surgery. Eur J Cardiothorac Surg 2006;29:456-60. [Crossref] [PubMed]