Safety and effectiveness of neoadjuvant immunotherapy combined with chemotherapy followed by surgical resection in patients with stage I–IIIA small-cell lung cancer: a retrospective single-arm clinical trial
Introduction
Small-cell lung cancer (SCLC) is closely related to a history of heavy smoking (1) and is considered a high-grade neuroendocrine lung carcinoma characterized by rapid growth, early metastasis, aggressiveness and poor differentiation (2,3), accounting for about 13–15% of all lung cancer (4). SCLC has been reckoned as “a graveyard for drug development” with limited successful therapeutic options and a dismal prognosis (2). Approximately one in three patients with SCLC present in the limited-stage (LS) (5), defined as without distant metastasis on the basis of the International Association for the Study of Lung Cancer staging criteria, which corresponds to stages I–IIIB of the TNM staging system (6). With regard to limited-stage SCLC (LS-SCLC), the recommended treatment is early concurrent chemotherapy plus radiotherapy (7). Despite a high sensitivity to chemotherapy and radiotherapy, the cancer almost invariably relapses, and outcomes are not satisfactory, with a 2-year survival of <50% and median survival of 16–24 months, emphasizing the urgent need to improve the efficacy and broaden the scope of current treatment strategies (8,9).
In recent years, immunotherapy with or without chemotherapy has been demonstrated to have a great effect in patients with SCLC, whether as first-line or multi-line therapy (10). The KEYNOTE-028 and KEYNOTE-158 studies showed that immunotherapy could be effective and tolerable for patients with previously treated SCLC (11,12). Results of the Checkmate-032 study demonstrated that immunotherapy as a third-line or later treatment for patients with SCLC could result in survival benefits, and the combination of two PD-L1/PD-1 immune checkpoint inhibitors could produce a better effect (13). The IMPOWER-133 and CASPIAN studies revealed that immunotherapy plus chemotherapy as first-line therapy for SCLC could yield a progression-free survival and overall survival (OS) that are much longer compared with chemotherapy alone (14,15).
Surgery as part of the multimodality therapy of cancer seems to be effective and can improve survival outcomes in patients with early LS-SCLC (16-18). Several studies have demonstrated that surgical resection produces favorable survival for patients with stage I–III LS-SCLC, with a 5-year OS of 27–52% (19-22) and 5-year OS of 58–66% for stage I, 18–56% for stage II, and 13–23% for stage III (23-25). Furthermore, neoadjuvant therapy followed by surgery has proven to be feasible and safe in resectable stage I–III SCLC (26), with a good tolerability and an overall 5-year survival of 33–48% (25,27,28). Fujimori et al. adopted 2–4 cycles of neoadjuvant platinum-based chemotherapy, with an ORR and surgical rate of 95.5% (26). Shepherd et al. demonstrated that the ORR and surgical rate in patients receiving preoperative chemotherapy were 80% and 52.8%, respectively (28). In view of this, we assumed that the tumor could shrink, even achieve down-staging after neoadjuvant immunochemotherapy, and then subsequent tumor resection could remove the tumor more thoroughly and achieve better results. However, there are still few data on whether surgery for stage I–IIIA SCLC can be performed after immunotherapy with chemotherapy. Therefore, we investigated the safety and effectiveness of neoadjuvant immunotherapy combined with chemotherapy followed by surgery in patients with stage I–IIIA SCLC in the hope of adding new ideas to the treatment of SCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1287/rc).
Methods
Patients
This study was designed as a retrospective single-arm clinical trial. Stage I–IIIA SCLC patients who received neoadjuvant immunotherapy with chemotherapy at the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine were consecutively enrolled between 2019 and 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine approved the study (2021 IIT No. 742), and we obtained written consent from patients to access their medical record information. The reportedly average ORR of immunochemotherapy for the first-line treatment of advanced SCLC was 55% (5). We estimate that the ORR of immunochemotherapy for SCLC neoadjuvant therapy is approximately 85%, taking α=0.05 (bilateral), 1−β =0.80, inferring an estimated sample size of 18. The main inclusion criteria for patients were: (I) age >18 and <80 years; (II) histopathologically confirmed SCLC by bronchoscopy or lung aspiration; (III) pretreatment clinical stage I–IIIA SCLC; (IV) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and (V) adequate organ function, sufficient lung and heart function. Patients with the following were excluded: (I) lack of pretreatment imaging assessment in the study hospital; (II) only one imaging assessment; (III) prior anticancer therapy, such as radiotherapy, interventional therapy or drug treatment; (IV) active autoimmune or infectious disease; (V) ongoing systemic immunosuppressive therapy; (VI) clinically significant concurrent malignant tumor; and (VII) active or untreated distant metastases. The primary endpoint of this study was the pathological remission rate after neoadjuvant therapy, and the secondary endpoints were ORR, surgical resection rate and adverse reactions. We obtained follow-up data from the patients’ regular examination or treatment in hospital. If we can’t complete it, contacting patients by telephone or WeChat would be adopted by us. Follow-up was not ended until 3 months after surgery, patient’s decision to cease treatment or termination of the study.
Neoadjuvant therapy procedure
Patients received 2–4 cycles (3 weeks per cycle) of immunotherapy combined with platinum-containing dual-drug chemotherapy (platinum + paclitaxel) before surgery. Immunotherapy comprised camrelizumab 200 mg, nivolumab 3 mg/kg, pembrolizumab 100 or 200 mg, sintilimab 200 mg or tislelizumab 200 mg. The platinum-based chemotherapy comprised cisplatin 75 mg/m2, carboplatin AUC (area under the ROC curve under the drug plasma concentration) =5 or nedaplatin 80 mg/m2, and the paclitaxel regimen was nab-paclitaxel 260 mg/m2 or paclitaxel 175–200 mg/m2. After 2 cycles of neoadjuvant therapy, patients were evaluated as candidates for surgical treatment. If the patient exhibited intolerance to neoadjuvant treatment, it could be altered or postponed as appropriate. If the tumor did not regress significantly, medical therapy continued and the surgical evaluation was performed after 1–2 cycles. If disease progression occurred, we recommended radiotherapy.
Tumor response evaluation
In the week before the neoadjuvant therapy, patients’ baseline data, were obtained, including computed tomography (CT) of the chest and abdomen, bronchoscopy and endoscopic ultrasound, positron emission tomography (PET)-CT, bone emission computed tomography, brain magnetic resonance imaging and abdominal ultrasound. Patients also underwent chest CT every 2 cycles until surgery or withdrawal from the treatment. We used the 8th edition of the AJCC TNM staging (29) to assess tumor location, degree of differentiation, cTNM, ycTNM and ypTNM. Evaluation of the tumor treatment response was performed on the basis of the Response Evaluation Criteria in Solid Tumor version 1.1 (RECST 1.1) (30) when target lesions existed. A complete response (CR) is all target lesions disappearing; partial remission (PR) is a minimum of 30% decline in the total diameter of the target lesions; progressive disease (PD) is enlargement ≥20% in the total diameter of target lesions or the emergence of new lesions; stable disease (SD) is the absence of CR, PR or PD.
Neoadjuvant therapy-related adverse events (AEs)
During therapy, there was continuous monitoring of therapy-related AEs, with routine blood and biochemical blood examinations every week, and myocardial enzyme spectrum, thyroid function, and coagulation function examinations every 3 weeks. Gastrointestinal reactions and skin reactions were self-reported by the patients.
Surgical treatment
Minimally invasive surgery with routine lymph node dissection (at least 2 fields) was the primary surgical approach for SCLC. Data on the duration of operation, estimated blood loss, and length of stay in hospital were recorded.
Pathological examination
Two investigators independently evaluated the pathological images and pathology report, to determine the pathological type, degree of differentiation, depth of invasion, resection margins, lymph nodes and tumor regression grade (TRG). The College of American Pathologists (CAP)/The National Comprehensive Cancer Network (NCCN) guidelines note that the TRG after neoadjuvant therapy is based on the approximate proportion of remaining viable tumor cells in the original cancer area. Furthermore, we divided TRG into four categories: TRG 0 (no remaining active tumor cells), TRG 1 (remaining viable tumor cells ≤10%), TRG 2 (10%< residual active cancer cells ≤50%) and TRG 3 (remaining viable cancer cells >50%). Generally, we considered pathological complete remission (pCR) as equal to TRG 0, and the major pathological response (MPR) referring to residual tumor cells ≤10% as equivalent to TRG 0–1.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, and continuous variables are shown as the median and interquartile range (IQR). In order to study the clinicopathological factors related to treatment response, we classified patients into PR and SD groups based on the tumor treatment response. The chi-square test was used to compare differences between PR and SD groups. For continuous variables, the differences between PR and SD groups were compared with the t-test or Wilcoxon test. All analyses were performed with R software (version 4.1.2). Two-sided P value <0.05 was assessed to be important.
Results
Patients and the treatment process
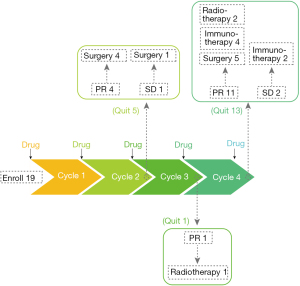
We enrolled 19 patients and an overview of the preoperative treatment process is shown in Figure 1. The operation rate was approximately 52.6% (10/19); 3 patients with PR were reluctant to undergo surgery and chose radiotherapy.
Response to neoadjuvant therapy
The response to treatment was rigorously evaluated in line with RECIST version 1.1. Among the total patients, 16 had PR, and 3 had SD; there were no cases of PD or CR. The objective response rate (ORR) was 84.2% (16/19). Based on the tumor treatment response, we classified the patients into two groups—PR and SD—and their baseline features are summarized in Table 1.
Table 1
| Characteristic | Total, n=19 | PR, n=16 | SD, n=3 |
|---|---|---|---|
| Median age (IQR), years | 66.0 (62.5–68.5) | 66.0 (61.5–68.25) | 64.0 (63.5–67.0) |
| Sex, n (%) | |||
| Male | 16 (84.21) | 13 (81.25) | 3 (100.0) |
| Female | 3 (15.79) | 3 (18.75) | 0 (0) |
| ECOG status, n (%) | |||
| 0 | 13 (68.42) | 11 (68.75) | 2 (66.67) |
| 1 | 6 (31.58) | 5 (31.25) | 1 (33.33) |
| Smoking status, n (%) | |||
| Never | 0 (0) | 0 (0) | 0 (0) |
| Ever | 19 (100.0) | 16 (100.0) | 3 (100.0) |
| Drinking status, n (%) | |||
| Never | 16 (84.21) | 14 (87.5) | 2 (66.67) |
| Ever | 3 (15.79) | 2 (12.5) | 1 (33.33) |
| Comorbidities, n (%) | |||
| Pulmonary disease | 2 (10.53) | 2 (12.5) | 0 (0) |
| Cardiac disease | 4 (21.05) | 4 (25.0) | 0 (0) |
| Diabetes mellitus | 2 (10.53) | 2 (12.5) | 0 (0) |
| Hypertension | 2 (10.53) | 2 (12.5) | 0 (0) |
| Clinical stage, n (%) | |||
| IA | 2 (10.53) | 2 (12.5) | 0 (0) |
| IB | 1 (5.26) | 1 (6.25) | 0 (0) |
| IIB | 2 (10.53) | 1 (6.25) | 1 (33.33) |
| IIIA | 14 (73.68) | 12 (75.0) | 2 (66.67) |
| Treatment cycle, n (%) | |||
| 2 | 5 (26.32) | 4 (25.0) | 1 (33.33) |
| 3 | 1 (5.26) | 1 (6.25) | 0 (0) |
| 4 | 13 (68.42) | 11 (68.75) | 2 (66.67) |
PR, partial remission; SD, stable disease; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group.
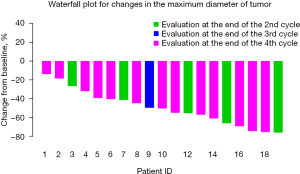
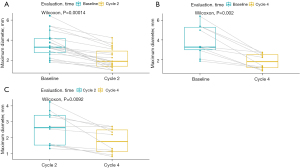
The percentage change in diameter of the maximum target lesion compared with the baseline tumor size is shown in Figure 2, showing that more significant decrease in the diameter of the lesion occurred after 3–4 cycles of neoadjuvant therapy than after 2 cycles of treatment. Therefore, we compared the tumor diameter in each cycle to evaluate the relationship between the number of treatment cycles and the change in the diameter of the tumor. As illustrated in Figure 3, significant shrinkage in tumor size occurred at the end of cycles 2 (Figure 3A), and 4 (Figure 3B) compared with baseline diameter. Tumor diameter at the end of the 4th cycle (Figure 3C) had reduced more than at the end of cycle 2.
The changes in the clinical stage of the patients before (cStage) and after (ycStage) neoadjuvant treatment are shown in Table 2. There was a significant reduction in the number of patients with T3, T2b and T2a, and an obvious increase in the number of patients with T1 after treatment. However, no significant difference in T stage before and after treatment was observed (P=0.345). The changes in N stage were as follows: the number of patients with N2 and N1 decreased, and the number of patients with N0 increased. Furthermore, the changes in N stage before and after treatment were significantly different (P<0.001). For the changes in TNM stage, we found that the number of patients in stage IIIA and IB decreased, and the number of patients in stage IA increased. Moreover, a significant difference in TNM stage before and after treatment was revealed (P=0.015).
Table 2
| Characteristic | cStage (n=19) | ycStage (n=19) | P value |
|---|---|---|---|
| T stage, n (%) | 0.345 | ||
| T1a | 0 (0) | 5 (26.32) | |
| T1b | 3 (15.79) | 7 (36.84) | |
| T1c | 3 (15.79) | 6 (31.58) | |
| T2a | 7 (36.84) | 1 (5.26) | |
| T2b | 4 (21.05) | 0 (0) | |
| T3 | 2 (10.53) | 0 (0) | |
| N stage, n (%) | 0.0004 | ||
| N0 | 3 (15.79) | 7 (36.84) | |
| N1 | 4 (21.05) | 2 (10.53) | |
| N2 | 12 (63.16) | 10 (52.63) | |
| Stage, n (%) | 0.015 | ||
| IA | 2 (10.53) | 7 (36.84) | |
| IB | 1 (5.26) | 0 (0) | |
| IIB | 2 (10.53) | 2 (10.53) | |
| IIIA | 14 (73.68) | 10 (52.63) |
SCLC, small-cell lung cancer.
Surgery and pathological response
Of the 19 patients, 10 eventually underwent surgery, and their outcomes and TRG are summarized in Table 3. The median time from last neoadjuvant therapy to surgery was approximately 27.5 days (IQR, 26.3–31.8 days). Of the 10 surgical patients, 8 underwent minimally invasive surgery, and 2 were converted to open surgery due to severe thoracic adhesions. The median operation time was 131.0 min (IQR, 117.2–156.0 min). The median estimated blood loss during the operation was 50 mL (IQR, 12.5–50.0 mL). Disregarding the lymph nodes used for intraoperative diagnosis, the median number of lymphatic node dissections during surgery was 16.0 (IQR, 11.3–25.8). In total, 9 patients (90.0%) had R0 resection, and 1 had R1 resection. The median length of hospital stay was 15.5 days (IQR, 10.5–13.7 days). There were no perioperative deaths, and 1 case of postoperative pyothorax. As for the pathological response, there were 3 patients (30.0%) with TRG 0, 1 (10.0%) with TRG 1, and 6 (60.0%) with TRG 2 and TRG 3. The rates of MPR and pCR were 40.0% and 30.0%, respectively.
Table 3
| Outcomes | Value (n=10) |
|---|---|
| Time from last neoadjuvant therapy to surgery, median (IQR), days | 27.5 (26.3–31.8) |
| Operation type, n (%) | |
| Minimally invasive | 8 (80.0) |
| Minimally invasive to open | 2 (20.0) |
| Operation time, median (IQR), min | 131.0 (117.2–156.0) |
| Estimated blood loss, median (IQR), mL | 50.0 (12.5–50.0) |
| Total No. of lymph node dissections during surgery, median (IQR), n | 16.0 (11.3–25.8) |
| Surgical margin, n (%) | |
| R0 resection | 9 (90.0) |
| R1 resection | 1 (10.0) |
| Length of hospital stay, median (IQR), days | 15.5 (10.5–13.7) |
| Postoperative complications, n (%) | |
| Pyothorax | 1 (10.0) |
| ypTNM stage, n (%) | |
| IA | 5 (50.0) |
| IIB | 2 (20.0) |
| IIIA | 3 (30.0) |
| Pathological response, n (%) | |
| TRG 0 | 3 (30.0) |
| TRG 1 | 1 (10.0) |
| TRG 2 and 3 | 6 (60.0) |
SCLC, small-cell lung cancer; IQR, interquartile range; TRG, tumor regression grade.
Toxicity
None of the patients withdrew from neoadjuvant therapy because of intolerable toxicity or PD or previously undocumented toxicities. The toxic effects of the neoadjuvant therapy are summarized in Table 4. Grade 1–2 AEs were most common, and fewer suffered grade 3 or 4 AEs (1 case of anemia and 1 case of constipation), with symptomatic management quickly resolving all cases.
Table 4
| Toxicities | None | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Hematologic | |||||
| Leukopenia | 17 | 2 | 0 | 0 | 0 |
| Agranulocytosis | 18 | 1 | 0 | 0 | 0 |
| Anemia | 7 | 7 | 4 | 0 | 1 |
| Thrombocytopenia | 19 | 0 | 0 | 0 | 0 |
| Gastrointestinal | |||||
| Nausea | 13 | 6 | 0 | 0 | 0 |
| Emesis | 13 | 6 | 0 | 0 | 0 |
| Diarrhea | 19 | 0 | 0 | 0 | 0 |
| Constipation | 9 | 5 | 4 | 1 | 0 |
| Hepatic injury | 15 | 4 | 0 | 0 | 0 |
| Renal injury | 18 | 1 | 0 | 0 | 0 |
| Skin reaction | 18 | 1 | 0 | 0 | 0 |
| Hypothyroidism | 19 | 0 | 0 | 0 | 0 |
Discussion
The role of surgery and the indications for surgical intervention in SCLC remains debatable. Two large, randomized, prospective trials conducted by the British Medical Research Council in the 1960s and 1970s compared surgery with radical radiotherapy in patients with LS-SCLC, and showed that radical radiotherapy was better than surgery with superior OS (31,32), which led to the cessation of treatment based on surgery alone. Then the Lung Cancer Study Group launched a large multicenter randomized phase III trial to estimate the effect of surgery in multimodal therapy (33). Patients after neoadjuvant chemotherapy were randomly assigned to a surgery or a non-surgery group. There was no difference in OS between the two arms and the 2-year survival for both was 20%. On the basis of that trial, surgery as part of multimodality therapy was largely uncommon. However, some prospective and retrospective studies subsequently affirmed the practicability of surgery as part of multimodality management, including before and after chemotherapy in patients with early LS-SCLC (23-26). In addition, a meta-analysis that enrolled 2 randomized trials and 13 retrospective studies demonstrated that surgery produced significant survival outcomes compared with the non-surgical approach in patients with stage I–III LS-SCLC (34). The NCCN Clinical Practice Guidelines now recommend surgical resection for patients in stage I (T1-2N0M0) (23).
It has also been shown previously that neoadjuvant therapy followed by surgery is feasible and effective in patients with stage I–IIIA SCLC (35). Shepherd et al. demonstrated that the ORR and surgical rate in patients receiving preoperative chemotherapy (cyclophosphamide, doxorubicin and vincristine or cisplatin and etoposide) were 80% and 52.8%, respectively (28). Lad et al. showed that in patients receiving preoperative chemotherapy (cyclophosphamide, doxorubicin, and vincristine), the ORR was 66% and the surgical rate was 83% (33). Fujimori et al. adopted 2–4 cycles of neoadjuvant platinum-based chemotherapy, with an ORR and surgical rate of 95.5% (26). Our study found that neoadjuvant immunotherapy and chemotherapy in patients with stage I–IIIA SCLC were associated with a high ORR (84.2%) and surgical rate (52.6%), similar to the other studies. We also found that the diameter of the lesion after 4 cycles of neoadjuvant therapy decreased more than after 2 cycles of treatment. Therefore, we suggest that more cycles of neoadjuvant management are feasible for patients without significant remission after 2 cycles of neoadjuvant treatment. The rates of pCR and MPR in our study were 30.0% and 40.0%, respectively. Lad et al. reported their pCR rate as 19% (33). The reason for the discrepancy may be due to the non-platinum chemotherapy regimen in the latter study. We also found that the number of patients in stage II–III decreased, and the number of patients in stage I increased. Among the enrolled patients, those with stage II–III accounted for 84.2%, but after treatment, it was 63.2%, which suggests that neoadjuvant treatment can lead to significant downstaging and increase the possibility of surgery.
We used the 8th edition of the AJCC TNM staging to stage the patients with LS-SCLC in our study. Currently, the widely accepted Veterans Administration staging criteria remains sufficient to guide radiotherapy and chemotherapy in patients with SCLC, but is not suitable when surgery is considered as a treatment modality, because it requires more detailed clinical staging classification. TNM classification can reflect the prognosis of LS-SCLC correctly, and is the most useful.
Immune-related AEs were manageable and tolerable. Most were grade 1–2. The incidence of grade 3 and grade 4 AEs was 5.3% for both. None of the patients in our study had previously undocumented toxicities.
The limitations of our study include the small sample size and retrospective nature, the absence of a randomized control group, short postoperative follow-up, and heterogeneity of patients and treatment regimens. These factors may limit the statistical power of this study and result in selection biases. To eliminate selection bias to a certain extent and make the results representative, we consecutively enrolled patients who met the inclusion criteria.
In conclusion, neoadjuvant immunotherapy combined with chemotherapy followed by surgical resection for patients with stage I–IIIA SCLC was effective and safe with a tolerable toxicity profile. However, larger, randomized controlled trials are required to confirm our findings. And whether a neoadjuvant therapy regimen gives a survival benefit should be confirmed by further follow-up.
Acknowledgments
Funding: This research was supported by the Zhejiang Province Major Science and Technology Special Program Project (grant No. 2020C03058), the Zhejiang Province Lung Tumor Diagnosis and Treatment Technology Research Supported by the Center (grant No. JBZX-202007), the Zhejiang Provincial Traditional Chinese Medicine (Integrated Traditional Chinese and Western Medicine) Key Discipline (grant No. 2017-XK-A33), and the Zhejiang Provincial Natural Science Foundation (grant No. LY19H160039).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1287/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1287/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1287/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine approved the study (2021 IIT No. 742), and we obtained written consent from patients for use of their medical record information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res 2014;12:3-13. [Crossref] [PubMed]
- Taniguchi H, Sen T, Rudin CM. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front Oncol 2020;10:741. [Crossref] [PubMed]
- Raso MG, Bota-Rabassedas N, Wistuba II. Pathology and Classification of SCLC. Cancers (Basel) 2021;13:820. [Crossref] [PubMed]
- Saltos A, Shafique M, Chiappori A. Update on the Biology, Management, and Treatment of Small Cell Lung Cancer (SCLC). Front Oncol 2020;10:1074. [Crossref] [PubMed]
- Wang S, Zimmermann S, Parikh K, et al. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin Proc 2019;94:1599-622. [Crossref] [PubMed]
- Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer 2002;37:271-6. [Crossref] [PubMed]
- Kubota K, Hida T, Ishikura S, et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet Oncol 2014;15:106-13. [Crossref] [PubMed]
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116-25. [Crossref] [PubMed]
- Welsh JW, Heymach JV, Guo C, et al. Phase 1/2 Trial of Pembrolizumab and Concurrent Chemoradiation Therapy for Limited-Stage SCLC. J Thorac Oncol 2020;15:1919-27. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:3740-8. [Crossref] [PubMed]
- Ott PA, Bang YJ, Piha-Paul SA, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol 2019;37:318-27. [Crossref] [PubMed]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]
- Ready NE, Ott PA, Hellmann MD, et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol 2020;15:426-35. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Zhong L, Suo J, Wang Y, et al. Prognosis of limited-stage small cell lung cancer with comprehensive treatment including radical resection. World J Surg Oncol 2020;18:27. [Crossref] [PubMed]
- Casiraghi M, Sedda G, Del Signore E, et al. Surgery for small cell lung cancer: When and how. Lung Cancer 2021;152:71-7. [Crossref] [PubMed]
- Farre N, Belda-Sanchis J, Guarino M, et al. The current role of surgery and SBRT in early stage of small cell lung cancer. J Clin Transl Res 2021;7:34-48. [PubMed]
- Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350-7. [Crossref] [PubMed]
- Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267-71. [Crossref] [PubMed]
- Badzio A, Kurowski K, Karnicka-Mlodkowska H, et al. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg 2004;26:183-8. [Crossref] [PubMed]
- Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615-9. [Crossref] [PubMed]
- Takenaka T, Takenoyama M, Inamasu E, et al. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung Cancer 2015;88:52-6. [Crossref] [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [Crossref] [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [Crossref] [PubMed]
- Fujimori K, Yokoyama A, Kurita Y, et al. A pilot phase 2 study of surgical treatment after induction chemotherapy for resectable stage I to IIIA small cell lung cancer. Chest 1997;111:1089-93. [Crossref] [PubMed]
- Hara N, Ohta M, Ichinose Y, et al. Influence of surgical resection before and after chemotherapy on survival in small cell lung cancer. J Surg Oncol 1991;47:53-61. [Crossref] [PubMed]
- Shepherd FA, Ginsberg RJ, Patterson GA, et al. A prospective study of adjuvant surgical resection after chemotherapy for limited small cell lung cancer. A University of Toronto Lung Oncology Group study. J Thorac Cardiovasc Surg 1989;97:177-86. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Miller AB, Fox W, Tall R. Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. Lancet 1969;2:501-5. [Crossref] [PubMed]
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 1973;2:63-5. [Crossref] [PubMed]
- Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S-3S. [Crossref] [PubMed]
- Liu T, Chen Z, Dang J, et al. The role of surgery in stage I to III small cell lung cancer: A systematic review and meta-analysis. PLoS One 2018;13:e0210001. [Crossref] [PubMed]
- Martucci N, Morabito A, La Rocca A, et al. Surgery in Small-Cell Lung Cancer. Cancers (Basel) 2021;13:390. [Crossref] [PubMed]