Identifying biomarkers of ventilator induced lung injury during one-lung ventilation surgery: a scoping review
Introduction
Thoracic surgery is part of curative therapy for lung cancer, which is the leading cause of cancer-related mortality worldwide (1). Thoracic surgery is also used to treat many other malignant and benign conditions, such as the respiratory failure induced by SARS-Cov-2 (2). The majority of thoracic surgery requires that the operated lung be isolated from the contralateral lung, and not ventilated during the operation. Thus, the non-operated lung experiences one-lung ventilation (OLV). It is known that undergoing mechanical ventilation puts patients at risk for ventilator induced lung injury (VILI) (3-5). This has been shown in patients undergoing prolonged two-lung ventilation (TLV) (i.e., many days), predominantly in the intensive care setting. Some evidence also exists for the role of VILI in patients undergoing surgery requiring shorter periods of exposure to TLV (4). Although the exact mechanisms that underlie VILI have yet to be elucidated, studies to date have suggested that the main risk factors for OLV-induced VILI are iatrogenic (6). These risk factors include high tidal volume (Vt), high airway pressures and high fraction of inspired oxygen (FiO2) all of which can contribute to lung parenchyma stress and mechanical injury that induces inflammation (7). Common pro-inflammatory cytokines, such as TNFα and IL-6, are increased in patients that have undergone OLV, while anti-inflammatory cytokines like IL-10 are decreased in abundance (8-10). Based on current knowledge and understanding, we hypothesize that patients undergoing OLV are at greater risk for VILI compared to patients undergoing TLV for two major reasons: (I) OLV is primarily used in a population that already has pre-existing lung damage; and (II) in OLV, all the potentially injurious factors are exerted on one lung rather than being distributed between two lungs.
One of the major causes of mortality after thoracic surgery is acute lung injury (ALI) or ARDS. ALI can occur in up to 4% of patients after OLV, with a mortality rate as high as 70% (4,5,11-13). Thus, OLV has potential to be a major risk factor for ALI after thoracic surgery, but the pathophysiology and determinants of OLV-induced VILI are poorly understood (4,12).
Studying and identifying these biomarkers is important as it will facilitate identification of high-risk patients for pre-operative, intra-operative and post-operative interventions to reduce the risk of VILI and respiratory complications. It is also important as a method of monitoring/tracking the response to such interventions. Moreover, studying and identifying these biomarkers is also important for studies that aim to understand the mechanisms behind such interventions. The aim of this scoping review is to identify biomarkers that have been linked to post-OLV lung injury. By providing an analysis of the current state of the literature, we hope to identify knowledge gaps, and provide guidance for further identification of reliable biomarkers for post-OLV VILI. We present the following article in accordance with the PRISMA-ScR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-20-2301/rc).
Methods
Study inclusion was based on PIO criteria. Population: All human and animal studies that used OLV as an exposure and investigated any analyte response as an outcome were considered. Studies using neonate models were excluded. Only English language studies or studies with an English translation were included. Intervention: Studies must utilize OLV. Studies that induced lung injury unrelated to VILI (i.e., lipopolysaccharide-induced injury) were excluded. Outcome: Studies must measure the effect of one lung ventilation on a biomarker.
In collaboration with a librarian (TG), a highly sensitive search strategy was used (Tables S1,S2). Electronic databases EMBASE, SCOPUS, and Medline were searched (inception to January 7, 2021). A gray literature search was conducted by an expert in this research area (BK). Bibliographies of included studies were hand-searched for relevant studies.
After abstract eligibility screening the data extracted from each study was; Title, year, authors, citation count, sample size, population type (type of animal or human), VILI induction method, blood product tested, intraoperative or post-operative measurement, histological findings, biomarker quantification method, biomarkers quantified and biomarker response including direction, magnitude, and statistical significance.
A calibration exercise was conducted initially for screening. During this calibration, screening of 50 studies was performed independently by 2 authors (BK and AB). Once calibration was achieved, all further screening was performed by a single author (AB) who assessed titles and abstracts for inclusion eligibility. Full text analysis, assessment for final inclusion and data extraction of the included studies was conducted independently by 2 authors (AB and RM). Chance-corrected agreement was calculated. All disagreements were resolved through discussion and consensus without the need for a third reviewer (BK).
Results
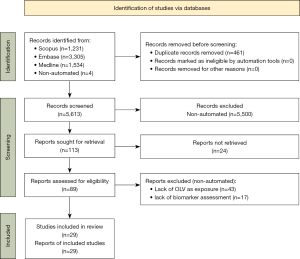
The search yielded 5,609 results after duplicates were removed with 4 manual additions. Twenty-nine studies were included for data synthesis as summarized in Table 1; the most common reason for exclusion was lack of OLV as an exposure, followed by lack of biomarker measurement (Figure 1).
Table 1
| Year, author (reference) | Model [n] | Intervention groups |
|---|---|---|
| 2011 Bastin (8) | Human [30] | OLV group: Vt 6.60±1.50 mL/kg; duration 147 min (121–196 min); plateau pressure 23.00±4.50 cmH2O |
| 2011 Breunig (14) | Human [15] | OLV group: Vt <7 mL/kg; PEEP 3–5 cmH2O; PIP <30 cmH2O; FiO2 set to 1.0 initially, gradually reduced based on arterial oxygen tension |
| 2020 Dai (15) | Pig [15] | Control: Vt 10 mL/kg; PEEP 5 cmH2O; FiO2 0.5; left pneumonectomy |
| Volume-control: Vt 20 mL/kg; PEEP 5 cmH2O; RR to maintain ETCO2 35–45 mmHg; FiO2 0.5; left pneumonectomy | ||
| Adaptive-control: ASV% of 60% minute ventilation of two lungs to maintain ETCO2; left pneumonectomy | ||
| 2015 Feng (16) | Human [30] | Propofol group: Vt 6–8 mL/kg; RR 14–16/min; I:E 1:2; ETCO2 35–45 mmHg; FiO2 1.0; anaesthetic: propofol |
| Sevoflurane group: Vt 6–8 mL/kg; RR 14–16 breath/min; I:E 1:2; ETCO2 35–45 mmHg; FiO2 1.0; anaesthetic: sevoflurane | ||
| 2018 Fiorelli (17) | Human [28] | OLV group: Vt 8–10 mL/kg; PEEP 5 cmH2O; PIP 35 cmH2O; FiO2 0.5 |
| 2017 de la Gala (18) | Human [174] | Propofol group: Vt 6 mL/kg; PEEP 5 cmH2O; FiO2 0.6–1; SaO2 >90%; permissive hypercapnia; anaesthetic: propofol |
| Sevoflurane group: Vt 6 mL/kg; PEEP 5 cmH2O; FiO2 0.6–1; SaO2 >90%; permissive hypercapnia; anaesthetic: sevoflurane | ||
| 2003 Gama de Abreu (19) | Female European rabbit [18] (Isolated perfused lung model) | OLV group: Vt 8 mL/kg; RR 30/min; PEEP 1 cmH2O; I:E 1:1; recruitment maneuver every 30 min |
| Protective OLV group: Vt 8 mL/kg; RR 30/min; PEEP 1 cmH2O; I:E 1:1; recruitment maneuver every 10 min | ||
| TLV group: Vt 8 mL/kg; RR 30/min; PEEP 1 cmH2O; I:E 1:1; recruitment maneuver every 10 min | ||
| 2015 García-de-la-Asunción (20) | Human [28] | OLV group: Vt 6 mL/kg; RR 12–14/min; PEEP 5–7 cmH2O; I:E 1:2; ETCO2 <40 mmHg; FiO2 0.5; SaO2 >92% |
| 2017 Liu (21) | Japanese white rabbit [36] | Sham: ventilatory parameters not specified |
| OLV: ventilatory parameters not specified | ||
| OLV + sevoflurane (four groups): 1%, 2%, 3%, 4% sevoflurane concentrations tested | ||
| 2018 Liu (22) | Dog [18] | OLV 100% collapsed: Vt 10–15 mL/kg; duration 2 h; RR 12–16/min; FiO2 1; ETCO2 35–45 mmHg; right lung collapsed fully |
| OLV 90% collapsed: Vt 10–15 mL/kg; duration 2 h; RR 12–16/min; FiO2 1; ETCO2 35–45 mmHg; right lung 90% collapsed | ||
| OLV 50% collapsed: Vt 10–15 mL/kg; duration 2 h; RR 12–16/min; FiO2 1; ETCO2 35–45 mmHg; right lung 50% collapsed | ||
| 2013 Liu (23) | New Zealand Rabbit [36] | OLV group: Vt 10 mL/kg; duration 0, 2, or 4 h; RR 50/min; PEEP 0 cmH2O; I:E 1:1; FiO2 0.4 |
| TLV group: Vt 10 mL/kg; duration 0, 2, or 4 h; RR 50/min; PEEP 0 cmH2O; I:E 1:1; FiO2 0.4 | ||
| 2018 Liu (9) | Human [60] | OLV group: Vt 10 mL/kg; RR 12/min; PEEP 0 cmH2O; I:E 1:1.5; ETCO2 35–40 mmHg; FiO2 1.0; no inspiratory time pause |
| OLV protective ventilation group: Vt 6 mL/kg; RR 12/min; PEEP 6 cmH2O; I:E 1:1.5; ETCO2 35–40 mmHg; FiO2 1.0; no inspiratory time pause | ||
| 2011 Mahmoud (24) | Human [50] | Propofol group: Vt 10 mL/kg; duration 80 min; RR set to maintain ETCO2 45 mmHg; PEEP 5 cmH2O; PIP 30 cmH2O; FiO2 0.8–1.0; PaO2 80 mmHg; anaesthetic: propofol |
| Isoflurane group: Vt 10 mL/kg; duration 78 min; RR set to maintain ETCO2 35–45 mmHg; PEEP 5 cmH2O; PIP 30 cmH2O; FiO2 0.8–1.0; PaO2 80 mmHg; anaesthetic: isoflurane | ||
| 2020 Pan (25) | Sprague-Dawley rat [30] | Sham: no ventilation |
| OLV-2 h: Vt 10 mL/kg; duration 2 h; RR 60/min; I:E 1:1.5; FiO2 1.0 | ||
| OLV-3 h: Vt 10 mL/kg; duration 3 h; RR 60/min; I:E 1:1.5; FiO2 1.0 | ||
| LIG-2 h: Vt 10 mL/kg; duration 2 h; RR 60/min; I:E 1:1.5; FiO2 1.0; Ligustrazine HCl injection 100 mg/kg 30 min prior OLV | ||
| LIG-3 h: Vt 10 mL/kg; duration 3 h; RR 60/min; I:E 1:1.5; FiO2 1.0; Ligustrazine HCl injection 100 mg/kg 30 min prior OLV | ||
| 2005 Schilling (26) | Human [32] | OLV tradition ventilation group: Vt 10 mL/kg; duration 71 min; RR set for PaCO2 35–45 mmHg; PEEP 0 cmH2O |
| OLV protective ventilation group: Vt 5 mL/kg; duration 68 min; RR set for PaCO2 35–45 mmHg; PEEP 0 cmH2O | ||
| 2007 Schilling (10) | Human [50] | Propofol group: Vt 10 mL/kg; duration 65 min; RR set for PaCO2 4.8–5.8 kPa; PEEP 5 cmH2O; PIP 35 cmH2O; FiO2 between 0.8 to 1.0; anaesthetic: propofol |
| Desflurane group: Vt 10 mL/kg; duration 61 min; RR set for PaCO2 4.8–5.8 kPa; PEEP 5 cmH2O; PIP 35 cmH2O; FiO2 between 0.8 to 1.0; anaesthetic: desflurane | ||
| 2011 Schilling (27) | Human [63] | Propofol group: RR set for PaCO2 36–44 mmHg; PEEP 5 cmH2O; PIP 30 cmH2O; FiO2 0.4–0.5; pressure-controlled ventilation; anaesthetic: propofol 1.5–2 mg/kg |
| Desflurane group: RR set for PaCO2 36–44 mmHg; PEEP 5 cmH2O; PIP 30 cmH2O; FiO2 0.4–0.5; pressure-controlled ventilation; anaesthetic: desflurane 1 min alveolar concentration per air | ||
| Sevoflurane group: RR set for PaCO2 36–44 mmHg; PEEP 5 cmH2O; PIP 30 cmH2O; FiO2 0.4–0.5; pressure-controlled ventilation; anaesthetic: sevoflurane 1 min alveolar concentration per air | ||
| 2006 Schreiber (28) | Male Wistar Rat [26] | High Vt group: Vt 20 mL/kg; OLV duration 2 h; RR 40 breath/min; PEEP 4.5 cmH2O; FiO2 0.5 |
| Low Vt group: Vt 8 mL/kg; OLV duration 2 h; RR 40 breath/min; PEEP 4.5 cmH2O; FiO2 0.5 | ||
| 2012 Siegl (29) | Female BALB/c and C57BL/6 mice [24] (isolated perfused lung model) | Balb/c groups |
| High pressure group: Vt 200 μL; duration 240 min; RR 90/min; EIP −25 cmH2O; EEP −3 cmH2O; deep breath (30 cmH2O) every 5 min; lungs perfused with 0.5 mM p38; MAPK inhibitor SB203580 | ||
| Low pressure group: Vt 200 μL; duration 240 min; RR 90/min; EIP −8 cmH2O; EEP −3 cmH2O; deep breath (30 cmH2O) every 5 min; lungs perfused with 0.5 mM p38; MAPK inhibitor SB203580 | ||
| C57BL/6 groups | ||
| High pressure group: Vt 200 μL; duration 240 min; RR 90/min; EIP −25 cmH2O; EEP −3 cmH2O; deep breath (30 cmH2O) every 5 min | ||
| Low pressure group: Vt 200 μL; duration 240 min; RR 90/min; EIP −8 cmH2O; EEP −3 cmH2O; deep breath (30 cmH2O) every 5 min | ||
| 2018 Tan (30) | Human [60] | Pressure controlled ventilation group: Pressure set for Vt of 6 mL/kg; PETCO2 30–45 mmHg; PEEP 0 cmH2O; FiO2 1.0; oxygen flow rate 1 L/min |
| Volume controlled ventilation group: Vt 6 mL/kg; PETCO2 30–45 mmHg; PEEP 0 cmH2O; FiO2 1.0; oxygen flow rate 1 L/min | ||
| 2015 Tojo (31) | Specific pathogen free male Sprague-Dawley rat [32] | Bilateral vs. unilateral ventilation experiment |
| Bilateral ventilation: Vt 8 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 1.0 reduced to 0.6 | ||
| Unilateral ventilation: Vt 8 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 1.0 reduced to 0.6. Right lung collapsed | ||
| 60% O2vs. 100% N2 high Vt experiment | ||
| Unilateral ventilation group: Vt 8 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 1.0 reduced to 0.6. Right lung collapsed | ||
| Bilateral 60% oxygen group: Left lung—Vt 8 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 0.6. Right lung—Vt 4 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 0.6 | ||
| Bilateral 0% oxygen group: Left lung—Vt 8 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 0. Right lung—Vt 4 mL/kg; RR 80/min; PEEP 4 cmH2O; FiO2 0 | ||
| 2017 Xu (32) | New Zealand Rabbit [30] | Sham TLV group: Vt 10 mL/kg; 3 h duration; RR 40/min; PEEP 0 cmH2O; I:E 1:2; FiO2 0.6 |
| 1.0 FiO2 OLV group: Vt 10 mL/kg; 3 h duration; RR 40/min; PEEP 0 cmH2O; I:E 1:2; FiO2 1.0 | ||
| 0.6 FiO2 OLV group: Vt 10 mL/kg; 3 h duration; RR 40/min; PEEP 0 cmH2O; I:E 1:2; FiO2 0.6 | ||
| 2018 Yang (33) | Japanese white rabbit [36] | Sham-operated group: TLV; Vt 20 mL/kg; duration 2 h; RR 30/min; I:E 1:2; FiO2 1 |
| OLV group: Right lung OLV; Vt 20 mL/kg; duration 2 h; RR 30/min; I:E 1:2; FiO2 1 | ||
| OLV + sevoflurane inhalation group: Vt 20 mL/kg; duration 2 h; RR 30/min; I:E 1:2; FiO2 1; sevoflurane 2.5% used | ||
| Club cells exfoliated + sham-operated group: TLV; Vt 20 mL/kg; duration 2 h; RR 30/min; I:E 1:2; FiO2 1; exposure to naphthalene vapour 100 mg/L for 12 h | ||
| Club cells exfoliated + OLV group: right lung OLV; Vt 20 mL/kg; duration 2 h; RR 30/min; I:E 1:2; FiO2 1 | ||
| Club cells exfoliated + OLV + sevoflurane inhalation group: Right lung OLV; Vt 20 mL/kg; duration 2 h; RR 30/min; I:E 1:2; FiO2 1; exposure to naphthalene vapour 100 mg/L for 12 h; sevoflurane 2.5% used | ||
| 2020 Yao (34) | Human [60] | Volume controlled ventilation group: VCV mode gradually increases flow rate and pressure; Vt 6 mL/kg; RR 14–18/min; PEEP; lung cancer patients undergoing thoracoscopic lobectomy |
| Pressure controlled ventilation-volume guaranteed group: PCV-VG mode delivers Vt at lowest preset pressure; Vt 6 mL/kg; RR 14–18/min; PEEP; lung cancer patients undergoing thoracoscopic lobectomy | ||
| 2019 Yin (35) | New Zealand white rabbit [24] | TLV-S group: Vt 6 mL/kg; RR 40/min; PEEP 3 cmH2O; PIP <20 cmH2O; FiO2 1.0; I:E 1:1.5. Treatment order: TLV 2.5 h. Intraperitoneal saline: 1.5 mL/kg; TLV 1 h |
| OLV-S group: Vt 6 mL/kg; RR 40/min; PEEP 3 cmH2O; PIP <20 cmH2O; FiO2 1.0; I:E 1:1.5. Treatment order: OLV 2.5 h. Intraperitoneal saline: 1.5 mL/kg; OLV 0.5 h; TLV 0.5 h | ||
| U-OLV group: Vt 6 mL/kg; RR 40/min; PEEP 3 cmH2O; PIP <20 cmH2O; FiO2 1.0; I:E 1:1.5. Treatment order: Intraperitoneal URB937 1.5 mL/kg; OLV 3 h; TLV 0.5 h | ||
| OLV-U group: Vt 6 mL/kg; RR 40/min; PEEP 3 cmH2O; PIP <20 cmH2O; FiO2 1.0; I:E 1:1.5. Treatment order: OLV 2.5 h; intraperitoneal URB937 1.5 mL/kg; OLV 0.5 h; TLV 0.5 h | ||
| 2012 You (36) | Japanese rabbit [30] | Sham OLV group: Vt 10 mL/kg; RR 40 /min; I:E 1:2; FiO2 1.0; Sham tracheostomy |
| OLV group: Vt 10 mL/kg; RR 40 /min; I:E 1:2; FiO2 1.0 | ||
| TLV group: Vt 10 mL/kg; RR 40 /min; I:E 1:2; FiO2 1.0 | ||
| OLV PDTC group: Vt 10 mL/kg; RR 40 /min; I:E 1:2; FiO2 1.0; pretreatment with 50 mg/kg NF-κB inhibitor pyrrolidine dithiocarbamate | ||
| TLV PDTC group: Vt 10 mL/kg; RR 40 /min; I:E 1:2; FiO2 1.0; pretreatment with 50 mg/kg NF-κB inhibitor pyrrolidine dithiocarbamate | ||
| 2019 Zeng (37) | Sheep [6] | OLV group: Vt 10 mL/kg; duration 8 h; PEEP 0–2 cmH2O; left thoracotomy performed for lung collapse; collapsed and aerated lungs compared |
| 2016 Zhang (38) | Human [60] | Inverse ratio group: Vt 7 mL/kg; RR 12 breath/min; PEEP 5 cmH2O; FiO2 1.0; I:E 2:1 |
| Control group: Vt 7 mL/kg; RR 12 breath/min; PEEP 5 cmH2O; FiO2 1.0; I:E 1:2 | ||
| 2020 Zhao (39) | Human [121] | Sham group: Vt 8–10 mL/kg; RR 10–15/min; FiO2 1.0; I:E 1.15; OLV with operated lung collapse; patients received the electrodes without electrical stimulation. |
| TEAS group: Vt 8–10 mL/kg; RR 10–15/min; FiO2 1.0; I:E 1.15; OLV with operated lung collapse; prior to anaesthesia patients received transcutaneous electrical acupoint stimulation. Patients received acupuncture at 6 locations, with electrical stimulation of 100 Hz for 10 s with 3 s intervals, with 20–25 mA |
OLV, one-lung ventilation; Vt, tidal volume; PEEP, positive end expiratory pressure; PIP, peak inspiratory pressure; FiO2, fraction of inspired oxygen; RR, respiratory rate; ETCO2, end-tidal CO2 partial pressure; ASV, adaptive support ventilation; I:E, inspiration:expiration ratio; TLV, Two-lung ventilation; SaO2, Oxygen saturation; HCl, hydrochloride; MAPK, mitogen-activated protein kinase; EIP, end-inspiratory pressure; EEP, end-expiratory pressure; PETCO2, patient end-tidal carbon dioxide; VCV, volume control ventilation; PCV-VG, pressure-controlled ventilation-volume guaranteed; OLV-S, one-lung ventilation + saline; U-OLV, intraperitoneal URB937 + one-lung ventilation; OLV-U, one-lung ventilation + intraperitoneal URB937; PDTC, pyrrolidine dithiocarbamate; TEAS, transcutaneous electrical acupoint stimulation.
Table 1 describes the study subjects, and the ventilatory parameters, ventilation mode (e.g., the methodological designs were limited to human observational studies, human interventional studies testing anaesthetic effects, and animal studies. The most common model was human patients (52% of studies). Of the animal studies 50% utilized rabbit and 22% used rat. Mouse, pig, sheep, and dog were each used by a single study (15,22,29,37). Of the human studies, the average sample size was 57, with an average Vt of 7.8 mL/kg in the VILI group. Of the animal studies, the average sample size was 25. A quarter of the human studies were interventional in nature, testing the effect of different anaesthetic methods on cytokine levels, or investigating the difference between two ventilation strategies.
Table 2 describes the analytical methodology used to detect markers and the major findings of each study. The most commonly used analytic method to detect biomarkers was enzyme-linked immunoassay, which was used in 69% (n=20) of studies. The most used sample type was blood (45%, n=13), followed by bronchoalveolar lavage fluid (BALF) (41%, n=12) and tissue (41%, n=12). Exhaled breath condensate was used in three studies (8,15,20) and plural fluid and perfusate were both used singularly by Gama de Abreu et al. (19).
Table 2
| Study | Marker test | Major finding |
|---|---|---|
| 2011 Bastin (8) | Plasma analytes: ELISA; vWF: immunoturbidimetric method (latex agglutination) | Exhaled breath condensate pH ↑. Plasma KL-6 and SP-D ↓. Plasma RAGE, vWF, and IL-6 ↑ |
| 2011 Breunig (14) | ELISA: BALF, pleural fluid, blood | BALF, blood, pleural fluid IL-6 ↑ post-operative BALF, pleural fluid IL-1RA ↑. Blood no change. GROα ↑ in pleural fluid, BALF no change, blood undetected |
| 2020 Dai (15) | ELISA: BALF, EBC, venous serum; RT-PCR: lung tissue miRNA | BALF IL-1β, IL-6, IL-8, and total protein ↑ all groups. BALF TNF-α no change all groups. Serum IL-1β, IL-6 ↑. EBC IL-1β and IL-6 ↑ after 3 h OLV. Tissue miR-144-5p, miR-449-3p and miR-451 ↑ in more damaging ventilatory mode |
| 2015 Feng (16) | Blood MDA: TBA method; total protein: Bradford assay; lung tissue HO-1 protein expression: Western blot; lung tissue HO-1 mRNA: RT-PCR | Venous blood MDA ↑ with sevoflurane, ↑↑ with propofol. Venous blood HO-1 ↑ with propofol, ↑↑ with sevoflurane. Lung tissue HO-1 mRNA ↑ with propofol, ↑↑ with sevoflurane |
| 2018 Fiorelli (17) | Markers analyzed with cytokine & growth factor arrays from Evidence Investigator Biochip Array technology® | BALF IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α, VEGF, EGF, and MCP-1 no change. BALF IL-8 and IFN-ɣ out of detection range |
| 2017 de la Gala (18) | Western blot: BALF, blood samples | BALF and arterial blood VEGF, IL-2, IL-4, IL-7, and IL-8 ↑ in all groups. Arterial MCP-1 ↑ in all groups. BALF MCP-1, IL-12 no change in all groups. BALF and arterial blood TNF-α, IL-1, and IL-6 ↑ with sevoflurane and ↑↑ with propofol. BALF and arterial blood IL-10 ↑ with sevoflurane, ↓ with propofol |
| 2003 Gama de Abreu (19) | Perfusate TXB2: ELISA | Lung perfusate TXB2 ↑, pH no change |
| 2015 García-de-la-Asunción (20) | EBC and plasma 8-iso-PGF2α : competitive enzyme immunoassay after alkaline hydrolysis | EBC H2O2 ↑ during 20 min after resuming TLV. EBC 8-iso-PGF2α ↑ 20 min before TLV resumption. Plasma 8-iso-PGF2α ↑ 5 min before TLV. EBC NO2 + NO3 ↑ 20 min before TLV. No change in plasma. EBC pH no change |
| 2013 Liu (23) | Western blot: lung tissue protein expression; RT-PCR: lung tissue mRNA | Tissue CCSP protein and mRNA ↓ in all groups compared to sham, but ↑ in sevoflurane groups compared to OLV. Tissue C-PLA2 protein and mRNA ↑ in all groups |
| 2017 Liu (21) | BALF TNF-α: ELISA; tissue TNF-α: immunohistochemistry techniques; total RNA: spectrophotometry and agarose gel electrophoresis; RNA expression: RT-PCR | BALF TNF-α ↑ and TNF-α mRNA ↑ |
| 2018 Liu (22) | Arterial cytokines: ELISA | Plasma TNF-α, IL-6, IL-10, and CRP ↑ |
| 2018 Liu (22) | ELISA: arterial serum cytokines | Arterial serum TNF-α, ICAM-1, IL-6 ↑ |
| 2011 Mahmoud (24) | BALF and blood cytokines: ELISA; SOD activity: pyrogallol auto-oxidation; albumin concentration: nephelometry; alveolar cell numbers: Coulter Counter | BALF and plasma TNF-α and IL-8 ↑ in all groups. BALF albumin and alveolar cell count ↑ in all groups. BALF and plasma SOD ↑ w/propofol, no change w/isoflurane. BALF and plasma MDA ↑ with isoflurane, ↓ with propofol |
| 2020 Pan (25) | IκBα: western blot; IκBα phosphorylation: RT-PCR; ELISA: tissue cytokines | Lung tissue IκBα and NFκB-65 phosphorylation ↓. Lung tissue TNF-α, IL-1β, IL-6, IL-8 ↑ |
| 2005 Schilling (26) | BALF IL-8, IL-10, and sICAM-1: ELISA; TNF-α and PMN cell elastase: immunoassay | BALF total protein concentration, albumin concentration, TNF-α, PMN elastase, intra-alveolar cell count, and IL-8 ↑; BALF IL-10 ↓ |
| 2007 Schilling (10) | BALF cytokines: ELISA and immunoassay; protein concentration: colorimetric detection assay; cell counts: flow cytometry | BALF TNF-α, IL-8, PMN elastase ↑ in all groups. BALF lymphocyte count ↓ in all groups. BALF post-operative intra-alveolar cell count and granulocyte count ↑ with propofol, no change with desflurane. BALF total protein, albumin, alveolar macrophage count no change in all groups. BALF sICAM-1 ↓ with desflurane, no change with propofol. BALF IL-10 ↓ with propofol, no change with desflurane |
| 2011 Schilling (27) | Multiplex bead immunoassay: arterial serum analytes, BALF | Serum IL-6 ↑. Serum TNF-α, IL-1β, IL-8, IL-10, IL-12 no change. Serum IL-1β no change. Serum IL-8 no change. Dependent lung BALF TNF-α, and IL-1β no change in volatile anaesthetic group, ↑ in propofol group. Dependent lung BALF IL-6 and IL-8 ↑ |
| 2006 Schreiber (28) | BALF IL6, TNF-α: PharMingen commercial rat assay; BALF protein concentration: turbidimetry; Neutrophils count: hemocytometer and cell smear using Greunwald stain | BALF protein concentration, neutrophil count, TNF-α, IL-6 ↑ |
| 2012 Siegl (29) | BALF analytes: ELISA; Kinase activity: Western blot; protein concentrations: BCA kit; RNA: RT-PCR | Lung tissue ERK, JNK, p38, and AKT phosphorylation ↑. Lung tissue IL-1β, Tnf, Cxcl1, Cxcl2, Areg mRNA ↑. BALF IL-1β, IL-6, CXCL-1, CXCL-2, and amphiregulin ↑. BALF TNF ↑ with C57BL/6 strain |
| 2018 Tan (30) | Serum analytes: ELISA | Arterial blood TNF-α, IL-6 and IL-10 ↑ |
| 2015 Tojo (31) | Lung tissue analytes: ELISA; RNA: RT-PCR and qPCR | Lung tissue TNF-α, CXCL-1, CCL-2, MPO ↑; HIF-1α ↑ in nonventilated lung; Cell culture NF-κB binding activity (atelectatic lung), HIF-1α, HIF-1 downstream gene VEGFA mRNA, GLUT1 mRNA ↑ |
| 2017 Xu (32) | Lung tissue analytes: ELISA; RNA: RT-PCR | Lung tissue MPO ↑. Lung tissue TNF-α, IL-6, ratio of NF-κB to β-actin expression↑ positive correlation with FiO2. Arterial pH no change |
| 2018 Yang (33) | ELISA: lung tissue arachidonic acid; RT-PCR: lung tissue mRNA | Lung tissue arachidonic acid ↑. Lung tissue C-PLA2 mRNA ↑. Lung tissue CCSP mRNA ↓ |
| 2020 Yao (34) | ELISA: venous blood analytes | Venous blood neutrophil elastase, TNF-a, and IL-8 ↓. Venous blood IL-6 ↑ |
| 2019 Yin (35) | ELISA, liquid chromatography mass spectrometer | Lung tissue anandamide no change. Lung tissue arachidonic acid, PGI2, TXA2, and LTB4 ↑. Lung tissue PGI2/TXA2 ↓ |
| 2012 You (36) | Protein concentration: Bradford assay; NF-κB DNA binding activity: electrophoretic mobility; NF-κB p65: Western blot; BALF TNF-α and IL-8: ELISA | Lung tissue NF-κB, NF-κB DNA binding activity, cytosolic p65 ↑. BALF TNF-α and IL-8 ↑ |
| 2019 Zeng (37) | Tissue sample RNA from each lung before and after ventilation: NextGen RNA sequencing | Collapsed lung tissue endothelial barrier gene set expression ↑. Collapsed lung tissue inflammation/immune response gene set expression ↓. Aerated lung tissue inflammation/immune response gene set expression ↑ |
| 2016 Zhang (38) | BALF analytes: ELISA | BALF IL-1β, IL-6 and IL-8 ↑ |
| 2020 Zhao (39) | Venous serum analytes: ELISA | Venous serum TNF-α, IL-6, IL-10 ↑ |
Analytical methodology used to detect markers and the major findings of each study. ↑ indicates that the analyte abundance or activity increased with OLV, and ↓ indicates that it decreased with OLV. ELISA, enzyme-linked immunosorbent assay; vWF, von Willebrand factor; BALF, bronchoalveolar lavage fluid; EBC, exhaled breath condensate; RT-PCR, reverse transcription polymerase chain reaction; miRNA, microRNA; MDA, malondialdehyde; TBA, thiobarbituric acid; mRNA, messenger RNA; TNF, tumor necrosis factor; RAGE, receptor for advanced glycation end products; SOD, superoxide dismutase; IL, interleukin; PMN, polymorphonuclear; BCA, bicinchoninic acid; qPCR, quantitative polymerase chain reaction; NF-κB, nuclear factor κB; KL-6, Krebs von den Lungen 6; SP-D, surfactant protein D; IL-1RA, interleukin 1 receptor antagonist; GROα, growth-regulated oncogene α; OLV, one-lung ventilation; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; MCP-1, monocyte chemoattractant protein-1; IFN-ɣ, interferon-ɣ; TXB2, thromboxane B2 ; TLV, two-lung ventilation; CCSP, clara cell secretory protein; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1; MPO, myeloperoxidase; PGI2, prostaglandin I2; TXA2, thromboxane A2 ; LTB4, leukotriene B4.
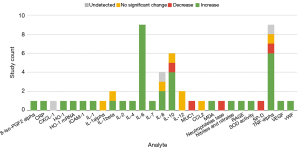
Figures 2,3 describe the change in abundance of each analyte in blood and BAL, respectively. A total of 27 analytes were investigated in blood across included studies. The studies investigating these analytes primarily used human subjects (69%, n=9). Only 6 analytes were investigated by ≥2 studies: IL-1β, IL-6, IL-8, IL-10, IL-12, and TNF-α. The bulk of markers investigated in blood were pro-inflammatory (81%, n=22 markers). Across studies investigating these markers 68% (n=30) of the assays found an increase. There were only three anti-inflammatory markers investigated in the blood (SOD, IL-10, and HO-1). Across studies 70% (n=7) of assays showed an increase in these markers.
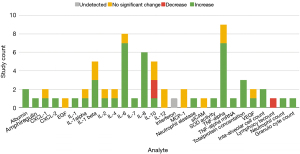
Twenty-nine analytes were investigated in BALF samples. There were 17 markers that were investigated by more than one study, and of those 10 had disagreement between studies. Much of this discordance is attributed to the 2018 study by Fiorelli et al. who found that all 12 of the cytokine analyzed in BALF had no change in abundance or were undetected after OLV (17). Eight out of 12 of the findings by Fiorelli et al. were in discordance with other studies, and they speculate that this is a result of positive end expiratory pressure (PEEP) playing a protective role against inflammation. Studies using BALF samples selected pro-inflammatory markers almost exclusively (90%, n=26). Across all studies, 66% (n=45) of findings showed an increase in inflammatory markers in lavage, with 24% of findings showing no significant change. Two anti-inflammatory markers were investigated (IL-10, SOD). Two studies found that IL-10 will either increase or decrease in BALF during OLV depending on the type of anaesthetic used (9,18), and three studies finding a decrease or no significant change (17,26,28).
Thirty-two markers were used in the analysis of lung tissue after OLV, but only nine of those markers were investigated by more than one study (arachidonic acid, C-PLA2, CCSP, IL-1β, IL-6, IL-8, MPO, NF-kB binding activity, and TNF-α). In those nine markers there was a 67% (n=6) consensus between studies regarding the effect of OLV on markers concentrations. The markers chosen by researchers to quantify in tissue were primarily inflammatory mediators (75%, n=24). Ninety-one percent (n=31) of the lung tissue inflammatory marker measurements across all studies found a statistically significant increase after OLV.
Twelve publications also used less common bio-samples (Table 3). Diverse biomarkers were assayed, including intracellular signaling pathways that control oxidative stress (HO-1, HIF-1α), gene transcription (NF-κB, HIF-1α), trophic and secretory cell responses (ERK, JNK, p38, AKT phosphorylation), as well as secreted cytokines, chemokines and growth factors (Table 3).
Table 3
| Sample type | Analyte | Finding |
|---|---|---|
| Human model | ||
| Lung tissue | HO-1 mRNA | ↑ Feng 2015 (16) |
| Exhaled breath condensate | pH | ↑ Bastin 2011 (8), no change García-de-la-Asunción 2015 (20) |
| H2O2 | ↑ García-de-la-Asunción 2015 (20) | |
| 8-iso-PGF2α | ↑ García-de-la-Asunción 2015 (20) | |
| NO2 + NO3 | ↑ García-de-la-Asunción 2015 (20) | |
| Pleural fluid | GROα | ↑ Breunig 2011 (14) |
| IL-6 | ↑ Breunig 2011 (14) | |
| IL-1RA | ↑ Breunig 2011 (14) | |
| Animal model | ||
| Sample type | Analyte | Finding |
| Lung tissue | Anandamide | No change; Yin 2019 (35) |
| Arachidonic acid | ↑ Yang 2018 (33), Yin 2019 (35) | |
| ERK, JNK, p38, AKT phosphorylation | ↑ Siegl 2012 (29) | |
| IL-1β, Tnf, Cxcl1, Cxcl2, Areg gene expression | ↑ Siegl 2012 (29) | |
| TNF-α | ↑ Tojo 2015 (31), Xu 2016 (32), Pan 2020 (25) | |
| CXCL-1 | ↑ Tojo 2015 (31) | |
| MCP-1 | ↑ Tojo 2015 (31) | |
| MPO | ↑ Tojo 2015 (31), Xu 2016 (32) | |
| IL-1β | ↑ Pan 2020 (25) | |
| IL-6 | ↑ Xu 2016 (32), Pan 2020 (25) | |
| IL-8 | ↑ Pan 2020 (25) | |
| HIF-1α | ↑ in nonventilated lung; Tojo 2015 (31) | |
| NF-κB, NF-κB DNA binding activity | ↑ You 2012 (36), ↓ Pan 2020 (25) | |
| NF-κB:β-actin expression | ↑ Xu 2016 (32) | |
| IF-κB phosphorylation | ↓ Pan 2020 (25) | |
| Cytosolic p65 | ↑ You 2012 (36) | |
| miR449b-3p, miR451-5p, miR144-5p microRNA | ↑ Dai 2020 (15) | |
| CCSP and CCSP mRNA | ↓ Liu 2013 (23), Yang 2018 (33) | |
| C-PLA2 and C-PLA2 mRNA | ↑ Liu 2013 (23), Yang 2018 (33) | |
| TXA2 | ↑ Yin 2019 (35) | |
| PGI2 | ↑ Yin 2019 (35) | |
| LTB4 | ↑ Yin 2019 (35) | |
| Immune response gene set expression | ↑ Yang 2018 (33) | |
| Lung perfusate | TXB2 | ↑ Gama de Abreu 2003 (19) |
| pH | No change; Gama de Abreu 2003 (19) | |
| Exhaled breath condensate | IL-1β | ↑ Dai 2020 (15) |
| IL-6 | ↑ Dai 2020 (15) | |
Analytes measured in parenchymal lung tissue, pleural fluid, lung perfusate, or exhaled breath condensate. ↑ indicates that the analyte abundance or activity increased with OLV, and ↓ indicates that it decreased with OLV. EBC, exhaled breath condensate; GROα, growth-regulated oncogene α; IL, interleukin; IL-1RA, interleukin 1 receptor antagonist; TNF, tumor necrosis factor; CXCL-1, chemokine (C-X-C motif) ligand 1; MCP-1, monocyte chemoattractant protein-1; MPO, myeloperoxidase; NF-κB, nuclear factor κB; CCSP, Clara cell secretory protein; TXA2, thromboxane A2 ; PGI2, prostaglandin I2; LTB4, leukotriene B4; OLV, one-lung ventilation.
If one considers the same analyte (i.e., IL-6) in a different sample medium (i.e., blood versus BALF) as a unique biomarker, there were a total of 98 markers tested. If not, there were 73 truly unique biomarker candidates: 29 were analyzed in the BALF, 27 in blood, 32 in lung tissue, and six in both the exhaled breath condensate and cell culture, 3 in pleural fluid, 2 in perfusate. Thirty-three analytes were investigated in more than one study, leaving 65 analytes examined by a single study. The most studied analytes were TNF-α (n=14), followed by IL-6 (n=13), IL-8 (n=9), and IL-10 (n=7). The majority of human studies utilized blood samples, while only a single animal study tested for analytes in blood. Animal studies utilized tissue samples more often (43% vs. 8%).
Discussion
We sought to determine the state of the evidence regarding potential biomarkers of VILI secondary to OLV. Although we identified 93 unique biomarker candidates, only 33 of these were measured in more than one study. Thus, 65% of the analytes were identified in only a single study. Of the markers identified in more than one study, fewer than half (n=16) showed concordance with respect to their change in abundance after an OLV intervention. For example, our synthesis of data from multiple studies showed that IL-10 levels may be either elevated, decreased or unchanged after OLV. It is unclear whether this discordance is driven by differences in analytic techniques, differences in exposures during OLV between studies, or both factors. If analytes in different sample mediums are considered distinct (e.g., IL-6 in blood vs. IL-6 in BALF), there was little overlap between analytes investigated by animal and human studies. This is primarily a consequence of the tendency of animal studies to use predominantly tissue samples and of human studies to use predominantly blood samples. There were four examples when animal and human studies did investigate the same analyte in the same sample medium (IL-6, IL-8, TNF-α, and total protein concentration in BALF). In these, there was consensus between human and animal results. There is gap in the literature regarding assessment of both BALF and blood in the same experiment. This is an important gap to address for future research. There may be important changes in both the systemic and local stress response; focusing only on just local (i.e., BALF) or systemic (i.e., blood) responses may result in an incomplete understanding of the processes involved in VILI after OLV.
The analytes most frequently assayed and identified as reliable biomarkers of OLV-induced lung injury in the BALF included the pro-inflammatory cytokines TNF-α (increased in 8 out of 10 studies), IL-8 (increased in 7 out of 7 studies), and IL-6 (increased in 7 out of 8 studies). In the lavage fluid inflammatory markers were found to increase in 67% of investigations. Biomarker results assessing the systemic response to OLV appear to be more consistent; in blood analyses, IL-6 (increased in 9 of 9 studies), and TNF-α (increased in 6 of 8 studies) appeared to be the most consistent biomarker candidates. TNF-α is a part of the primary immune response and can induce synthesis and release of other proinflammatory cytokines in the lung during injury. (40). IL-6 has been shown to have a dual nature in animal models of lung injury, as it’s effect can be inflammatory, or anti-inflammatory, depending on the model employed. (40). Systemic IL-10 levels increased in 4 out of 6 studies that investigated it, while local findings were much less concordant. IL-10 was found to decrease or not significantly change in three studies, and in two studies it was found that the direction of change from baseline was dependent on the anaesthetic used during ventilation. IL-10 is an anti-inflammatory cytokine that can inhibit the expression of inflammatory mediators while having no effect on anti-inflammatory mediators (41,42). Studies of lung injury using animal models have shown that IL-10 can reduce lung injury and plays a protective role in systemic inflammation (43). The most commonly used analytical method was enzyme-linked immunosorbent assay (ELISA) (72% of studies), which lends itself well to protein biomarker detection, as it is highly sensitive and can be calibrated to determine absolute concentrations (44). The most common sample types analyzed were bronchoalveolar lavage (BAL) (55% of studies) and blood (43%), samples routinely taken during thoracic surgery, and that represent local and systemic responses to OLV-induced VILI, respectively.
Fifty-two percent of the studies were conducted in humans. Human biomarker studies may be more relevant for clinical applications but present their own challenges. For ethical reasons, the acceptable experimental exposure human patients may be subjected to is significantly less damaging than animal models, which may explain why only a third (5 of 15) of human studies compared a protective ventilation protocol to a more historical and damaging one (Vt 10–15 mL/kg) (3). Ethically, it is not possible to justify purposefully exerting anesthetic exposures that are thought to be injurious. Thus, the literature is limited to observational studies that have retrospectively assessed the effect of potentially injurious historical ventilation practices.
The effects of OLV exposure on the anti-inflammatory cytokine IL-10 in BALF was the most discordant finding of the studies we reviewed. This may be due to the varying immunomodulatory effects of the anaesthetics employed during mechanical ventilation. For example, de la Gala et al. found that IL-10 increased with inhaled sevoflurane, and decreased with propofol (18). The authors suggest that sevoflurane protects against mechanical forces on the lung tissue by reducing alveolar capillary permeability, in agreement with the findings of Voigtsberger et al. (45). De la Gala et al. note that propofol may induce less of an inflammatory response than volatile anaesthetic in non-thoracic surgery.
There are limitations to our findings. Some of these are limitations of the scoping review but many are inextricably linked to the limitations in the data synthesized for this review. Discordant findings between studies, a relatively small number of studies on this topic, small sample sizes of the existing studies, and the short period of observation of the studies all contribute to limiting the strength of conclusions, and in uncovering the critical gaps in knowledge. Discordant findings between studies may be due to differences in OLV procedure and/or analytical method. Variations in experimental design between studies, such as the difference between studies that only employed protective ventilation practices and studies that used traditional practices, may have led the researchers to different conclusions with or without the availability of a well-defined control. Furthermore, even in those studies that managed to have control groups, ethical limitations in purposefully exerting “harmful” ventilation practices may have resulted in “experimental” and “control” groups experiencing exposures that were too similar; this has the potential of dampening or suppressing a true effect of clinically relevant OLV. Another limitation is that included studies did not account for potential interaction between pre-ventilation lung function and each analyte.
Another important caveat is that our review reports the response of analytes to OLV-induced lung injury. The FDA’s Biomarker Qualification Program requires preclinical biomarkers to not only show correlation with changes induced in the biological process, but it must be demonstrated that the response of the biomarker is exclusive to the change placed on the process (46). Our study cannot fully conclude that these biomarker profiles are entirely driven by exposure to OLV, as some component of inflammatory changes may be driven by the surgical insult. This is an issue which needs to be addressed in the design of future studies.
Conclusions
We sought to identify the evidence regarding candidate biomarkers of VILI caused by OLV. The candidate biomarkers with the most evidence and greatest reliability are general markers of inflammation, such as IL-6 and TNF-α. There is a gap in the literature regarding assessment of both local and systemic response in the same experiment. There remains a substantial body of biomarkers that remain unknown, including the response of individual lungs to OLV. Future studies should assess both in order to obtain more complete understanding of the processes involved in VILI after OLV. A reliable constellation of biomarkers for OLV-induced VILI could allow for rapid preclinical diagnosis to better allocate resources after surgery. Such biomarkers may allow identification of patients at highest risk for developing VILI after thoracic surgery and therefore allocate nursing, monitoring and preventive resources to these patients. Biomarkers for OLV-induced VILI may be used to measure effect of and response to novel preventative or therapeutic interventions. Finally, reliable biomarkers may also one day provide targets for novel interventions, such as immune-modulating drugs that could be used to prevent or reduce the risk of ARDS/ALI after thoracic surgery.
Acknowledgments
This work was presented at the 2018 Annual Surgery Research Day in Winnipeg, Manitoba, Canada (January 17, 2018).
Funding: This work was supported by the Optimization of Perioperative Care in Thoracic Surgery Grant (University Medical Group, University of Manitoba), which served as start-up research funds for the Senior Investigator (BK).
Footnote
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-20-2301/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-20-2301/coif). AJGB, RM, THG, SKS, and LT report that this work was supported by the Optimization of Perioperative Care in Thoracic Surgery Grant (University Medical Group, University of Manitoba) Optimization of Perioperative Care in Thoracic Surgery Grant (University Medical Group, University of Manitoba). AJH participated in a Canadian Thoracic Society advisory panel for a topic unrelated to this publication, sponsored by Boehringer Ingelheim Canada Inc., and received honorarium for lecture facilitation in local research rounds featuring an external speaker from AstraZeneca Canada (topic of presentation not relevant to work in manuscript), and reports grants from Canada Research Chairs Program and Children’s Hospital Research Institute of Manitoba (CHRIM). BK and GB received honoraria from AstraZeneca as part of a consultancy board concerning lung cancer treatment and report Optimization of Perioperative Care in Thoracic Surgery Grant (University Medical Group, University of Manitoba). The other authors have no conflict of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Tobin MJ. Basing Respiratory Management of COVID-19 on Physiological Principles. Am J Respir Crit Care Med 2020;201:1319-20. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Licker M, Fauconnet P, Villiger Y, et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 2009;22:61-7. [Crossref] [PubMed]
- Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376-80. [Crossref] [PubMed]
- Beitler JR, Malhotra A, Thompson BT. Ventilator-induced Lung Injury. Clin Chest Med 2016;37:633-46. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Bastin AJ, Sato H, Davidson SJ, et al. Biomarkers of lung injury after one-lung ventilation for lung resection. Respirology 2011;16:138-45. [Crossref] [PubMed]
- Liu W, Huang Q, Lin D, et al. Effect of lung protective ventilation on coronary heart disease patients undergoing lung cancer resection. J Thorac Dis 2018;10:2760-70. [Crossref] [PubMed]
- Schilling T, Kozian A, Kretzschmar M, et al. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth 2007;99:368-75. [Crossref] [PubMed]
- STS General Thoracic Surgery Database. Available online: https://www.sts.org/registries-research-center/sts-national-database/sts-general-thoracic-surgery-database. Published June 2018, accessed April 9, 2019.
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [Crossref] [PubMed]
- Breunig A, Gambazzi F, Beck-Schimmer B, et al. Cytokine & chemokine response in the lungs, pleural fluid and serum in thoracic surgery using one-lung ventilation. J Inflamm (Lond) 2011;8:32. [Crossref] [PubMed]
- Dai YL, Hsu RJ, Huang HK, et al. Adaptive support ventilation attenuates postpneumonectomy acute lung injury in a porcine model. Interact Cardiovasc Thorac Surg 2020;31:718-26. [Crossref] [PubMed]
- Feng H, Wang GM, Qiao Y, et al. Effects of sevoflurane preconditioning on lung injury during one lung ventilation. Int J Clin Exp Med 2015;8:13634-8. [PubMed]
- Fiorelli S, Defraia V, Cipolla F, et al. Short-term one-lung ventilation does not influence local inflammatory cytokine response after lung resection. J Thorac Dis 2018;10:1864-74. [Crossref] [PubMed]
- de la Gala F, Piñeiro P, Reyes A, et al. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br J Anaesth 2017;119:655-63. [Crossref] [PubMed]
- Gama de Abreu M, Heintz M, Heller A, et al. One-lung ventilation with high tidal volumes and zero positive end-expiratory pressure is injurious in the isolated rabbit lung model. Anesth Analg 2003;96:220-8. table of contents. [PubMed]
- García-de-la-Asunción J, García-del-Olmo E, Perez-Griera J, et al. Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: a study of exhaled breath condensate and blood. Eur J Cardiothorac Surg 2015;48:e37-44. [Crossref] [PubMed]
- Liu J, Zeng Y, Cui F, et al. The impact of spontaneous ventilation on non-operative lung injury in thoracic surgery: a randomized controlled rabbit model study. Eur J Cardiothorac Surg 2017;52:1083-9. [Crossref] [PubMed]
- Liu G, Wang H, Lu X, et al. Correlation between controlled lung collapse and early lung injury in dogs. Exp Ther Med 2018;16:3027-33. [Crossref] [PubMed]
- Liu R, Yang Y, Li Y, et al. Effects of sevoflurane on pulmonary cytosolic phospholipase A2 and clara cell secretory protein expressions in rabbits with one-lung ventilation-induced lung injury. Nan Fang Yi Ke Da Xue Xue Bao 2013;33:469-73. [PubMed]
- Mahmoud K, Ammar A. Immunomodulatory Effects of Anesthetics during Thoracic Surgery. Anesthesiol Res Pract 2011;2011:317410. [Crossref] [PubMed]
- Pan W, Zhang L, Li T, et al. The Protective Effect Of Ligustrazine On Lung Of One Lung Ventilation Rats In NF-KB Related Inflammatory Factor Signaling Pathway. Acta Medica Mediterranea 2020;36:3065-72.
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 2005;101:957-65. [Crossref] [PubMed]
- Schilling T, Kozian A, Senturk M, et al. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology 2011;115:65-74. [Crossref] [PubMed]
- Schreiber T, Niemann C, Schmidt B, et al. A novel model of selective lung ventilation to investigate the long-term effects of ventilation-induced lung injury. Shock 2006;26:50-4. [Crossref] [PubMed]
- Siegl S, Uhlig S. Using the one-lung method to link p38 to pro-inflammatory gene expression during overventilation in C57BL/6 and BALB/c mice. PLoS One 2012;7:e41464. [Crossref] [PubMed]
- Tan J, Song Z, Bian Q, et al. Effects of volume-controlled ventilation vs. pressure-controlled ventilation on respiratory function and inflammatory factors in patients undergoing video-assisted thoracoscopic radical resection of pulmonary carcinoma. J Thorac Dis 2018;10:1483-9. [Crossref] [PubMed]
- Tojo K, Nagamine Y, Yazawa T, et al. Atelectasis causes alveolar hypoxia-induced inflammation during uneven mechanical ventilation in rats. Intensive Care Med Exp 2015;3:56. [Crossref] [PubMed]
- Xu Z, Gu L, Bian Q, et al. Oxygenation, inflammatory response and lung injury during one lung ventilation in rabbits using inspired oxygen fraction of 0.6 vs. 1.0. J Biomed Res 2016;31:56-64. [PubMed]
- Yang Y, Wang WF, Li YH, et al. Sevoflurane attenuates ventilator-induced lung injury by regulating c-PLA2 expression. Mol Med Rep 2018;18:2923-8. [Crossref] [PubMed]
- Yao W, Yang M, Cheng Q, et al. Effect of Pressure-Controlled Ventilation-Volume Guaranteed on One-Lung Ventilation in Elderly Patients Undergoing Thoracotomy. Med Sci Monit 2020;26:e921417. [Crossref] [PubMed]
- Yin H, Li X, Xia R, et al. Posttreatment With the Fatty Acid Amide Hydrolase Inhibitor URB937 Ameliorates One-Lung Ventilation-Induced Lung Injury in a Rabbit Model. J Surg Res 2019;239:83-91. [Crossref] [PubMed]
- You Z, Feng D, Xu H, et al. Nuclear factor-kappa B mediates one-lung ventilation-induced acute lung injury in rabbits. J Invest Surg 2012;25:78-85. [Crossref] [PubMed]
- Zeng C, Ribeiro G, Hinoshita T, et al. Transcriptomic Response to Lung Collapse During Experimental Single-Lung Ventilation. American Thoracic Society International Conference 2019.
- Zhang WP, Zhu SM. The Effects of Inverse Ratio Ventilation with PEEP on Respiratory Function and Inflammatory Cytokines in Patients during One-lung Ventilation. J Pulm Respir Med 2016;54:1-5.
- Zhao F, Wang Z, Ye C, et al. Effect of Transcutaneous Electrical Acupoint Stimulation on One-Lung Ventilation-Induced Lung Injury in Patients Undergoing Esophageal Cancer Operation. Evid Based Complement Alternat Med 2020;2020:9018701. [Crossref] [PubMed]
- Chen J, Lin J, Luo H, et al. Effects of Human Interleukin-10 on Ventilator-Associated Lung Injury in Rats. Inflammation 2019;42:538-47. [Crossref] [PubMed]
- Goldman JL, Sammani S, Kempf C, et al. Pleiotropic effects of interleukin-6 in a "two-hit" murine model of acute respiratory distress syndrome. Pulm Circ 2014;4:280-8. [Crossref] [PubMed]
- Eskdale J, Kube D, Tesch H, et al. Mapping of the human IL10 gene and further characterization of the 5' flanking sequence. Immunogenetics 1997;46:120-8. [Crossref] [PubMed]
- Grob NM, Aytekin M, Dweik RA. Biomarkers in exhaled breath condensate: a review of collection, processing and analysis. J Breath Res 2008;2:037004. [Crossref] [PubMed]
- Hosseini S, Vázquez-Villegas P, Rito-Palomares M, et al. Advantages, Disadvantages and Modifications of Conventional ELISA. SpringerBriefs in Applied Sciences and Technology 2017:67-115.
- Voigtsberger S, Lachmann RA, Leutert AC, et al. Sevoflurane ameliorates gas exchange and attenuates lung damage in experimental lipopolysaccharide-induced lung injury. Anesthesiology 2009;111:1238-48. [Crossref] [PubMed]
- Sauer JM, Porter ACBiomarker Programs, Predictive Safety Testing Consortium. Preclinical biomarker qualification. Exp Biol Med (Maywood) 2018;243:222-7. [Crossref] [PubMed]