Prolonged air leak after robotic lung resection: a narrative review
Introduction
Background
In the last decades, the number of patients affected by non-small cell lung cancer (NSCLC) receiving minimally invasive surgical (MIS) treatment has progressively increased. The first reported robotic pulmonary lobectomy dates back to 2002 (1); since that time, the indications to the use of robotic-assisted thoracic surgery (RATS) in the field of pulmonary oncology have continuously expanded.
The limited availability of the robotic system and presumably a steep learning curve were initially indicated as major limitations of the robotic technique. However, its clear advantages, including magnified tridimensional vision, hand tremor filtration, and improved dexterity compared to manual video-assisted thoracic surgery (VATS), contributed to the spread of the RATS in the thoracic surgery community (2). Several retrospective studies and meta-analyses demonstrated similar perioperative results of robotic surgery and VATS, and a significant improvement in early outcomes when compared to open approach: namely, they reported a reduction in postoperative pain, analgesics consumption, chest tube duration, and postoperative length of stay (LOS) (3,4). Furthermore, postoperative quality of life (QoL) after RATS turned out superior over open thoracotomy up to 12 months after the procedure (5).
Recently, these results were confirmed by the first multicenter randomized study that evaluated the perioperative outcomes of robotic lobectomy compared to VATS technique in the treatment of early stage NSCLC (ROMAN study). No significant differences between the techniques were reported concerning intraoperative thoracotomic conversion and immediate complications (6).
Another major issue regarding robotic surgery is related to hospital costs. We analyzed in a previous research 23 patients treated by robot, 41 by VATS, and 39 by open surgery. The estimated economic burden was 82%, 68% and 69%, respectively for robot, VATS and open approaches, of the regional 3 Italian health service reimbursement. This study demonstrated that, although higher than other techniques, the cost of robotic thoracic surgery still allowed profit in a system paid by national health system reimbursements; moreover, the RATS group has benefited of lower in-hospital LOS and more extensive lymph node dissection than VATS and open surgery (7).
Although there is no unique threshold to define prolonged air leaks (PAL), most studies describe them as the evidence of a chest tube air leak exceeding 5 to 7 days after surgery (8). The development of PAL is one of the most impactful complications in the immediate postoperative period after pulmonary resection. In fact, patients with PAL show significantly worse morbidity rates when compared to those with uneventful postoperative course, including longer hospitalization and chest tube duration, increased rates of respiratory complications and pleural empyema, and higher percentage of reoperation, readmission and in-hospital mortality (9-12). In addition, several authors underlined that the development of PAL after lung cancer surgery represents a major economic burden for the hospital, owing to both direct and indirect costs (10,12-14).
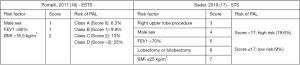
Both patient-related variables and intraoperative characteristics including the surgical approach resulted to be related to a higher risk of postoperative PAL (10,12,15). As a result, the European Society of Thoracic Surgeons (ESTS) and the Society of Thoracic Surgeons (STS) independently developed two scoring systems to stratify the risk of PAL onset following lung resection based on a number of preoperative characteristics (Figure 1), so to implement preventive measures in selected subjects judged at higher risk (16,17). We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-818/rc).
Aims of the narrative review
Considering the rapid spread of robotic lung resections in recent years, the purpose of this review is to collect and discuss the available data on the incidence and clinical impact of PAL following RATS as opposed to VATS and open approaches. Furthermore, we will go through the preoperative and intraoperative risk factors for the prediction and prevention of PAL and provide an updated insight on the management of this postoperative complication in the field of robotic surgery. We present the following article in accordance with the Narrative Review reporting checklist.
Methods
A research on PubMed/MEDLINE and Scopus database from inception until May, 27th 2022 was performed to assess the studies that evaluated the incidence and the management of postoperative air leaks after robotic pulmonary resection (Table 1). The following keywords were used: robotic surgery, lobectomy, segmentectomy, air leak, PAL, complication, management, comparative study. We included in our evaluation all the literature published in English, with available full-text or abstract, without restrictions concerning article type. All the material retrieved by the literature search was reviewed by the authors to select the studies that were included in the final analysis.
Table 1
| Items | Specification |
|---|---|
| Date of search | May 27th, 2022 |
| Databases and other sources searched | PubMed/MEDLINE |
| Scopus | |
| Search terms used | (“robotic surgery” [MeSH]) AND “air leak” [MeSH] |
| (“robotic surgery” [MeSH]) AND “lobectomy” [MESH] AND “air leak” [MeSH] | |
| (“robotic surgery” [MeSH]) AND “segmentectomy” [MESH] AND “air leak” [MeSH] | |
| (“robotic surgery” [MeSH]) AND “PAL” [MeSH] | |
| (“robotic surgery” [MeSH]) AND “complication” [MeSH] | |
| (“air leak” [MeSH]) AND “management” [MeSH] | |
| (“robotic surgery” [MeSH]) AND “comparative study” [MeSH] | |
| Timeframe | Inception of database – May, 27th 2022 |
| Inclusion criteria | English language |
| Available full-text or abstract | |
| Studies including patients ≥18-year-old | |
| Exclusion criteria | Non-English language |
| Pediatric population studies | |
| Selection process | Data search and selection: GV, PM, SV |
| Assembly of data: PM, SV, FR | |
| Interpretation: PM, GV, FR |
Prolonged air leaks following robotic surgery: a literature review
Incidence and predictive factors of PAL in patients treated with robotic lung resection
Several retrospective series demonstrated RATS safety for the treatment of lung cancer patients. Still, data on PAL incidence following robotic lung resection are ambiguous. In the study by Hoeijmakers et al., RATS was claimed to be associated to a two-fold increase in the probability of PAL when compared to VATS at multivariate analysis (18). However, as mentioned by the authors, this result should be carefully evaluated, considering that the study cohort included patients operated in Centers with a wide heterogeneity in the technical expertise in robotic surgery and in the adoption of fissureless approaches for lung resection.
Contrasting with this result, a retrospective multicenter study on more than 1,200 patients operated in four American and European leading centers in the field of robotic thoracic surgery, demonstrated that the incidence of major perioperative complications (≥ grade III of Clavien-Dindo classification) following RATS anatomic resection for NSCLC was about 4% overall, with PAL occurring in only 0.9% of them (19). In this study, male gender, preoperative pulmonary function, history of neoadjuvant treatment and the extension of lung resection were identified as risk factors for postoperative morbidity including PAL. Surgeon’s experience, evaluated on a learning curve of 20 procedures, was instead not related to the incidence of severe complications.
Su and colleagues recently analyzed the potential association between proficiency with robotic surgery and the occurrence of PAL. The trial involved 305 patients undergoing robotic pulmonary lobectomy; the overall incidence of PAL was 8.8%. This analysis showed a significant 15% reduction in PAL onset every 10 additional cases performed (OR =0.85, P=0.04), which became even more evident for the operators with an experience of more than 50 cases behind. This stems from the greater confidence with the robotic system that enabled them to overcome the absence of tactile feedback when manipulating the pulmonary parenchyma and during dissection (20). Therefore, a definitive evaluation on the role of learning curve on the incidence of PAL is challenging. It is possible that with the completion of the learning curve in RATS, surgeons acquire confidence with complex cases, such as sublobar anatomical resections, large tumors and post-induction resections. When evaluating the perioperative outcomes of proficient RATS and VATS, no difference was found between the techniques in terms of risk of PAL development (6).
The evaluation of other potential risk factors for PAL after robotic lung resection related to patient’s characteristics, including male gender, older age, low spirometry values indicative for emphysematous disease, and BMI, showed contrasting results (20,21).
PAL onset after robotic surgery vs. open surgery and VATS
Several studies in the literature explored the performance of robotic surgery compared to open surgery and manual VATS in the prevention of PAL after major anatomical lung resections (Table 2). In the last years, with the worldwide growing diffusion of the robotic system, this technique was largely demonstrated to lead to an improvement in short term outcomes including PAL after lung resection compared to thoracotomic approach. Still, clear results of RATS vs. VATS are uncertain.
Table 2
| Author, year | No. of patients | PAL incidence (%) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Open | VATS | RATS | Open | VATS | RATS | Open vs. RATS | VATS vs. RATS | Open vs. VATS | |||
| Farivar, 2014 (22) | 5,913 | 4,612 | 181 | 10.7 | 8.9 | 6.1 | 0.049* | 0.22 | NR | ||
| Agzarian, 2016 (23)** | 11,826 | 9,545 | 758 | 10.7–10.8 | 8.9–23.7 | 5.2–25.4 | 0.049–0.05* | 0.17–1.00 | NR | ||
| Kneuertz, 2018 (24) | 312 | – | 287 | 10 | – | 6 | 0.047* | – | – | ||
| Ng, 2019 (25)** | 10,103 | 8,659 | – | 8.1 | 7.1 | – | NR | 0.86 | 0.001* | ||
| – | 12,402 | 1,237 | – | 9.8 | 9.9 | ||||||
| Aiolfi, 2021 (26)** | 88,865 | 79,171 | 15,390 | 7.4 | 7.4 | 7.8 | NR | NR | NR | ||
*, statistical significant difference; **, meta-analysis. PAL, prolonged air leak; VATS, video-assisted thoracic surgery; RATS, robotic-assisted thoracic surgery; NR, not reported.
In the multicenter study of Farivar and colleagues, patients who underwent robot-assisted pulmonary lobectomy were compared with those treated by thoracotomy and VATS included in the STS database (22). Robotics allowed a significant reduction in the rate of PAL (6.1%) with respect to the open surgery group (10.7%, P=0.049), while PAL incidence in robotic and VATS cases (8.9%) resulted comparable. Nevertheless, such difference contributed to a reduction in postoperative chest tube duration and in-hospital LOS in favor of robotic surgery.
In another trial on 599 patients receiving robotic (n=287) versus open (n=312) lung lobectomy, the former group showed a significantly lower overall rate of respiratory complications, with almost half the incidence of postoperative PAL compared to the latter group (16 pts – 6% vs. 31 pts – 10%, P=0.047) (24). Moreover, in high-risk patients showing limited preoperative respiratory function, incidence of PAL in robotic cases turned out to be only one-third of that of the open surgery group (5% vs. 15%, P=0.02).
The meta-analyses by Agzarian, Ng and Aiolfi confirmed the superiority of MIS in preventing PAL onset after major lung resections with respect to open surgery, even though no reliable difference was identified between RATS and VATS (23,25,26). Even if high concordance between these analyses was encountered, it should be underlined that the results could be flawed because of the high heterogeneity between the trials included in the analyses. In fact, most of them were single-center retrospective studies including patients who underwent different types of anatomical and not anatomical lung resection. The operative approach in RATS and VATS cases was also variable (uniportal, multiportal, 3- or 4-robotic arms). Therefore, the final message of these meta-analyses should be interpreted with caution, and more prospective studies are needed to confirm their results.
PAL in patients undergoing robotic sublobar resection
The worldwide establishment of lung cancer screening programs in high-risk individuals resulted in the detection of an increasing number of small pulmonary nodules. The proportion of early stage lung cancer indeed reached almost 80% overall in the cohort of patients enrolled in the COSMOS trial (27). For this reason, in the last 15 years several retrospective studies questioned the indications for pulmonary lobectomy. Recently, the Japan Clinical Oncology Group (JCOG) demonstrated both non-inferiority and superiority in terms of 5-year overall survival in the first multicenter randomized trial comparing the survival and perioperative outcomes of anatomical segmentectomy as opposed to pulmonary lobectomy (JCOG0802/WJOG4607L) for the treatment of early stage NSCLC (28). Despite the overall incidence of postoperative complications did not differ among the groups, a higher frequency of PAL was observed in the segmentectomy arm (6.5% vs. 3.8%, P=0.04). Complex segmentectomy (i.e., those in which two or more intersegmental planes are divided) was identified as an independent risk factor for the onset of pulmonary complications, including PAL (OR 2.07, P=0.023) (28,29).
MIS techniques confirmed substantial advantages in terms of perioperative results and comparable oncological efficacy compared to open thoracotomy in patients treated with sublobar resection (30). Nevertheless, even very experienced thoracoscopists recognize that VATS learning curve is rather challenging when facing lung sparing surgery, especially in case of complex segmentectomies requiring deeper, peripheral dissection of the segmental hilum. Hence, a major limitation of the technique is the possibility to achieve adequate surgical margins and lymph node dissection while providing precise dissection in order to obviate PAL onset (31).
The spread of robotic surgery programs led to an increase in the number of sublobar anatomical resections in patients affected by limited disease, in particular among surgeons who completed the learning curve (32). A recent study by Zhou and colleagues compared the perioperative outcomes of RATS, VATS and open anatomical segmentectomies for the treatment of limited NSCLC (33). During the study period, still about half of the cases were approached by thoracotomy, but robotic surgery became the preferred technique at this center in recent years. Atypical segmentectomies increased from 18.5% to 37.5% overall at the end of the enrollment. Of note, a significantly higher proportion of patients in the RATS group received a complex segmentectomy with respect to VATS and open surgery groups (45% vs. 15% vs. 22%, P<0.001). Nonetheless, the incidence of PAL in the former group was remarkably lower than that of other techniques (3.9% vs. 12.5% with VATS, and 13.3% with thoracotomy), even if this result did not reach a statistically significant threshold (P=0.069). According to the authors, the reason for the reduction in overall postoperative complications (including PAL) was the precise dissection of segmental structures allowed by the robotic platform and the use of dedicated staplers.
PAL management in robotic thoracic surgery
Although several methods have been proposed over years for the treatment of PAL, every effort should be made to prevent their onset. Preoperative identification of individuals at risk for developing an air leak may guide surgeons in the selection of patients in whom to take intraoperative prophylactic measures; this is the reason why the aforementioned scoring systems were developed based on several risk factors (16,17).
Despite surgeons’ efforts, prevention of air leaks is not always possible, especially in case of high-risk patients, such as COPD and diabetic patients or heavy smokers. Intraoperative air leaks may be detected with a visual submersion test (or bubbling test) or a mechanical ventilation test (MVT, i.e., difference between inspired and expired tidal volume). Recently, a new method for the detection of air leaks by means of aerosolized indocyanine green (ICG) has been proposed in a canine pleural defect model (34). The researchers administered aerosolized ICG into the airway and were able to identify alveolar-pleural fistulas using a near-infrared light camera. Surely, further studies will be required to prove the applicability of this method in humans. Still, if these results will be confirmed, robotic surgery may take full advantage of this new air leak detection technique, being the robotic endoscope able to provide not only visible light but also near-infrared light.
Several strategies have been outlined in the literature for the treatment of intraoperative air leaks: these include direct parenchymal suturing, the use of different sealants and pleural mesh patches, staple line buttressing, pleural tenting (in case of upper lobectomy/bilobectomy), and prophylactic pneumoperitoneum (in case of lower lobectomy/bilobectomy) (35). When a parenchymal suture is needed to control aerostasis, the improved dexterity of robotic arms can show improved results, in particular when compared with straight manual thoracoscopic instruments.
In the context of robotic pulmonary resections, another preventive measure to obviate air leakage onset is the choice of staplers: during lung resections, surgeons may either decide to use robotic staplers or hand-held staplers. Although widely considered comparable in terms of outcomes, robotic stapling technology not only enables greater precision and a wider degree of movement, but it also uses a tissue feedback mechanism to ensure appropriate tissue thickness before stapling. In a recent study published in 2021, Zervos and colleagues compared perioperative outcomes and costs of robotic vs. hand-held staplers during robotic lobectomy (36). They reported that the use of robotic staplers is associated with a significantly lower rate of bleeding, conversion to open thoracotomy, and development of air leaks and other complications, with comparable overall hospitalization costs.
Recently, the perioperative outcome including incidence of PAL was analyzed in a comparative study between patients undergoing interlobar fissure division by traditional staplers or the new robot-dedicated vessel sealing system (VSS) (37). The authors demonstrated that the use of VSS to complete fused fissures was able to significantly lower the occurrence of overall postoperative complications, and allowed benefits on the onset of PAL (0% vs. 10%, P=0.058) and reduction of surgery costs due to the lower number of stapler recharges used in this group of patients.
Among the conservative procedures, autologous blood patch (ABP) for the treatment of PAL in patients with pneumothorax was first reported in 1987 (38). Ever since, multiple studies demonstrated its beneficial effect, with a success rate exceeding 89% (39). In our experience, this is the treatment of choice in case of unsolvable PAL after switching from water seal to one-way Heimlich valve. The procedure is simple, readily available, and cost-effective and can be reproduced in every facility: 50–250 mL (most often 100–150 mL) of peripheral venous blood drawn from the patient are instilled in the pleural cavity through the chest drain, followed by 30–45 mL of saline to keep the tube patent. Afterwards the chest tube is either clamped or, more often, suspended over a drip stand above the patient’s chest level (this allows air to exit and blood to remain in the pleural cavity) (39,40). In a systematic review by Hugen et al., most reported complications of ABP were Clavien-Dindo grade I or II, like fever (most prevalent), pneumonia, empyema, or prolonged pleural effusion, with none of the patients requiring surgical treatment (39). A recent meta-analysis by Karampinis et al. analyzed the performance of ABP for PAL after lung resection. Most patients suffered PAL for over 7 days before pleurodesis. The success rate of the procedure reached 85.7% after 48 hours from treatment, with low rates of minor complications and only 1.5% incidence of post-procedural pleural empyema (41).
Conclusions
The onset of postoperative PAL is still a major problem of thoracic surgery for lung cancer. Patients suffering from PAL have higher rates of complications and longer chest tube duration and hospital stay. As a result, the direct and indirect costs linked to this event have increased.
The development of robotic thoracic surgery programs introduced an alternative technique to open surgery and VATS for the treatment of lung cancer. Several trials demonstrated the efficacy of robotic surgery in preventing and lowering the incidence of PAL compared to traditional open approach. While the results between robotic and VATS techniques overlap when facing major pulmonary resections, the former seems to favor PAL prevention in case of anatomical sublobar resections thanks to more precise dissection of deep structures even in complex segmentectomies. Nevertheless, possible selection bias and heterogeneity of retrospective studies are major limitations of this review. In fact, complex resections at high risk for PAL development may be avoided by surgeons at the beginning of their learning curve with RATS. Therefore, additional prospective studies evaluating the performance of robotic surgery and VATS are advocated to confirm our considerations.
Keeping in mind that the best strategy to avoid PAL after lung resection is to prevent them, patient- and procedure-related risk factors should be considered so to undertake intraoperative precautions. The introduction of dedicated robotic staplers seems to be a promising tool to achieve this purpose, along with the use of ancillary techniques to guide the dissection such as preoperative planning based on CT scan 3D-reconstruction and the ICG-guided intersegmental plane identification.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Crisci, Alessandro Brunelli, Florian Augustin, Francesco Zaraca) for the series “Prolonged Air Leak after Lung Surgery: Prediction, Prevention and Management” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-818/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-818/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-818/coif). The special series “Prolonged Air Leak after Lung Surgery: Prediction, Prevention and Management” was sponsored by Bard Limited. Bard Limited has no interference on the contents of the special series. GV received grants from Umberto Veronesi Foundation, Intuitive Surgical Inc., AIRC, Italian Ministry of Health and Inail. GV is a consultant for Ab Medica SpA, Medtronic and Johnson & Johnson. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. [Crossref] [PubMed]
- Novellis P, Maisonneuve P, Dieci E, et al. Quality of Life, Postoperative Pain, and Lymph Node Dissection in a Robotic Approach Compared to VATS and OPEN for Early Stage Lung Cancer. J Clin Med 2021;10:1687. [Crossref] [PubMed]
- Veronesi G, Abbas AE, Muriana P, et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front Oncol 2021;11:726408. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Shintani Y, Funaki S, Ose N, et al. Preoperative variables for predicting prolonged air leak following pulmonary resection. J Thorac Dis 2019;11:S1891-3. [Crossref] [PubMed]
- Liang S, Ivanovic J, Gilbert S, et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:948-54. [Crossref] [PubMed]
- Yoo A, Ghosh SK, Danker W, et al. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clinicoecon Outcomes Res 2017;9:373-83. [Crossref] [PubMed]
- Attaar A, Luketich JD, Schuchert MJ, et al. Prolonged Air Leak After Pulmonary Resection Increases Risk of Noncardiac Complications, Readmission, and Delayed Hospital Discharge: A Propensity Score-adjusted Analysis. Ann Surg 2021;273:163-72. [Crossref] [PubMed]
- Yotsukura M, Okubo Y, Yoshida Y, et al. Predictive factors and economic impact of prolonged air leak after pulmonary resection. Gen Thorac Cardiovasc Surg 2022;70:44-51. [Crossref] [PubMed]
- Brunelli A, Chapman K, Pompili C, et al. Ninety-day hospital costs associated with prolonged air leak following lung resection. Interact Cardiovasc Thorac Surg 2020;31:507-12. [Crossref] [PubMed]
- Wood DE, Lauer LM, Layton A, et al. Prolonged length of stay associated with air leak following pulmonary resection has a negative impact on hospital margin. Clinicoecon Outcomes Res 2016;8:187-95. [Crossref] [PubMed]
- Attaar A, Tam V, Nason KS. Risk Factors for Prolonged Air Leak After Pulmonary Resection: A Systematic Review and Meta-analysis. Ann Surg 2020;271:834-44. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Seder CW, Basu S, Ramsay T, et al. A Prolonged Air Leak Score for Lung Cancer Resection: An Analysis of The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2019;108:1478-83. [Crossref] [PubMed]
- Hoeijmakers F, Hartemink KJ, Verhagen AF, et al. Variation in incidence, prevention and treatment of persistent air leak after lung cancer surgery. Eur J Cardiothorac Surg 2021;61:110-7. [Crossref] [PubMed]
- Cao C, Louie BE, Melfi F, et al. Outcomes of major complications after robotic anatomic pulmonary resection. J Thorac Cardiovasc Surg 2020;159:681-6. [Crossref] [PubMed]
- Su L, Ho H, Stock CT, et al. Surgeon Experience Is Associated With Prolonged Air Leak After Robotic-assisted Pulmonary Lobectomy. Ann Thorac Surg 2022;114:434-41. [Crossref] [PubMed]
- Patel RA, Velez-Cubian FO, Ng E, et al. Perioperative Factors Associated with Prolonged Air Leaks after Robotic-Assisted Thoracoscopic Pulmonary Lobectomy. J Am Coll Surg 2016;223:S25.
- Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Agzarian J, Fahim C, Shargall Y, et al. The Use of Robotic-Assisted Thoracic Surgery for Lung Resection: A Comprehensive Systematic Review. Semin Thorac Cardiovasc Surg 2016;28:182-92. [Crossref] [PubMed]
- Kneuertz PJ, D'Souza DM, Moffatt-Bruce SD, et al. Robotic lobectomy has the greatest benefit in patients with marginal pulmonary function. J Cardiothorac Surg 2018;13:56. [Crossref] [PubMed]
- Ng CSH, MacDonald JK, Gilbert S, et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations (Phila) 2019;14:90-116. [Crossref] [PubMed]
- Aiolfi A, Nosotti M, Micheletto G, et al. Pulmonary lobectomy for cancer: Systematic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach. Surgery 2021;169:436-46. [Crossref] [PubMed]
- Veronesi G, Maisonneuve P, Spaggiari L, et al. Diagnostic performance of low-dose computed tomography screening for lung cancer over five years. J Thorac Oncol 2014;9:935-9. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Kumar A, Deng JZ, Raman V, et al. A National Analysis of Minimally Invasive Vs Open Segmentectomy for Stage IA Non-Small-Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2021;33:535-44. [Crossref] [PubMed]
- Petersen RH. Is complex segmentectomy safe? Eur J Cardiothorac Surg 2021;61:108-9. [Crossref] [PubMed]
- Gergen AK, White AM, Mitchell JD, et al. Introduction of robotic surgery leads to increased rate of segmentectomy in patients with lung cancer. J Thorac Dis 2021;13:762-7. [Crossref] [PubMed]
- Zhou N, Corsini EM, Antonoff MB, et al. Robotic Surgery and Anatomic Segmentectomy: An Analysis of Trends, Patient Selection, and Outcomes. Ann Thorac Surg 2022;113:975-83. [Crossref] [PubMed]
- Yokota N, Go T, Fujiwara A, et al. A New Method for the Detection of Air Leaks Using Aerosolized Indocyanine Green. Ann Thorac Surg 2021;111:436-9. [Crossref] [PubMed]
- Sridhar P, Litle VR, Okada M, et al. Prevention of Postoperative Prolonged Air Leak After Pulmonary Resection. Thorac Surg Clin 2020;30:305-14. [Crossref] [PubMed]
- Zervos M, Song A, Li Y, et al. Clinical and Economic Outcomes of Using Robotic Versus Hand-Held Staplers During Robotic Lobectomy. Innovations (Phila) 2021;16:470-6. [Crossref] [PubMed]
- Miyajima M, Shindo Y, Tsuruta K, et al. Interlobar division using vessel-sealing system in robot-assisted pulmonary lobectomy. JTCVS Tech 2022;13:211-6. [Crossref] [PubMed]
- Robinson CL. Autologous blood for pleurodesis in recurrent and chronic spontaneous pneumothorax. Can J Surg 1987;30:428-9.
- Hugen N, Hekma EJ, Claessens NJM, et al. Efficacy of an Autologous Blood Patch for Prolonged Air Leak: A Systematic Review. Ann Thorac Surg 2022;114:1064-71. [Crossref] [PubMed]
- Hasan IS, Allen MS, Cassivi SD, et al. Autologous blood patch pleurodesis for prolonged postoperative air leaks. J Thorac Dis 2021;13:3347-58. [Crossref] [PubMed]
- Karampinis I, Galata C, Arani A, et al. Autologous blood pleurodesis for the treatment of postoperative air leaks. A systematic review and meta-analysis. Thorac Cancer 2021;12:2648-54. [Crossref] [PubMed]