Recent developments in video-assisted thoracoscopic surgery for pulmonary nodule management
Introduction
With increasing availability and accessibility of advanced radiological investigations, as well as with the advent of lung screening programs, the incidence of newly diagnosed pulmonary nodules is on the rise. A far-cry from the days when patients often present with advanced disease, thoracic surgeons are now receiving more referrals concerning management of incidental findings of pulmonary nodules on various modalities of imaging. Consequently, the demand on diagnostic and therapeutic thoracic procedures to manage these nodules is equally on the rise. The management of pulmonary nodules is very much dependent on the nature of the lesion, although ethnic and regional variations in what the nature of the lesion may entail can be quite variable. Malignant lung lesions have to be staged accordingly and if deemed resectable should be dealt with surgically according to oncological principles. The importance of obtaining good histology is paramount for appropriate management of lung nodules, for suspicious nodules, patients should be offered options of image-guided biopsy or surgical biopsy. However, the prospect of undergoing major surgery with lengthy incisions for the sole purpose of diagnosis often deters patients from surgery. This disregards the fact that surgical biopsy can yield more reliable and representative tissue for diagnosis, and also deprives patients of the possibility of same session completion lobectomy for primary malignant disease. Furthermore, in the current era when identification of the subtype and specific characteristics of adenocarcinomas can have significant prognostic and clinical significance, surgical excisional biopsy may more readily and accurately provide this information. Recent advancements in thoracic anaesthesia, minimally invasive surgical access, intra-operative identification techniques and the increasing evidence for sublobar resections in selected cases have all contributed to a paradigm shift in surgical management of small pulmonary nodules. The emergence of video-assisted thoracoscopic surgery (VATS) more than two decades ago perhaps started this evolution. Compared to thoracotomy, VATS is associated with advantages such as less post-operative pain, shorter hospital stay, less impairment of lung function and better preserved immune function postoperatively. Furthermore, VATS have been shown to result in at least comparable if not better long term survival than traditional open approaches following major lung resection for early stage non-small cell lung carcinoma. More recently, advances in instrumentation, anaesthesia technique and perioperative imaging have provided new opportunities for further refinement of VATS. The advent of uniportal VATS and the introduction of complimenting adjuncts such as non-intubated VATS, hybrid theatre & image guided VATS have broadened horizons and introduced new opportunities for future minimally invasive thoracic surgical procedures. This article shall provide an up-to-date perspective on the innovations and research into these recent developments in VATS pulmonary nodule management.
Uniportal VATS
The introduction of VATS in the 1990s has gone a long way to dispel unnecessary fear patients have of a painful and lengthy recovery process following thoracic surgery. VATS is preferred to open thoracotomy because of the smaller incisions, resulting in shorter length of hospital stay, and more rapid postoperative recovery (1). In practice, for patients with an indeterminate solitary pulmonary nodule, adequate lung function and low anaesthetic risks, it is reasonable to suggest, or at least provide the option of “upfront” VATS excision for diagnosis, as minimally invasive techniques no longer entail significant postoperative morbidity or mortality. Excision biopsy has the advantage of avoiding sampling error that may occur in other biopsy modalities, and is more likely to provide detailed histological information such as the invasiveness or subtype of adenocarcinoma. Furthermore, upon diagnosing malignancy at intraoperative frozen section, the patient may proceed to completion anatomical lung resection in the same general anaesthesia session.
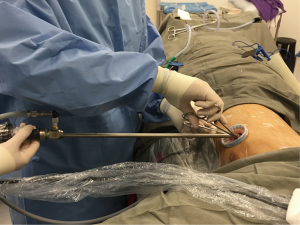
In conventional VATS, the camera port is situated inferiorly in the mid axillary line at 7th to 8th intercostal space with the anterior utility and posterior retraction ports situated in positons to form a triangle to facilitate maneuverability, creating a baseball diamond configuration with the operating site within the chest. Although conventional VATS was a significant improvement on open thoracotomy, nevertheless the procedure involved multiple ports and access through numerous intercostal spaces that could still result in chronic pain and neuralgia. In fact, stepwise evolution saw thoracic surgeons adopting the two port VATS approach by omitting the posterior retraction port, focusing most of the operative maneuvers via the anterior utility port. The two port approach was successful for major lung resections, nonetheless the technique still required two incisions (2). In 2004, Gaetano Rocco was the pioneer who first reported the uniportal VATS approach for lung wedge resection, utilizing only a single incision and intercostal space (3,4). With the potential advantages of less postoperative pain, better cosmesis and quicker recovery, he further explored the role of uniportal VATS by pioneering other procedures such as pericardial window, mediastinal surgery and pleural surgery by this approach. Initial case series and retrospective studies comparing uniportal VATS to multiport VATS in minor thoracic procedures such as sympathectomy and pleurodesis were encouraging, and showed lower pain scores and less residual paresthesia with the uniportal access (5). A major breakthrough came when Dr. Diego Gonzalez from Coruna performed the first lobectomy via uniportal approach in 2010, and since then with increasing experience and expertise, he has also reported uniportal complex lung resections including sleeve lobectomies, lung with chest wall resections and pulmonary artery resections (6,7). In addition, retrospective and small scaled comparative studies have found the uniportal VATS to be feasible and effective in anatomical resection of lung cancer, and is a safe alternative to multiport VATS (8-12) (Figure 1). From the operating surgeon’s perspective, another advantage of uniportal VATS is that the approach to the thorax through a single-incision actually mimics that of open surgery, which for some can be more familiar and ergonomically friendly than the multiport VATS approach which has the torsional dihedral angulated optical plane that is not favorable for two dimensional monitors (13). To date, there are no large-scale randomized controlled trials available to demonstrate statistically significant advantages of uniport VATS over conventional multiport VATS (14). Such trials to investigate the potential advantages of lesser postoperative pain and faster recovery are on-going at our institute (15). Nonetheless, with patient demand for less invasive procedures, and increasing popularity amongst thoracic surgeons, the future of uniportal VATS remains promising and is an attractive alternative for the diagnosis and treatment of patients with lung nodules in expert hands (16,17).
Non-intubated VATS
Apart from the physiological stress from surgery, one of the considerations patient face while opting for thoracic surgery is the need for general anaesthesia, and intubation for one lung ventilation during surgery. Theoretically single lung ventilation can be associated with elevated risk of pneumonia, airway damage, barotrauma, atelectasis and neuromuscular problems. During general anesthesia, paralysis impairs the efficiency of diaphragmatic contraction which results in an increased risk of atelectasis and also affects ability to cough and clear sputum (18). The non-intubated VATS approach was introduced to avoid the side effects of intubated general anesthesia and promote quicker recovery postoperatively (Figure 2). The rationale of this approach and proposed advantages include superior respiratory function early after surgery, reduced morbidity and mortality, shortened hospital stay and ultimately translation into lower hospital cost (19). Patients are awake and spontaneously ventilating under controlled sedation while given regional anesthesia. Modes of regional anesthesia include thoracic epidural anesthesia, intercostal or paravertebral nerve blocks in addition to controlled sedation with monitoring of cerebral activity. Vagal nerve blocks are also given to abolish cough reflexes. Anesthetic studies report minimal impairment of ventilatory mechanics and gaseous exchange in patients with chronic obstructive pulmonary disease after given thoracic epidural anesthesia (20). No detrimental effects have been seen on tidal volume and peak inspiratory flow. In addition, the effect on oxygenation is limited and easily amenable. Multiple reports and case series demonstrated non-intubated VATS to be feasible for simple and minor thoracic procedures such as segmentectomies and lung biopsies (21-23). Rates of conversion to intubated general anesthesia were reported to be between 2.3% to 10% (24). However, the role of non-intubated VATS remains controversial for major lung resection. Gonzalez-Rivas et al. demonstrated the feasibility of single incision VATS approach for major lung resection in a non- intubated patient with no use of epidural and vagus blockade. In the case report, a right middle lobectomy was performed with the patient fitted with a laryngeal mask and sedated with remifentanil (25). However, to date there is still lack of strong evidence supporting its use in major lung surgeries. Main contraindications of non-intubated thoracic surgery (NITS) include hemodynamic instability, morbidly obese patients, expected dense and extensive pleural adhesions, non-compliant patients, central and large tumors and contraindications to regional anesthesia such as coagulopathy. In a comparative study by Pompeo’s group, 60 patients with undetermined pulmonary nodule were allocated to two groups with 30 patients in the double lumen intubation group and another 30 in the NITS awake group. Results showed better postoperative satisfaction score and shorter median hospital stay in the NITS awake group, with 47% of the NITS awake group discharged 2 days following surgery compared with 17% in the intubated group (26). Perhaps the largest randomized controlled trial comparing intubated VATS to NITS VATS was conducted by Liu et al., in which 354 patients undergoing bullectomy, wedge resections and lobectomy were randomized to NITS thoracic epidural anesthesia with sedation versus under standard anaesthesia (27). The study found that independent of the type of surgical procedure, NITS resulted in shorter duration of hospital stay.
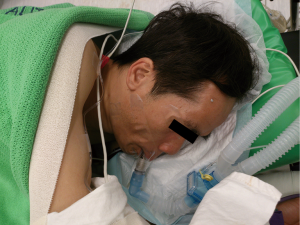
All in all, the role of NITS in major lung surgeries remains controversial. However, in the context of management of lung nodules planned for excision biopsy or sublobar resections, NITS can be a good alternative to standard anesthesia and should be offered to patients. It is noteworthy that for safe and successful execution of this technique, a surgeon experienced with VATS and an anesthetist who is experienced with sedation and can readily convert to intubated general anesthesia in case of emergency is essential.
Hybrid operating theatre and VATS
Screening for lung cancer in patients at risk can reduce cancer-specific mortality (28). Furthermore, around 8% to 51% of patients screened on CT scan will present with an indeterminate nodule (29), many of which will be smaller than 2cm, and may have partial solid component that require diagnosis (30). Some of these nodules are too small for accurate image guided biopsy, or those with significant ground glass component may provide inadequate or non-representative biopsy sample. Studies show that sensitivity of cancer diagnosis using percutaneous image guided biopsy decreases with size, from 90% for 3 cm diameter lesions to 70% for 1 cm lesions. Although other emerging technologies including electromagnetic navigational bronchoscopy can sometimes aid diagnosis, surgical excision biopsy remains the only reliable option in a significant number of cases.
Small lesions, especially those smaller than 1 cm or deep lesions can be difficult to localize intra-operatively, and conversion to larger wounds or even thoracotomy is occasionally required to ensure localization for complete resection (31). Since the surgical access and extent of surgery is very much dependent on the ability of the surgeon to identify the nodule, throughout the years, different methods have been described to “mark” the small lung nodules to guide surgery. Methods include percutaneous injection of dye such as methylene blue adjacent to the nodule so that it can be seen intraoperatively, or percutaneous hook wires and microcoils placement prior to surgery which provides visual, tactile as well as fluoroscopic guidance to the target lesion (32,33). These methods to better “image” the nodule have been found to improve tumor localization for resection.
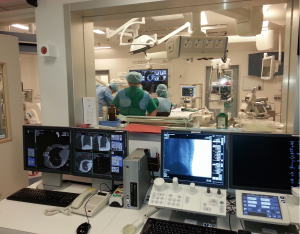
The combination of image guided localization techniques with VATS is known as iVATS. The emergence of iVATS allows for lung sparing surgery, decreases duration of the operation, reduces chance of conversion to thoracotomy, and plays an important role in identification of the nodule. However, the challenges of performing iVATS is that different expertise from multi-disciplines are required, and problems may occur when the locations for performing preoperative localization and surgical resection are at a distance apart. The spatial and temporal separation of the latter may put the patient at increased risk from wound contamination, migration of coils or hookwire, and complications from puncturing of the lung such as pneumothorax (tension) or hemothorax. The ideal strategy would be to have the localization and resection of the lung lesion in the same location and at the same session. This paves the way for the development of the hybrid operating theatre in the management of lung nodules (Figure 3). Mostly used during cardiac and vascular procedures, the hybrid operating theatre combines imaging capabilities with the setting of a standard operating theatre. In a study conducted by Gill et al., a new workflow for intraoperative marker guided iVATS in the CT scan equipped hybrid operating theatre is derived from their experience with 23 patients. The mean size of the lesions was 1.3 cm and all cases had complete resections done. T bars were inserted under intraoperative CT guidance and wedge resections of the nodules were performed in the same theatre right after localization. The median length of stay in their series was 4 days, and 3 (13%) patients had minor postoperative complications including air leak and pneumonia (34). The overall complication rates for iVATS are comparable to standard VATS. In their series, postoperative CT scan is repeated in the hybrid OR to ensure complete resection of the lesion, which is a definite advantage of having available “hands-on” real-time imaging. This landmark study demonstrated the safety and efficacy of using hybrid OR for resection of very small pulmonary nodules. In the same year, Ng et al.’s group also reported successfully using iVATS technique in hybrid theatre setting for single port VATS major lung resection in the treatment of small early stage lung cancer (35). However, to set up a successful hybrid OR iVATS program requires a multidisciplinary team of experts. Apart from a skilled surgeon and anesthetist, other specialists such as interventional radiologists, technicians and dedicated nurses are also important for a successful hybrid OR iVATS program. Combining the advantages of real-time imaging within hybrid operating theatre and specialized localization techniques, image guided VATS resection can potentially herald a shift in treatment paradigm in surgery for solidary pulmonary nodules and small early stage lung tumors.
Fluorescence thoracoscopy
The standard accepted treatment for lung cancer is lobectomy, as a randomized controlled trial in 1995 by the Lung Cancer Study Group (LCSG) reported a three- fold increase in local recurrence for sublobar resection (35). This study has since been criticized for being misleading as large tumors were also being included in the sublobar resection group. Subsequent reports and studies showed sublobar resection can have equivalent oncological outcomes in carefully selected cases of lung cancer with high percentage GGO, and for smaller and peripheral tumors (36). However, the role of VATS sublobar resection remains controversial as it is associated with higher recurrence rates. Proposed factors influencing recurrence and survival following sublobar resection include the status of the lymph nodes and the surgical margins (37). Lymph node status is related and considered important in the determination of the extent of resection, and hence intraoperative localization and identification of lymph nodes has become a topic of interest for surgeons looking to perform sublobar resections for small lung nodules.
The sentinel lymph node (SLN) is the first node within the lymphatic drainage from the tumor that would allow assessment and is often representative of the disease status of the remaining nodes. Sentinel node navigation surgery is already an accepted part of routine practice in skin cancers, breast cancer and gastrointestinal cancers. Its role in lung cancer is garnering interest lately, with its role in guiding therapy (38). To accurately identify the SLN, new intraoperative imaging modalities are needed to provide real time assessment of lymph node status and tumor borders. The near infrared (NIR) fluorescence light can be used to visualize structures during surgery. NIR fluorescent light can provide deep tissue penetration and low auto-fluorescence, thereby providing sufficient contrast to differentiate tissues. The use of NIR fluorescence imaging in combination with exogenous NIR fluorescent agents, such as indocyanine green (ICG), methylene blue and fluorescein, can detect various oncologically important structures in real-time. Following injection of fluorescent contrast around the tumor, NIR fluorescence imaging enables SLN mapping by real time intraoperative detection. Amongst the NIR fluorescent agents, ICG is currently the most popular for tumor surgery due to its low toxicity and lower incidence of allergic reactions.
The clinical application of NIR and ICG in thoracic surgery relates to guidance on extent of resection for pulmonary nodules diagnosed as lung cancer. The rationale is that if the SLN identified by intraoperative ICG injection is positive for metastasis, a sublobar resection approach may be associated with higher recurrence, and a more extensive surgical resection may be warranted. In a retrospective study by Moroga et al. the accuracy of sentinal lymph node biopsy (SLNB) is 80% (39). Eighty-three stage IA patients who underwent segmentectomies, 20 patients had SLNB, and 63 did not. The technique of identification involved injection of ICG around the tumor intra-operatively. ICG NIR fluorescence system was used to identify fluorescence 10 minutes after injection. Green fluorescent nodes were dissected as sentinel nodes prior to thoracoscopic resections. Overall in the SLNB group there was no recurrence. No cases of segmentectomy were converted to lobectomy. In the group without SLNB, locoregional relapse occurred in 6.3% of patients but the difference between the SLNB group was statistically insignificant. The conclusion from this study was that SLNB could potentially be useful in guiding segmentectomies, and that larger studies with more patients and longer follow up are needed to draw further conclusions. Other smaller series has found roughly similar accuracy rate of SLNB in terms of corresponding to the final pathology obtained from systematic lymph node dissection. Reasons for failed identification of SLN or inaccurate SLNB results, include leakage of ICG from tumor, dense adhesions and incomplete fissure making identification of SLN difficult. For the former misdemeanor, in some studies the tumor injection sites were immediately closed with endoloop or endoclip to prevent spillage and dispersal of ICG.
Overall, image guided thoracic surgery represents an exciting prospect for management of lung masses, paving the way for more specific and tailored patient care. With more advancements and improvements in NIR and contrast agents, we can expect increased utilization of fluorescence thoracoscopy not solely in determining extent of resection but also in localization of small lung nodules which very often represents a challenge in approach and resection during VATS.
Conclusions
With the increasing incidence of pulmonary nodules, there is a need for VATS to evolve to meet the challenges in both the diagnoses and treatment of these lesions. In the era of minimal invasive surgery, the call to innovate and improve on the old guard is gaining momentum. With the emergence of new approaches such as uniportal and non-intubated VATS, and exciting innovations in intra-operative imaging, VATS not only remains a reliable management option for patients with pulmonary nodules, but also an increasingly attractive one as side effects from anaesthesia and surgical access trauma are further minimized, and surgical accuracy improves. These contemporary developments should help keep VATS at the forefront of the diagnostic and therapeutic algorithm of lung nodules.
Acknowledgements
Funding: This work was supported by Direct Grant for Research, Ref No. 20132046; Research Grants Council General Research Fund, RGC GRF No. 14117715, Government of Hong Kong SAR.
Footnote
Conflicts of Interest: Calvin S. H. Ng has produced workflow booklet with Siemen for hybrid theatre use in thoracic surgery which was non-compensated. The other author has no conflicts of interest to declare.
References
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Rocco G, Khalil M, Jutley R. Uniportal video-assisted thoracoscopic surgery wedge lung biopsy in the diagnosis of interstitial lung diseases. J Thorac Cardiovasc Surg 2005;129:947-8. [Crossref] [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Ng CS. Thoracoscopic sleeve resection-the better approach? J Thorac Dis 2014;6:1164-6. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Ng CS, Kim HK, Wong RH, et al. Single-Port Video-Assisted Thoracoscopic Major Lung Resections: Experience with 150 Consecutive Cases. Thorac Cardiovasc Surg 2016;64:348-53. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]
- Salati M, Brunelli A, Rocco G. Uniportal video-assisted thoracic surgery for diagnosis and treatment of intrathoracic conditions. Thorac Surg Clin 2008;18:305-10. vii. [Crossref] [PubMed]
- Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg 2015;99:1739-44. [Crossref] [PubMed]
- Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5 Suppl 3:S214-6. [PubMed]
- Young R, McElnay P, Leslie R, et al. Is uniport thoracoscopic surgery less painful than multiple port approaches? Interact Cardiovasc Thorac Surg 2015;20:409-14. [Crossref] [PubMed]
- Ng CS, Gonzalez-Rivas D, D'Amico TA, et al. Uniportal VATS-a new era in lung cancer surgery. J Thorac Dis 2015;7:1489-91. [PubMed]
- Ng CS, Lau KK, Gonzalez-Rivas D, et al. Evolution in surgical approach and techniques for lung cancer. Thorax 2013;68:681. [Crossref] [PubMed]
- Ng CSH. Uniportal VATS in Asia. J Thorac Dis 2013;5:S221-5. [PubMed]
- Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013;117:133-41. [Crossref] [PubMed]
- Pompeo E. State of the art and perspective in non-intubated thoracic surgery. Asvide 2014;1:350.
- Gruber EM, Tschernko EM, Kritzinger M, et al. The effects of thoracic epidural analgesia with bupivacaine 0.25% on ventilatory mechanics in patients with severe chronic obstructive pulmonary disease. Anesth Analg 2001;92:1015-9. [Crossref] [PubMed]
- Pompeo E, Dauri M. Awake Thoracic Surgery Research Group. Is there any benefit in using awake anesthesia with thoracic epidural in thoracoscopic talc pleurodesis? J Thorac Cardiovasc Surg 2013;146:495-7.e1. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Gill RR, Jaklitsch MT, Jacobson FL. Controversies in lung cancer screening. (Internet). J Am Coll Radiol 2013;10:931-6. [Crossref] [PubMed]
- Libby DM, Smith JP, Altorki NK, et al. Managing the small pulmonary nodule discovered by CT. Chest 2004;125:1522-9. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Santambrogio R, Montorsi M, Bianchi P, et al. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg 1999;68:218-22. [Crossref] [PubMed]
- Zhou JH, Li WT, Chen HQ, et al. CT-guided hookwire localization of small solitary pulmonary nodules in video-assisted thoracoscopic surgery. Zhonghua Zhong Liu Za Zhi 2009;31:546-9. [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Nomori H, Ikeda K, Mori T, et al. Sentinel node navigation segmentectomy for clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2007;133:780-5. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Video-assisted thoracoscopic indocyanine green fluorescence imaging system shows sentinel lymph nodes in non-small-cell lung cancer. J Thorac Cardiovasc Surg 2011;141:141-4. [Crossref] [PubMed]
- Moroga T, Yamashita S, Tokuishi K, et al. Thoracoscopic segmentectomy with intraoperative evaluation of sentinel nodes for stage I non-small cell lung cancer. Ann Thorac Cardiovasc Surg 2012;18:89-94. [Crossref] [PubMed]


