Zhengyuan capsules for the treatment of chemotherapy-induced cancer-related fatigue in stage IIIB–IV unresectable NSCLC: study protocol for a randomized, multi-center, double-blind, placebo-controlled clinical trial
Introduction
Over 28.4 million new cancer cases will be projected worldwide in 2040 (1). Modern treatments have prolonged survival for most cancers, and consequently, the demands of quality of life (QoL) have increased. Thus, there has been considerable interest in recent years to improve the QoL of patients undergoing cancer therapy and managing its side effects more effectively.
While lung cancer remains the leading cause of cancer-related deaths, the 3-year relative survival of non-small cell lung cancer (NSCLC) patients rose from 19% in 2001 to 31% in 2017 due to extensive application of targeted therapy and immunotherapy (2). However, for NSCLC patients without driver genes and/or with low PD-L1 expression, and resistance to targeted therapy or immunotherapy, chemotherapy remains the primary approach for lung cancer (LC) at present (3). Although it has considerable toxic side effects due to the toxicity of anticancer drugs. For instance, the common symptoms of advanced LC that impair QoL, including pain, breathlessness, anorexia, nausea, vomiting, and fatigue are primarily induced by chemotherapy (4). One study showed chemotherapy as an independent factor of cancer-related fatigue (CRF), which is a common and severe symptom in patients receiving chemotherapy regimens (5). Notably, CRF is constantly present over 14 days after chemotherapy, increases cumulatively with consecutive cycles, and may even persist for years after treatment (6). For example, platinum-based chemotherapy drugs, which are widely used to treat LC, including NSCLC, can result in CRF due to platinum accumulation.
CRF has been defined by the National Comprehensive Cancer Network (NCCN) as a distressing, persistent, and subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is disproportionate to routine activities and frequently interferes with usual functions. About 80% of cancer patients experience CRF during treatment. Normally, CRF is rarely isolated and often accompanies symptoms such as pain, emotional distress, anemia, and sleep disturbances. Unlike ordinary fatigue, CRF cannot be relieved by sleep or rest, and its impact on daily life is decidedly worse. Patients in the advanced stages of cancer experience higher levels of physical and mental fatigue than those at the earlier stages and cancer survivors (7). Furthermore, it is closely related to the patients’ QoL and treatment expectations and can decrease survival (8,9). Although CRF is highly prevalent among cancer patients, it is not only underdiagnosed but also managed sub-optimally.
The pathogenesis of CRF is complex and unclear. Various clinical studies have implicated its underlying mechanisms as skeletal muscle metabolism, inflammatory response, and central nervous system function (10). Fatigue is always accompanied by dysregulated glycogen metabolism in the liver and muscle, and the ensuing reduction in hepatic glycogen levels is associated with an inflammatory tumor microenvironment (11). Studies show anemia, the most common hematological toxicity observed during chemotherapy (12,13), is associated with severe CRF (14,15). The accumulation of platinum-based chemotherapy drugs increases IL-8 production, which can trigger inflammation or aggravate existing anemia, resulting in CRF (16). In addition, psychosocial factors (pre-treatment fatigue, depression, sleep disturbance, dysfunctional coping and appraisal processes, loneliness, early life stress) and biobehavioral factors (physical inactivity, elevated body mass index) are also associated with CRF (17).
To date, efforts have been made to assess and treat CRF, but there is a lack of standard protocol. Current management of CRF is mainly pharmacological and non-pharmacological interventions (18). The latter includes cognitive-behavioral interventions, exercise, rest, and sleep, have the advantage of being free of side effects and easy to implement, as well as the disadvantage of being less effective in the short term and inapplicable to patients with severe fatigue. Pharmacological treatments are mainly hematopoietic growth factors, psychostimulants, and antidepressants. Specific drugs such as erythropoietin, methylphenidate, modafinil and paroxetine have been studied in various trials that have found the efficacy of drugs, but also the limitations of being effective in specific populations and the potential risk of long-term addiction.
In addition, traditional Chinese therapies such as acupuncture, moxibustion, and herbal medicines can effectively reduce CRF during and after cancer treatment, but it is difficult to identify the specific mechanism of fatigue relief due to the individual nature of traditional Chinese medicine (TCM) (19-22). The treatments above have been implemented on a small part of the population and are not widely used due to the complexity of the treatment or the different physical conditions. In summary, there are no standardized treatment options for patients worldwide. Therefore, it is essential to develop a novel strategy to relieve CRF effectively and conveniently and improve the QoL of patients.
Zhengyuan capsules are a patented Chinese medicine developed by the Yangtze River Pharmaceutical Group Co., Ltd. to prevent and treat CRF. Zhengyuan capsules is an oral formulation that can be administered with ease, the main ingredients of which are Epimedium, raw sun-dried ginseng, honey-fried Astragalus, turtle shell powder, Ligustrum lucidum, Atractylodes macrocephala and dried tangerine peel. CRF belongs to the category of “consumptive disease” in TCM. Patients suffer from Qi consumption and Blood damage because chemotherapy can impair the spleen and stomach and lead to liver and kidney deficiency. Zhang Zhongjing emphasized that treatment of CRF should focus on warming spleen-kidney.
Zhengyuan capsules have the effect of nourishing qi to invigorate spleen and nourishing kidney essence. The therapeutic effects of Zhengyuan capsules against chemotherapy-induced CRF have been demonstrated in a mouse model of A549 lung adenocarcinoma. Clinical trials also suggest that Chinese herbal medicines (CHM) are effective and safe for CRF. However, current evidence is insufficient to draw a definite conclusion regarding the safety and efficacy of proprietary Chinese medicines, and further trials are warranted.
Therefore, we have designed a randomized controlled trial to assess the efficacy and safety of Zhengyuan capsules in advanced NSCLC patients with CRF and provide preliminary evidence for clinical applications. The primary objective will be the Brief Fatigue Inventory (BFI) score, while secondary objectives will include the Revised Piper’s Fatigue Scale (RPFS) score, QoL score, clinical symptom score, inflammation and metabolism index, and progression-free survival (PFS).
Methods
Study design
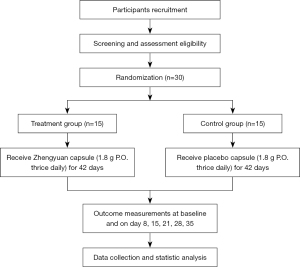
This multi-center, randomized, double-blind, placebo-controlled clinical trial is primarily designed to assess the efficacy and safety of Zhengyuan capsules for treating CRF in stage IIIB–IV unresectable NSCLC patients undergoing chemotherapy. The study also aims to evaluate the effect of the Zhengyuan capsules on the QoL of patients and investigate other relevant indicators of CRF. The flowchart of the study design is presented in Figure 1. Thirty participants meeting all inclusion criteria will be randomized into a Zhengyuan capsule (treatment) or placebo capsule (control) group and receive the respective capsules for 42 consecutive days starting from the first day of the first chemotherapy cycle.
Setting and participants
Participants will be recruited from The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Hunan Provincial Tumour Hospital, and Yunnan Provincial Tumour Hospital. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine has approved this trial (No. ZYYECK[2020]096), all participating hospitals/institutions were informed and agreed the study. Informed consent will be obtained before each participant is enrolled, and participants will be free to withdraw at any time.
Randomization, allocation concealment, and blinding
Participants will be randomly divided into control or treatment groups using the centralized interactive web response system (IWRS) and identified by a random number generated through a block randomization sequence by SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) in a 1:1 ratio. Randomization will take place by minimization, and different chemotherapy regimens [pemetrexed-cisplatin (AP), paclitaxel-cisplatin (TP), and docetaxel-cisplatin (DP)] and degree of fatigue (mild 1–3, moderate 4–7, and severe 8–10) will be used as the stratification factors. Project sponsors, experimental technicians, outcome analysts, data managers, and statisticians will be unaware of the treatment allocations. To ensure blinding, the real capsule and the corresponding placebo will have the same appearance, packaging, label, and logo.
Diagnostic criteria
Diagnostic criteria for NSCLC
The diagnostic criteria for NSCLC will adopt “The Diagnosis and Treatment Criterion for Common Cancer in China” compiled by the Department of Medical Affairs of the Ministry of Health of the People’s Republic of China.
Diagnostic criteria for CRF
According to the criteria proposed by the Tenth Conference in the Revision of International Classification of Diseases (ICD-10), CRF is diagnosed when fatigue symptoms recur for more than two weeks with five or more of the following symptoms: (I) weakness or heaviness in the body, (II) lack of concentrated attention, (III) lack of enthusiasm, depression and decreased interest, (IV) insomnia or lethargy, (V) lack of vitality regardless of proper sleep and rest, (VI) difficulty in movement, (VII) emotional responses including sadness and frustration, (VIII) inability to conduct routine activities that were previously possible, (IX) short-term memory loss, and (X) fatigue symptoms that last for several hours without relief.
Inclusion criteria
- Stage IIIB–IV unresectable NSCLC with negative driving genes diagnosed by pathology or cytology.
- Estimated life expectancy ≥3 months, Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤2, age between 18 and 65 years.
- Consent to receive platinum-based chemotherapy regimen (taxanes in combination with cisplatin/carboplatin for patients with squamous cell carcinoma, pemetrexed combined with cisplatin/carboplatin for non-squamous cell carcinoma patients, tumor angiogenesis inhibitor or immune checkpoint inhibitors may be used).
- Willingness to accept the treatment during the trial, ability to insist on the treatment according to the doctor’s advice, and good compliance.
- Confirmed by diagnostic criteria for CRF, BFI score ≥4.
Exclusion criteria
- Any condition hindering participants from completing the clinical trial, including but not limited to severe and uncontrollable organic disease or infection, unstable angina, and congestive heart failure.
- Severe abnormal liver and renal dysfunction [serum creatinine ≥1.5 times upper limit of normal (ULN), ALT or AST ≥3 times ULN; Bilirubin ≥1.5 times ULN], neurological symptoms, psychiatric illness or disorder with unmanageable symptoms, or hypothyroidism with no signs of improvement.
- Patients with cognitive dysfunction and speech expression disorder and without the ability to complete the questionnaire independently or with the help of others.
- Concurrent chemoradiotherapy.
- Pregnant or lactating women, or those planning to get pregnant.
- Allergies to the components of the trial drug.
- Participation in other clinical trials within three months.
- Infectious diseases such as Hepatitis B, HIV, or syphilis.
- Other malignant tumours.
- TCM syndrome differentiation criteria in line with the hyperactivity of fire excess from yin deficiency.
- General unsuitability to participate in the trial as judged by the investigator.
Withdrawal/termination criteria
- Any serious adverse reactions to the study drug during the treatment regimen, or lack of improvement after active treatment.
- Voluntary withdrawal by the patient.
Interventions
Description of trial drugs
Treatment group: Zhengyuan capsules (0.45g/one tablet) orally administered three times a day, four tablets each time. Components: Epimedium, raw sun-dried ginseng, honey-fried Astragalus, turtle shell powder, Carapax Trionycis, Ligustrum lucidum, Atractylodes macrocephala, and dried tangerine peel manufactured by the Yangtze River Pharmaceutical Group Guangzhou Hairui Pharmaceutical Co. Ltd.
Control group: Zhengyuan capsule simulant (specification: 0.45 g/one capsule) orally administered three times a day, four tablets each time. Components: Lactose, caramel, carotene, and ultrapure water, manufactured by the Yangtze River Pharmaceutical Group Guangzhou Hairui Pharmaceutical Co. Ltd
Treatment regimen
Eligible patients will be randomly divided into the treatment and control groups at a 1:1 ratio and receive conventional platinum-based dual-drug chemotherapy (21 days cycle) and Zhengyuan capsules or Zhengyuan-simulant, respectively, as detailed above. The capsules will be taken for 42 consecutive days from day 1 of chemotherapy in the first week.
Squamous cell carcinoma patients will receive taxanes in combination with cisplatin/carboplatin, and non-squamous cell carcinoma patients will receive pemetrexed in combination with cisplatin/carboplatin. The detailed regimen is outlined in Table 1. The chemotherapy dose will be lowered in case of CTCAE grade 3 adverse reaction and ceased if CTCAE grade 4 adverse reactions are observed. The trial drug will be continued even if the chemotherapy regimen requires change due to tumour progression.
Table 1
| Type | Chemotherapy regimen | Dose | Time | Cycle |
|---|---|---|---|---|
| Squamous cell carcinoma | TP regimen | Every 21 days for a cycle | ||
| Paclitaxel/Paclitaxel albumin-bound type/Paclitaxel liposome | 135–175 mg/m2; 260 mg/m2; 135–175 mg/m2 |
Day 1 | ||
| Cisplatin/Carboplatin | Cisplatin 75 mg/m2 | Day 1–3 | ||
| Carboplatin AUC =5–6 | Day 1 | |||
| DP regimen | Every 21 days for a cycle | |||
| Docetaxel | 75 mg/m2 | Day 1 | ||
| Cisplatin/Carboplatin | Cisplatin 75 mg/m2 | Day 1–3 | ||
| Carboplatin AUC =5–6 | Day 1 | |||
| Non-squamous cell carcinoma | AP regimen | Every 21 days for a cycle | ||
| Pemetrexed | 500 mg/m2 | Day 1 | ||
| Cisplatin/Carboplatin | Cisplatin 75 mg/m2 | Day 1–3 | ||
| Carboplatin AUC =5–6 | Day 1 |
NSCLC, non-small cell lung cancer; TP, paclitaxel-cisplatin; DP, docetaxel-cisplatin; AP, pemetrexed-cisplatin; AUC, area under the curve.
Combination therapy
- CHM, including oral and injectable preparations, and containing ginseng, Codonopsis Pilosula, Astragalus membranaceus, and other similar ingredients that strengthen and invigorate Qi will not be administered during the study period.
- Drugs that can improve immune function, such as lentinan, placental polypeptide, and thymosin, will not be administered.
- The following proprietary antineoplastic proprietary Chinese medicines can be administered: Brucea Javanice soft capsule, Brucea Javanica Oil Emulsion, compound Kushen injection, compound antharides sodium vitamin B6 injection, Cinobufacini injection, Xihuang Pills, Jinlong Capsule, and Huaier granules.
- Transfusion of plasma, red blood cells, platelets, and other blood products will be permitted if a patient suffers from severe anaemia or thrombocytopenia during treatment. Colony stimulating factor can be used in patients with neutropenia.
- Cancer-related pain, vomiting, and other symptoms can be managed when needed, and interventions must be accurately documented.
Data collection
The data collection process is outlined in Table 2.
Table 2
| Study schedule | Screening | First course of treatment | Second course of treatment | 6-week follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-3-0 | D1±1 | D8±1 | D15±1 | D21±1 | D28±1 | D35±1 | D43±1 | D84±1 | ||||
| Vital signs | X | X | X | X | ||||||||
| BFI | X | X | X | X | X | X | X | X | ||||
| Piper | X | X | X | X | X | X | X | X | ||||
| EORTC QLQ-C30v3.0, QLQ-LC13 | X | X | X | X | ||||||||
| Clinical symptom score | X | X | X | X | ||||||||
| Haematology exploratory index | X | X | X | X | ||||||||
| AEs* | X | X | X | X | X | X | X | X | – | |||
*, record according to the actual situation. BFI, brief fatigue inventory; EORTC QLQ-C30, Organization for Research and Treatment of Cancer Quality of Life Questionnaire Module C30; QLQ-LC13, Quality of Life Questionnaire-Lung Cancer 13; AE, adverse event.
Outcome measurements and assessment
The primary outcome is the change in the BFI score during chemotherapy cycles and the difference in BFI scores between the two groups. Secondary outcomes include the RPFS-Chinese Version, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Module C30 (EORTC QLQ-C30), Lung Cancer 13 (QLQ-LC13), clinical symptoms, the haematology exploratory index, PFS, and safety.
Baseline data
Baseline data including vital signs, BFI, RRFS, EORTC QLQ-C30 v3.0, and EORTC QLQ-LC13, clinical symptom score, blood analysis, urine routine, stool routine, liver and kidney function tests, coagulation function, and electrocardiograms will be recorded. Serum samples will be stored for further evaluation.
BFI
BFI is used to rapidly assess the severity and impact of CRF on daily functioning over a 24-h period. It has adequate reliability, validity, and internal responsiveness when used to assess CRF in patients hospitalized for chemotherapy (23). The degree and impact of CRF is scored using a scale from 1 to 10 (1 indicating the absence of fatigue and 10 indicating maximum fatigue). The global fatigue score is obtained by calculating the average of all the items, and fatigue is accordingly classified as mild (1 to 3 points), moderate (4 to 6 points), and severe (7 to 10 points). Researchers will assess the BFI score at baseline, on days 8, 15, 21, 28, and 35 during treatment, and on days 43 and 84 during the follow-up. The score and time-dependent changes will be compared between the control and experimental groups.
RPFS
RPFS is one of the most widely used instruments to evaluate CRF, and consists of 22 questions, including five open-ended questions in four dimensions: Behavioural/severity (six items), affective meaning (five items), sensory (five items), and cognitive/mood (six items) (24). The scores range from 0 to 10 points for each item, and the total score is the sum of the average score for each question. Higher scores indicate more severe fatigue. RPFS will be assessed at the same time as BFI. The difference between the two groups across multiple dimensions, and the difference and trend in the change of the primary and subscales can be used to evaluate the improvement in CRF after treatment.
EORTC QLQ-C30 and QLQ-LC13
The International Consortium for Health Outcomes Measurement has chosen the EORTC QLQ-C30 and QLQ-LC13 for scoring patient-reported outcomes. Both scales can also be used as outcome indicators for lung cancer (25). The patient-reported parameters, including QoL, function and behaviour, symptoms, and side effects of treatment, will be assessed at baseline, and on days 21, 43, and 84 of treatment.
EORTC QLQ-C30 is a 30-item quality-of-life questionnaire on function, symptoms, and general health, and is routinely used to monitor clinical outcomes due to its ease of use and brevity (26). Higher scores for the functional and general health items indicate better outcomes, while higher symptom scores are indicative of more severe symptoms.
QLQ-LC13 is a standard tool for measuring the QoL of patients with lung cancer (27), assessing 13 typical symptoms of lung cancer patients, such as coughing, pain, dyspnoea, sore mouth, peripheral neuropathy, and hair loss. Higher scores indicate worse symptoms.
Clinical symptom score
Patients will be instructed to report CRF symptoms such as fatigue and listlessness, shortness of breath and laziness to speak, poor appetite, sore waist and soft legs, dizziness, and spontaneous perspiration in the form of ePRO. These symptoms will be scored to evaluate treatment effects at baseline and on days 1, 28, 43, and 84 of treatment.
Haematology exploratory index
Peripheral blood will be collected at baseline and days 1–3 before each chemotherapy cycle. The serum will be stored at −20 ℃ and tested for IL-6, CRP, IL-1β, IL-1α, TNF-α, HGB, IL-8, and other CRF-related indicators using suitable kits.
PFS
The survival follow-up phase will be initiated after the trial, and the investigator will not interfere with any treatments during the follow-up. The treatment plan, PFS, and survival will be recorded. PFS will be evaluated by computed tomography (CT), magnetic resonance (MR), and other imaging techniques.
Safety assessment
Safety indicators including blood, urine and fecal routine, liver and kidney function, coagulation and electrocardiogram will be performed before chemotherapy. Any adverse reactions that occur during treatment will be assessed by the National Cancer Institute Common Toxicity Criteria (NCI CTC 4.0).
Estimation of sample size
Based on past observational data, we hypothesize the mean difference of BFI between the Zhenyuan group and placebo group is 1.7, with a standard deviation of 1.5. With a significance level (alpha) of 0.050 and 80% power, the sample size was calculated to be 13. Given the 10% dropout rate, 15 patients will be enrolled in each group.
Statistical analysis
We will analyze data in both intention-to-treat (ITT) and per-protocol (PP) principles and discuss the difference between the IIT and PP principles. The homogeneity of variance test will test all measurement data. T-test and paired sample t-test will be used for inter-group and intra-groups comparisons, respectively, if the variance is homogeneous and the data points are normally distributed. Otherwise, the Mann-Whitney U test or t-test will be used to compare between the two groups, and the Wilcoxon test signed-rank test or Wilcoxon signed rank test will be used for intra-group comparison. Since BFI, RRFS, EORTC QLQ-C30, EORTC QLQ-LC13, and the haematology exploratory index require repeated measurements and may fluctuate with the chemotherapy cycle, a generalized linear model will be used to determine the interaction between groups and the time-dependent changes to better distinguish between the effects of treatment and time. Adjustment factors including age (<60, ≥60 y), chemotherapy regimens, and research centers will be used.
All statistical tests are two-sided, and P value <0.05 will be considered statistically significant. Empowerstats (www.empowerstats.com; X&Y Solutions Inc., Boston, MA, USA) and R (http://www.R-project.org) will be used for statistical analyses. For missing data, the potential reasons will be analysed, and imputation adjustment methods will be used. The last observation method (LOCF) will be used to estimate the missing values, and different sensitivity analyses will be performed to assess the impact of missing data on the experimental results after the main analysis.
Adverse events (AEs)
The drug exposure history, general physical examination status, vital signs, laboratory tests, and electrocardiograms of patients will be used as the indicators for safety evaluation. An AE is any untoward medical occurrence in a subject administered the trial drug and is not necessarily causally related to this treatment. Once patients sign the informed consent, the AEs will be assessed at each visit during the study period through non-dominant questioning, self-reporting, physical examination, laboratory examination, or other methods. The investigator will record the patients’ symptoms, severity, time of occurrence, duration, and treatment measures in the original medical record and evaluate their relevance to the trial drug. All AEs will be classified according to the NCI CTC 4.0.
Quality control and trial management
The principal investigator will ensure all researchers have received appropriate training related to the research, any information related to the research is relayed to the relevant personnel, and proper training records are kept. The original patient medical records will be kept for reference, and a case report form will be completed for each screened and selected case. Researchers will ensure the accuracy and reliability of data collection. If inconsistency in the detection and measurement methods of laboratory data from the different centers occurs, the Bland-Altman method will be used for correction.
Discussion
CRF is challenging to manage during and after cancer treatment and may even be exacerbated by other cancer-related AEs such as depression, anxiety, sleep disturbance, and pain (28). It not only lowers the motivation of patients to complete treatment but also adversely impacts their functional status and QoL, which is an independent prognostic factor for overall survival in advanced NSCLC (29,30). Chemoradiotherapy, female sex, insomnia, neuroticism, pain, depression, and poor PSs are some of the risk factors for CRF, the latter of which is the most significant (31). In the TCM Syndrome Differentiation, most patients with CRF have Qi deficiency or blood deficiency with clinical manifestations such as lack of strength, spontaneous sweating, dizziness, shortness of breath and laziness to speak, pale tongue, and vacuous pulse, which significantly contribute to the poor PSs. TCM is recommended for CRF management in clinical practice, although further evidence is required to confirm its positive effects.
Pharmacological interventions for CRF include psychostimulants, antidepressants, corticosteroids, and erythropoietin-stimulating agents (ESAs) (32). However, these drugs carry harmful side effects and some target only specific CRF. For instance, ESAs can effectively improve chemotherapy-induced anemia to relieve CRF but have little effect on patients without anemia. Therefore, TCM formulations that benefit most patients with fewer side effects are ideal for the management of CRF since they primarily focus on strengthening the constitution and physical strength of recipients. In China, patients prefer TCM to treat disease and relieve uncomfortable symptoms. Single herbs like Panax ginseng (33) and Panax quinquefolius (34), decoctions such as Bojungikki-Tang (35) and Sipjeondaebo-Tang (36), and proprietary formulations like the Shenfu injection (37) are proven to be effective for CRF with minimal side effects. Zhengyuan capsules invigorate Qi, the spleen, and kidneys, filling essence, softening firmness, and dispelling knots to improve symptoms of fatigue by enhancing post-chemotherapy hepatic glycogen levels and improving hematopoietic and immune function. However, they are unsuitable for patients with fire excess from yin deficiency.
This study has some limitations. First, small sample size may lead to false-negative results. Second, CRF is a chronic symptom and may persist long after the treatment is over. Since the intervention and follow-up will last only six weeks, it will be difficult to prove the long-term benefits of Zhengyuan capsules. Third, other chemotherapy regimens for NSCLC are used in the clinical setting and have not been included in the study design. Finally, we cannot completely control the impact of non-drug interventions on the results of this trial. Despite these limitations, we believe this study will provide preliminary data on Zhengyuan capsules regarding their safety, efficacy, and potential as an effective therapeutic option for CRF.
Acknowledgments
We acknowledge the Yangtze River Pharmaceutical Group Guangzhou Hairui Pharmaceutical Co. Ltd. to supply the Zhengyuan capsules and placebo. We appreciated Hunan Provincial Tumour Hospital, and Yunnan Provincial Tumour Hospital for recruitment and enrolment. We also acknowledge the China Academy of Chinese Medical Science, China Center for Evidence-Based Traditional Chinese Medicine for technical support.
Funding: This work is supported by the National Administration of Traditional Chinese Medicine: 2019 Project of building evidence-based practice capacity for TCM (No. 2019XZZX-ZXZL-5).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1263/coif). All authors report that Yangtze River Pharmaceutical Group Co., Ltd. provided Zhengyuan capsules and a placebo for the study while not participating in research design, data collection, data analysis, data interpretation, or article writing. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). This trial has been approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (No. ZYYECK[2020]096), all participating hospitals/institutions were informed and agreed the study. Informed consent will be obtained before each participant is enrolled, and participants will be free to withdraw at any time.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Pirker R. Chemotherapy remains a cornerstone in the treatment of nonsmall cell lung cancer. Curr Opin Oncol 2020;32:63-7. [Crossref] [PubMed]
- Henson LA, Maddocks M, Evans C, et al. Palliative Care and the Management of Common Distressing Symptoms in Advanced Cancer: Pain, Breathlessness, Nausea and Vomiting, and Fatigue. J Clin Oncol 2020;38:905-14. [Crossref] [PubMed]
- Minton O, Strasser F, Radbruch L, et al. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage 2012;43:226-35. [Crossref] [PubMed]
- Miller M, Maguire R, Kearney N. Patterns of fatigue during a course of chemotherapy: results from a multi-centre study. Eur J Oncol Nurs 2007;11:126-32. [Crossref] [PubMed]
- de Raaf PJ, de Klerk C, Timman R, et al. Differences in fatigue experiences among patients with advanced cancer, cancer survivors, and the general population. J Pain Symptom Manage 2012;44:823-30. [Crossref] [PubMed]
- Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 2011;103:1851-8. [Crossref] [PubMed]
- Adam S, van de Poll-Franse LV, Mols F, et al. The association of cancer-related fatigue with all-cause mortality of colorectal and endometrial cancer survivors: Results from the population-based PROFILES registry. Cancer Med 2019;8:3227-36. [Crossref] [PubMed]
- Yang S, Chu S, Gao Y, et al. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019;8:738. [Crossref] [PubMed]
- Hirai K, Ishiko O, Tisdale M. Mechanism of depletion of liver glycogen in cancer cachexia. Biochem Biophys Res Commun 1997;241:49-52. [Crossref] [PubMed]
- Bahl A, Sharma DN, Julka PK, et al. Chemotherapy related toxicity in locally advanced non-small cell lung cancer. J Cancer Res Ther 2006;2:14-6. [Crossref] [PubMed]
- Kosmidis P, Krzakowski M. ECAS Investigators. Anemia profiles in patients with lung cancer: what have we learned from the European Cancer Anaemia Survey (ECAS)? Lung Cancer 2005;50:401-12. [Crossref] [PubMed]
- Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology 2005;69:2-7. [Crossref] [PubMed]
- Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer 2014;111:33-45.
- Zhang Y, Huang X, Feng S, et al. Platinum Accumulation and Cancer-Related Fatigue, Correlation With IL-8, TNF-α and Hemocytes. Front Pharmacol 2021;12:658792. [Crossref] [PubMed]
- Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597-609. [Crossref] [PubMed]
- Mohandas H, Jaganathan SK, Mani MP, et al. Cancer-related fatigue treatment: An overview. J Cancer Res Ther 2017;13:916-29. [PubMed]
- Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470-6. [Crossref] [PubMed]
- Oh B, Butow P, Mullan B, et al. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann Oncol 2010;21:608-14. [Crossref] [PubMed]
- Lee S, Jerng UM, Liu Y, et al. The effectiveness and safety of moxibustion for treating cancer-related fatigue: a systematic review and meta-analyses. Support Care Cancer 2014;22:1429-40. [Crossref] [PubMed]
- Su CX, Wang LQ, Grant SJ, et al. Chinese herbal medicine for cancer-related fatigue: a systematic review of randomized clinical trials. Complement Ther Med 2014;22:567-79. [Crossref] [PubMed]
- Nunes AF, Bezerra CO, Custódio JDS, et al. Clinimetric Properties of the Brief Fatigue Inventory Applied to Oncological Patients Hospitalized for Chemotherapy. J Pain Symptom Manage 2019;57:297-303. [Crossref] [PubMed]
- Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum 1998;25:677-84. [PubMed]
- Bozcuk H, Dalmis B, Samur M, et al. Quality of life in patients with advanced non-small cell lung cancer. Cancer Nurs 2006;29:104-10. [Crossref] [PubMed]
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 2009;20:17-25. [Crossref] [PubMed]
- Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development. Cancer 2015;121:4300-23. [Crossref] [PubMed]
- Kuhnt S, Ernst J, Singer S, et al. Fatigue in cancer survivors--prevalence and correlates. Onkologie 2009;32:312-7. [Crossref] [PubMed]
- Qi Y, Schild SE, Mandrekar SJ, et al. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. J Thorac Oncol 2009;4:1075-82. [Crossref] [PubMed]
- Daly LE, Dolan RD, Power DG, et al. Determinants of quality of life in patients with incurable cancer. Cancer 2020;126:2872-82. [Crossref] [PubMed]
- Ma Y, He B, Jiang M, et al. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int J Nurs Stud 2020;111:103707. [Crossref] [PubMed]
- Klasson C, Helde Frankling M, Lundh Hagelin C, et al. Fatigue in Cancer Patients in Palliative Care-A Review on Pharmacological Interventions. Cancers (Basel) 2021;13:985. [Crossref] [PubMed]
- Yennurajalingam S, Tannir NM, Williams JL, et al. A Double-Blind, Randomized, Placebo-Controlled Trial of Panax Ginseng for Cancer-Related Fatigue in Patients With Advanced Cancer. J Natl Compr Canc Netw 2017;15:1111-20. [Crossref] [PubMed]
- Barton DL, Soori GS, Bauer BA, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer 2010;18:179-87. [Crossref] [PubMed]
- Jeong JS, Ryu BH, Kim JS, et al. Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integr Cancer Ther 2010;9:331-8. [Crossref] [PubMed]
- Lee JY, Kim EH, Yoon JH, et al. Traditional Herbal Medicine, Sipjeondaebo-Tang, for Cancer-Related Fatigue: A Randomized, Placebo-Controlled, Preliminary Study. Integr Cancer Ther 2021;20:15347354211040830. [Crossref] [PubMed]
- Gu Y, Xu H, Zhao M. Clinical study on effect of Shenfu injection treating cancer-related fatigue of patients with advanced carcinoma. Zhongguo Zhong Yao Za Zhi 2010;35:915-8. [PubMed]