
Gender differences in the effective dose of alfentanil in painless bronchoscopy
In a previous edition of this Journal, an interesting study (1) was presented addressing the effective dose of alfentanil in suppressing bronchoscopy responses to painless bronchoscopy with an i-gel supraglottic airway device. Their main findings suggested that the ED50 of alfentanil for suppressing responses to painless bronchoscopy in females and males was 13.68±4.75 and 17.96±3.45 µg/kg, respectively, and no significant difference (P=0.078) was observed between them. These results may provide a reference for clinicians to use alfentanil in painless bronchoscopy with no need to consider the influence of gender factors. As animal and human studies have suggested that there existed gender differences in opioid-induced analgesia and associated adverse events (2-5), clinicians need to be aware of the gender differences when administering opioids (6). The authors have studied a very interesting topic and provided novel evidence on this matter, though more investigations are needed to confirm the results.
Coincidentally, we are doing a similar study, and some of the results are very similar, but not consistent with the result of gender differences in alfentanil efficacy as previously reported (1), and we hope to discuss this issue. Our randomized, double-blinded clinical trial was designed and registered (ChiCTR2100049052) to assess the safety and efficacy of a fixed dose of midazolam (0.05 mg/kg) combined with different doses of alfentanil (10–30 µg/kg) in diagnostic flexible bronchoscopy (DFB) sedation. A total of 270 adult patients (135 females vs. 135 males; 18–65 years; ASA grade I or II) were recruited for this study, through a randomization software program, and then divided by gender and then assigned randomly into 9 different dose groups containing 15 patients each. All patients undergoing fiberoptic bronchoscopy received topical anesthesia with 2% lidocaine and sedation regimes with the combination of midazolam (0.05 mg/kg) plus alfentanil at 9 different dosages (10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5, 30 µg/kg). The intravenous sedative midazolam was administered two minutes before alfentanil, and patients breathed spontaneously at all times with a nasopharyngeal tube for oxygen supply or manual assisted ventilation when necessary. An independent third-party observer was responsible for rating his perception of the patient’s severity of cough during the procedure on a 10-point visual analogue scale (VAS) (7), where 0 represented no cough and 10 represented incessant intolerable cough resulting in procedural interference. The primary outcome in our study was cough severity evaluated by VAS, which refers to the intense stress irritated by the insertion of bronchoscope into larynx and respiratory tract, and was dangerous to patients and might affect the operation of bronchoscope. A bronchoscopy VAS score ≤1 was defined as a negative reaction to the bronchoscopy, and our probit analysis showed that the ED50 of alfentanil for suppressing responses to painless bronchoscopy in female and male was 18.47 µg/kg [95% confidence interval (CI): 17.1 to 19.75 µg/kg] and 22.21 µg/kg (95% CI: 18.94 to 27.73 µg/kg), respectively, with a significant difference (P=0.048) between them.
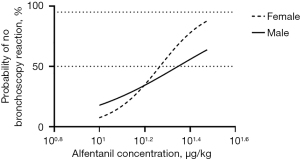
As shown in Figure 1, our results suggested a gender difference in the effective dose of alfentanil in painless bronchoscopy. Although there are differences between our study and the earlier study (1) in the combined use of drugs (propofol vs. midazolam), ventilation mode (manually ventilated via the i-gel supraglottic airway device when necessary vs. spontaneous ventilation with a nasopharyngeal tube for oxygen supply), negative criteria (the bronchoscopy score combining three variables including movement of the vocal cords, cough occurrence, and limb movement vs. cough VAS score) and measurement of ED50 value (an Up-and-Down Sequential Allocation Trial vs. Bliss method), some of the results are similar or comparable. For example, we agree with the authors that the optimal dose of alfentanil required for painless bronchoscopy is significantly higher than in some other studies, as Yu et al. (8) reported that the optimal dose of alfentanil in combination with propofol was down to 10 µg/kg. However, with regard to the gender difference in alfentanil, our findings are obviously different from those of the earlier study (1), and this discrepancy needs to be clarified for the importance of gender differences in clinical application of alfentanil.
Many other factors may affect the ED50 value of alfentanil, such as the patient's disease state, age, degree of obesity, and the level of the bronchoscopy operator, but obviously the earlier study (1) has some shortcomings, including a large age span, a small sample size, and no respiratory disease information for the enrolled patients, as airway hyperresponsiveness is common in patients with acute respiratory infection, while in patients with chronic pulmonary infection such as bronchiectasis or tuberculosis, airway tolerance was significantly higher. In our study, the categories of respiratory disease in the enrolled patients include pneumonia (47%), bronchiectasis (16.7%), pulmonary shadow (14.4%), hemoptysis (6.7%), and miscellaneous (15.2%), and no significant difference in disease categories was found between male and female groups (P=0.157). These factors should be taken into account when citing our findings.
In summary, the study by Chen et al. (1) provided interesting and novel data on the effective dose and gender differences of alfentanil in painless bronchoscopy, and their results suggested that there were no obvious differences of the effective dose of alfentanil (ED50 value) between men and women. Our similar study is consistent with their findings in that the optimal dose of alfentanil required for painless bronchoscopy is significantly higher than in some other studies, but do not support their conclusion in that there was no gender difference in alfentanil. Considering that the gender difference of alfentanil has a great impact on its clinical application, further research is needed to confirm this discrepancy. As the authors (1) pointed out, future studies are needed to investigate the analgesic effects of alfentanil at different doses and in the different genders with multi-center and large-sample studies.
Acknowledgments
Funding: This work was supported by the Medical and Health Science and Technology Project of Zhejiang Province, China (No. 2020KY917).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1460/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen N, Wang X, Chen L, et al. Estimation of the median effective dose and the 95% effective dose of alfentanil required to inhibit the bronchoscopy reaction during painless bronchoscopy with i-gel supraglottic airway device: an Up-and-Down Sequential Allocation Trial. J Thorac Dis 2022;14:1537-43. [Crossref] [PubMed]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 2004;8:397-411. [Crossref] [PubMed]
- Escorial M, Muriel J, Margarit C, et al. Sex-Differences in Pain and Opioid Use Disorder Management: A Cross-Sectional Real-World Study. Biomedicines 2022;10:2302. [Crossref] [PubMed]
- Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav 2010;58:72-81. [Crossref] [PubMed]
- Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain 2004;8:413-25. [Crossref] [PubMed]
- Lau T, Hayward J, Vatanpour S, et al. Sex-related differences in opioid administration in the emergency department: a population-based study. Emerg Med J 2021;38:467-73. [Crossref] [PubMed]
- Leiten EO, Martinsen EM, Bakke PS, et al. Complications and discomfort of bronchoscopy: a systematic review. Eur Clin Respir J 2016;3:33324. [Crossref] [PubMed]
- Yu AL, Critchley LA, Lee A, et al. Alfentanil dosage when inserting the classic laryngeal mask airway. Anesthesiology 2006;105:684-8. [Crossref] [PubMed]


