Risk factors and clinical outcomes associated with acquired hypofibrinogenemia in patients administered hemocoagulase batroxobin for hemoptysis
Highlight box
Key findings
• Low levels of baseline plasma fibrinogen and a prolonged and higher total dose of batroxobin were associated with the development of acquired hypofibrinogenemia. Acquired hypofibrinogenemia was associated with increased 30-day mortality (hazard ratio, 4.164; 95% confidence interval, 1.318–13.157).
What is known and what is new?
• Batroxobin is used to prevent hemostasis or bleeding in surgical and trauma patients.
• Prolonged use and higher total doses of batroxobin were associated with the development of acquired hypofibrinogenemia. Acquired hypofibrinogenemia was associated with increased 30-day mortality in patients with hemoptysis.
What is the implication, and what should change now?
• The plasma fibrinogen levels in patients who were administered batroxobin for hemoptysis should be monitored, and batroxobin should be discontinued if hypofibrinogenemia occurs.
Introduction
The term hemoptysis refers to the expectoration of blood from the lower respiratory tract or lung parenchyma (1). Hemoptysis is most commonly caused by respiratory infection, bronchiectasis, lung cancer, and pulmonary tuberculosis (2-4). The majority of hemoptysis cases are self-limiting; however, massive hemoptysis can be life-threatening (5,6). Treatment for patients with massive hemoptysis requires bronchial arterial embolization or surgery, while conservative management may be considered for patients with non-life-threatening hemoptysis (5,6). Systemic hemostatic drugs, such as pituitrin, hemocoagulase, and tranexamic acid, can also be administered simultaneously (6). Tranexamic acid is used to prevent bleeding associated with traumatic injury, surgery, and congenital fibrinogen deficiency (7,8).
Hemocoagulase batroxobin is a hemostatic drug that reduces bleeding time and the need for blood transfusion in patients with trauma or those undergoing surgery (9-12). Batroxobin forms a fibrin clot by degrading fibrinogen into fibrin, which exerts a hemostatic effect (13,14). Batroxobin can be topically administered using bronchoscopy with an infusion or systemically to patients with hemoptysis (6,15-17). However, there have been reports of bleeding associated with acquired hypofibrinogenemia following batroxobin administration (12,18-20). The risk factors and prognosis related to acquired hypofibrinogenemia in hemoptysis patients treated with batroxobin are not well established. In this study, we evaluated the risk factors for and prognosis of acquired hypofibrinogenemia in hemoptysis patients treated systemically with batroxobin. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-717/rc).
Methods
Study population and design
This study retrospectively evaluated the data of hospitalized patients who were administered hemocoagulase batroxobin for hemoptysis in a single tertiary hospital in South Korea from January 2020 to April 2021. Plasma fibrinogen was measured in all enrolled patients before the administration of intravenous batroxobin and at least once after receiving treatment. Patients with a baseline plasma fibrinogen level of <150 mg/dL, those without plasma fibrinogen measurements, or those aged <18 years were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board at Chonnam National University Hospital, Gwangju, Republic of Korea approved the study (ID: CNUH-2022-367). The requirement for informed consent was waived because of the retrospective nature of the study.
Data collection
We reviewed the medical charts of the patients during the study period. The patients’ age, sex, body mass index, smoking status and duration, underlying diseases (hypertension, diabetes, ischemic heart disease, liver disease, malignancy, and drugs associated with bleeding tendency), presence of disseminated intravascular coagulation (DIC) at admission, causes of hemoptysis (bronchiectasis, old tuberculosis, mycobacterial disease, aspergilloma, lung cancer, lung abscess, drugs associated with hemoptysis, and cryptogenic causes), initial respiratory parameters (respiratory rate, partial pressure of oxygen/fraction of inspired oxygen ratio, pH, and partial pressure of dioxide), treatment for hemoptysis (bronchial artery embolization and surgery), presence of massive hemoptysis (≥100 mL/24 hours of hemoptysis), admission to the intensive care unit (ICU), transfusions (packed red blood cells, fresh frozen plasma, and cryoprecipitates), baseline laboratory findings (fibrinogen and platelet levels, activated partial thromboplastin time, prothrombin time, total bilirubin, aspartate transaminase, alanine transferase, and albumin levels), follow-up plasma fibrinogen levels, usage period and dosage of batroxobin, in-hospital death, and 30-day death after initiation of batroxobin were investigated.
Definitions
Acquired hypofibrinogenemia was defined as a baseline plasma fibrinogen level of >150 mg/dL, dropping to a follow-up level of <150 mg/dL after batroxobin administration (21,22). We also classified the hypofibrinogenemina as mild (100–200 mg/dL), moderate (50–100 mg/dL), and severe (10–50 mg/dL) (23). The Fibrinogen Clauss assay was used to measure plasma fibrinogen concentration. The definition of uncontrolled bleeding was hemoptysis that persisted despite treatment or recurred after treatment. Cryptogenic hemoptysis was defined as an absence of any cause after investigation of chest computed tomography and bronchoscopy for hemoptysis (1). Massive hemoptysis was defined as hemoptysis of more than 100 mL/24 h (6,24). DIC was diagnosed following the algorithm of the International Society on Thrombosis and Haemostasis (25).
Batroxobin dosage and administration route
Batroxobin was administered according to the clinician’s judgment regardless of bronchial artery embolization. Batroxobin at 10–20 National Institute of Health Unit (NIHU) was diluted with 500 mL of normal saline and administered intravenously for 24 hours. Batroxobin administration was stopped after hemoptysis improved clinically.
Statistical analysis
All data were expressed as the median [interquartile range (IQR)] or number (percentage). Demographic and clinical variables were compared between the hypofibrinogenemia group and non-hypofibrinogenemia group using the chi-square test (for categorical variables) or the Mann-Whitney U test (for continuous variables). A receiver operating characteristic (ROC) curve was plotted based on baseline plasma fibrinogen levels to predict hypofibrinogenemia. The Youden index was used to calculate optimal baseline fibrinogen cut-offs, sensitivities, and specificities in the ROC curve. Factors associated with acquired hypofibrinogenemia were selected using univariate logistic regression analysis. Subsequent multivariate logistic regression analyses included variables with P values of <0.25 in the univariate analysis using a forward method. Although DIC and chronic liver disease had P values greater than 0.25 in the univariate analysis, we included both variables in the multivariate logistic regression analysis as they were associated with acquired hypofibrinogenemia. Owing to the interaction between the total dosage and the duration of batroxobin administration, two models were developed, each utilizing each variable separately. We used the Kaplan-Meier analysis to evaluate the 30-day mortality. Factors associated with acquired hypofibrinogenemia were identified using a Cox-regression analysis that included variables with P values of <0.25 in the univariate analysis using the forward method. All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA) and MedCalc version 20.023 (Ostend, Belgium); a P value of <0.05 was considered statistically significant.
Results
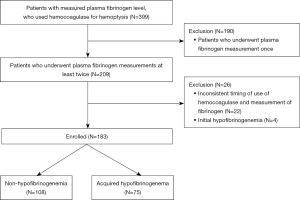
During the study period, 399 patients were administered batroxobin for hemoptysis and had available plasma fibrinogen measurements (Figure 1). Of these, 216 were excluded for the following reasons: plasma fibrinogen level was only measured once (n=190), inconsistent timing of batroxobin use and fibrinogen measurements (n=22), and initial hypofibrinogenemia ≤150 mg/dL (n=4). Finally, 183 patients were enrolled, of whom 75 had acquired hypofibrinogenemia after the administration of batroxobin.
Mild hypofibrinogenemia occurred in 28 patients (15.3%) and moderate hypofibrinogenemia in 60 patients (32.8%) after batroxobin use. There were no cases of severe hypofibrinogenemia.
Differences in baseline characteristics between the non-hypofibrinogenemia and hypofibrinogenemia groups
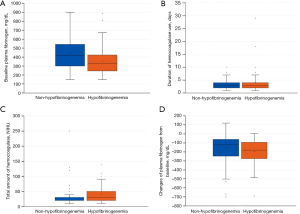
Table 1 summarizes the baseline characteristics of the two groups. The median age of the patients in the non-hypofibrinogenemia and hypofibrinogenemia groups was 72.0 and 74.0 years, respectively, without a statistically significant difference. Men were significantly more common in the non-hypofibrinogenemia group (73.1% vs. 50.7%; P=0.003). There was no significant difference in underlying diseases between the two groups. None of the patients in either group had hematologic disorders. The most common cause of hemoptysis in both groups was bronchiectasis. The proportions of patients who underwent bronchial artery embolization were similar in the non-hypofibrinogenemia and hypofibrinogenemia groups (18.5% vs. 20.0%, respectively). The patients in the hypofibrinogenemia group showed more ICU admissions (11.1% vs. 22.7%; P=0.041) and tended to have more massive hemoptysis than those in the non-hyperfibrinogenemia group (23.1% vs. 36.0%; P=0.068). The patients in the hypofibrinogenemia group required more transfusions of packed red blood cells, frozen fresh plasma, and cryoprecipitates than those in the non-hyperfibrinogenemia group. Moreover, the number of patients with persistent or recurrent hemoptysis was higher in the hypofibrinogenemia group than in the non-hyperfibrinogenemia group. The baseline plasma fibrinogen levels were significantly lower in the hypofibrinogenemia group than in the non-hypofibrinogenemia group (332.1 vs. 418.0 mg/dL; P<0.000). The patients in the hypofibrinogenemia group received batroxobin at significantly higher total doses and for longer periods of time than those in the non-hypofibrinogenemia group (shown in Figure 2). The decrease in plasma fibrinogen level from baseline was greater in the hypofibrinogenemia group than in the non-hypofibrinogenemia group (−121.5 vs. −180.0 mg/dL; P=0.011) (shown in Figure 2). In-hospital mortality was also significantly higher in the hypofibrinogenemia group than in the non-hyperfibrinogenemia group (4.6% vs. 14.7%; P=0.030).
Table 1
| Variables | Non-hypofibrinogenemia (N=108) | Hypofibrinogenemia (N=75) | P |
|---|---|---|---|
| Age, years | 72.0 (60.0–80.0) | 74.0 (64.0–82.0) | 0.157 |
| Men | 79 (73.1) | 38 (50.7) | 0.003 |
| Body mass index, kg/m2 | 21.8 (18.8–23.7) | 20.8 (18.6–23.9) | 0.785 |
| Current smoker | 12 (11.2) | 3 (4.0) | 0.103 |
| Pack-years | 0 (0–20.0) | 0 (0–0) | 0.002 |
| Hypertension | 37 (34.3) | 31 (41.3) | 0.354 |
| Diabetes | 20 (18.5) | 19 (25.3) | 0.277 |
| Ischemic heart disease | 26 (24.1) | 23 (30.7) | 0.396 |
| Liver disease | 6 (5.6) | 3 (4.0) | 0.739 |
| Malignancy | 12 (11. 2) | 6 (8.2) | 0.617 |
| DIC | 2 (1.9) | 3 (4.0) | 0.402 |
| Bronchiectasis | 33 (30.6) | 24 (32.0) | 0.872 |
| Old tuberculosis | 26 (24.1) | 19 (25.3) | 0.863 |
| Mycobacterial disease | 17 (15.7) | 10 (13.3) | 0.679 |
| Aspergilloma | 4 (3.7) | 6 (8.0) | 0.321 |
| Lung cancer | 4 (3.7) | 3 (4.0) | 1.000 |
| Lung abscess | 6 (5.6) | 3 (4.0) | 0.739 |
| Cryptogenic | 5 (4.6) | 4 (5.3) | 1.000 |
| Medication | 4 (3.7) | 1 (1.3) | 0.650 |
| Initial respiratory parameters | |||
| Respiratory rate | 20 (20–20) | 20 (20–20) | 0.216 |
| PaO2/FiO2 ratio | 335 (263–403) | 313 (195–426) | 0.312 |
| pH | 7.43 (7.39–7.45) | 7.42 (7.39–7.44) | 0.168 |
| pCO2 | 38.9 (35.0–43.8) | 40.0 (33.7–45.0) | 0.875 |
| Treatment | |||
| BAE | 20 (18.5) | 15 (20.0) | 0.850 |
| Surgery | 1 (0.9) | 0 (0) | 1.000 |
| Massive hemoptysis | 25 (23.1) | 27 (36.0) | 0.068 |
| ICU admission | 12 (11.1) | 17 (22.7) | 0.041 |
| PRC transfusion | 11 (10.2) | 29 (38.7) | <0.000 |
| FFP | 3 (2.8) | 17 (22.7) | <0.000 |
| Cryoprecipitates | 2 (1.9) | 15 (20.0) | <0.000 |
| Uncontrolled bleeding | <0.000 | ||
| Continuous bleeding | 4 (3.7) | 16 (21.3) | |
| Rebleeding | 1 (0.9) | 8 (10.7) | |
| Baseline fibrinogen, mg/dL | 418.0 (301.2–542.3) | 332.1 (246.4–423.6) | <0.000 |
| Platelet, /mm3 | 227.5 (177.2–280.7) | 209.0 (166.0–258.0) | 0.210 |
| aPTT | 29.1 (26.5–32.9) | 28.9 (26.1–31.1) | 0.238 |
| PT (INR) | 1.07 (1.01–1.20) | 1.06 (1.00–1.17) | 0.533 |
| Total bilirubin, mg/dL | 0.66 (0.45–0.96) | 0.60 (0.45–0.79) | 0.234 |
| ALT, U | 17.0 (12.0–25.0) | 20.0 (14.0–25.0) | 0.950 |
| AST, U | 28.0 (22.0–39.0) | 27.0 (22.0–36.0) | 0.314 |
| Albumin, mg/dL | 3.6 (3.1–4.1) | 3.9 (3.4–4.2) | 0.121 |
| Duration of batroxobin use, days | 3.0 (2.0–4.0) | 3.0 (2.0–4.7) | 0.007 |
| Total doses of batroxobin, NIH units | 30.0 (20.0–30.0) | 30.0 (20.0–50.0) | <0.000 |
| In-hospital mortality, n (%) | 5 (4.6) | 11 (14.7) | 0.030 |
| 30-day mortality, n (%) | 5 (4.6) | 10 (13.3) | 0.057 |
Data are presented as the median (interquartile range) or number (%). DIC, disseminated intravascular coagulation; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; PCO2, partial pressure of carbon dioxide; BAE, bronchial artery embolization; ICU, intensive care unit; PRC, packed red cell; FFP, fresh frozen plasma; aPTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NIH, national institute of health.
Risk factors associated with acquired hypofibrinogenemia
Risk factors for acquired hypofibrinogenemia are shown in Table 2. Because the duration of batroxobin use and total dose of batroxobin were significantly different between the non-hypofibrinogenemia and hypofibrinogenemia groups, two models, including each variable, were used. In the multivariate analysis with logistic regression, age, male sex and low levels of baseline plasma fibrinogen were associated with acquired hypofibrinogenemia. In addition, prolonged batroxobin use and higher total doses of batroxobin increased the risk of acquired hypofibrinogenemia.
Table 2
| Variables | Model 1a | Model 2b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||||
| Age | 1.016 (0.994–1.038) | 0.153 | 1.025 (1.000–1.051) | 0.043 | 1.016 (0.994–1.038) | 0.153 | 1.026 (1.001–1.051) | 0.043 | |||
| Men | 2.652 (1.425–4.937) | 0.002 | 3.277 (1.633–6.579) | 0.001 | 2.652 (1.425–4.937) | 0.002 | 2.329 (1.095–4.956) | 0.028 | |||
| Current smoker | 0.330 (0.090–1.212) | 0.095 | 0.330 (0.090–1.212) | 0.095 | |||||||
| Pack-years | 0.967 (0.944–0.991) | 0.008 | 0.967 (0.944–0.991) | 0.008 | |||||||
| DIC | 0.453 (0.074–2.778) | 0.392 | 0.453 (0.074–2.778) | 0.392 | |||||||
| Massive hemoptysis | 1.867 (0.975–3.577) | 0.060 | 1.867 (0.975–3.577) | 0.060 | |||||||
| Baseline fibrinogen | 0.996 (0.994–0.998) | <0.000 | 0.996 (0.994–0.999) | 0.002 | 0.996 (0.994–0.998) | <0.000 | 0.996 (0.994–0.999) | 0.001 | |||
| Platelet | 0.998 (0.995–1.001) | 0.153 | 0.998 (0.995–1.001) | 0.153 | |||||||
| PT (INR) | 0.575 (0.221–1.496) | 0.257 | 0.575 (0.221–1.496) | 0.257 | |||||||
| aPTT | 0.961 (0.906–1.018) | 0.177 | 0.961 (0.906–1.018) | 0.177 | |||||||
| Duration of batroxobin use | 1.201 (1.015–1.421) | 0.033 | 1.244 (1.019–1.519) | 0.032 | |||||||
| Total doses of batroxobin | 1.012 (1.000–1.024) | 0.057 | 1.014 (1.000–1.029) | 0.049 | |||||||
a, model 1 include duration of batroxobin use; b, model 2 include total doses of batroxobin. OR, odds ratio; CI, confidence interval; DIC, disseminated intravascular coagulation; PT, prothrombin time; INR, international normalized ratio.
The ROC curve to predict acquired hypofibrinogenemia based on baseline plasma fibrinogen levels. The area under the curve was 0.662, with statistical significance (P<0.000) (shown in Figure 3). The sensitivity and specificity for the cut-off value of 413 mg/dL of the baseline plasma fibrinogen level were 74.7% and 50.9%, respectively. The patients with baseline fibrinogen levels of 413 mg/dL showed a high risk of acquired hypofibrinogenemia [odds ratio (OR) 4.276, P<0.000] (Table 3).
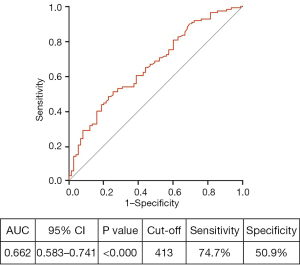
Table 3
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Baseline plasma fibrinogen <413 mg/dL | |||
| Unadjusted | 2.854 | 1.511–5.389 | 0.001 |
| Adjusteda | 3.005 | 1.543–5.839 | 0.001 |
| Adjustedb | 4.137 | 1.909–8.965 | <0.000 |
| Adjustedc | 4.181 | 1.903–9.186 | <0.000 |
| Adjustedd | 4.276 | 1.943–9.410 | <0.000 |
a, age, and sex; b, age, sex, smoking status, pack-years, hypertension, diabetes, ischemic heart disease, liver disease, bronchiectasis, old tuberculosis, mycobacterial diseases, aspergilloma, lung cancer, lung abscess, cryptogenic, medication, and disseminated intravascular coagulation; c, age, sex, smoking status, pack-years, hypertension, diabetes, ischemic heart disease, liver disease, bronchiectasis, old tuberculosis, mycobacterial diseases, aspergilloma, lung cancer, lung abscess, cryptogenic, medication, disseminated intravascular coagulation, and dose of batroxobin; d, age, sex, smoking status, pack-years, hypertension, diabetes, ischemic heart disease, liver disease, bronchiectasis, old tuberculosis, mycobacterial diseases, aspergilloma, lung cancer, lung abscess, cryptogenic, medication, disseminated intravascular coagulation, and duration of batroxobin. OR, odds ratio; CI, confidence interval.
Risk factors for 30-day mortality
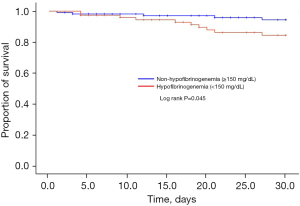
In the Kaplan-Meier survival analysis, 30-day mortality was higher in the hypofibrinogenemia group than in the non-hypofibrinogenemia group (log lank P=0.045) (shown in Figure 4). In the Cox proportional hazard model, a low levels of baseline plasma fibrinogen [hazard ratio (HR), 1.004; 95% confidence interval (CI), 1.001–1.007; P=0.006], total bilirubin (HR, 1.916; 95% CI, 1.322–2.777; P=0.001), DIC (HR, 5.271; 95% CI, 1.458–19.059; P=0.011), and acquired hypofibrinogenemia (HR, 4.164; 95% CI, 1.318–13.157; P=0.015) were associated with increased 30-day mortality (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.023 (0.981–1.066) | 0.296 | |||
| Men | 1.379 (0.490–3.882) | 0.543 | |||
| Baseline fibrinogen levels | 1.002 (1.000–1.005) | 0.076 | 1.004 (1.001–1.007) | 0.006 | |
| Platelet | 0.993 (0.987–1.000) | 0.036 | |||
| aPTT | 0.999 (0.964–1.035) | 0.946 | |||
| PT (INR) | 1.098 (0.694–1.737) | 0.689 | |||
| Total bilirubin | 1.676 (1.179–2.383) | 0.004 | 1.916 (1.322–2.777) | 0.001 | |
| ALT | 1.000 (0.997–1.003) | 0.907 | |||
| AST | 1.000 (0.999–1.001) | 0.904 | |||
| Albumin | 0.345 (0.185–0.645) | 0.001 | |||
| Body mass index | 0.957 (0.840–1.090) | 0.506 | |||
| Current smoker | 0.994 (0.131–7.567) | 0.995 | |||
| Pack-years | 1.012 (0.982–1.044) | 0.438 | |||
| Uncontrolled bleeding | 2.745 (0.937–8.043) | 0.066 | |||
| Hypertension | 2.010 (0.729–5.543) | 0.178 | |||
| Diabetes | 1.525 (0.485–4.793) | 0.470 | |||
| Ischemic heart disease | 1.650 (0.587–4.638) | 0.342 | |||
| Liver disease | 1.215 (0.159–9.252) | 0.851 | |||
| Malignancy | 1.486 (0.332–6.640) | 0.604 | |||
| DIC | 9.664 (2.725–34.280) | <0.000 | 5.271 (1.458–19.059) | 0.011 | |
| Massive hemoptysis | 0.576 (0.162–2.041) | 0.393 | |||
| Bronchiectasis | 0.334 (0.075–1.479) | 0.148 | |||
| Old tuberculosis | 0.448 (0.101–1.984) | 0.290 | |||
| Mycobacterial diseases | 0.039 (0.000–17.579) | 0.297 | |||
| Aspergilloma | 1.208 (0.159–9.192) | 0.855 | |||
| Lung cancer | 0.047 (0.000–4532.678) | 0.601 | |||
| Lung abscess | 0.3961 (0.888–17.668) | 0.071 | |||
| Cryptogenic | 0.047 (0.000–5,064.288) | 0.605 | |||
| Medication | 0.048 (0.000–6,257.979) | 0.672 | |||
| BAE | 0.654 (0.148–2.900) | 0.577 | |||
| Dose of batroxobin | 1.001 (0.985–1.017) | 0.889 | |||
| Duration of batroxobin use | 1.029 (0.900–1.177) | 0.674 | |||
| Hypofibrinogenemia | 2.851 (0.974–8.341) | 0.056 | 4.164 (1.318–13.157) | 0.015 | |
HR, hazard ratio; CI, confidence interval; aPTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DIC, disseminated intravascular coagulation; BAE, bronchial artery embolization.
Discussion
To the best of our knowledge, this is the first study to evaluate the risk factors for and prognosis of acquired hypofibrinogenemia associated with batroxobin administration to treat hemocoagulase in patients with hemoptysis. We found that the administration of batroxobin for a prolonged period and with high total doses and lower baseline plasma fibrinogen levels were associated with acquired hypofibrinogenemia in patients with hemoptysis. The patients in the acquired hypofibrinogenemia group had a higher rate of ICU admission, transfusion, and in-hospital mortality than those in the non-hypofibrinogenemia group. Acquired hypofibrinogenemia was associated with increased 30-day mortality in patients with hemoptysis.
Hemocoagulase batroxobin is a thrombin-like serine protease extracted from snake venom (26). Thrombin forms fibrin monomers by degrading the alpha and beta chains of fibrinogen, whereas batroxobin only degrades the alpha chain of fibrinogen (13,14). Batroxobin has a 10-fold higher affinity for fibrinogen than thrombin (27). Batroxobin reduces the bleeding time and the need for blood transfusion in patients with trauma or those undergoing surgery (2,9-12). Bronchoscopy with the bronchial infusion of batroxobin was also effective in treating patients with massive hemoptysis (15-17). Additionally, batroxobin can be administered systemically to patients with hemoptysis to control bleeding (2,6,20,28). Patients with massive hemoptysis are also recommended to be administered batroxobin systemically in combination with bronchial artery embolization or surgery (6). However, the use of batroxobin leads to a reduction in plasma fibrinogen levels due to increased fibrinogen consumption (27). In this study, plasma fibrinogen levels decreased from baseline in both the patients from the hypofibrinogenemia and non-hypofibrinogenemia groups after batroxobin administration. Previous studies also reported a decrease in plasma fibrinogen after the administration of hemocoagulase batroxobin (12,18-20). Plasma fibrinogen decreased significantly more in the acquired hypofibrinogenemia group than in the non-hypofibrinogenemia group in this study.
Our study found that more uncontrolled bleeding was observed in the patients in the hypofibrinogenemia group than in those in the non-hypofibrinogenemia group, suggesting that the development of acquired hypofibrinogenemia following batroxobin administration is associated with a bleeding tendency. Wei et al. found that patients with postoperative hypofibrinogenemia had a higher incidence of intracranial hematomas and received more blood and plasma transfusions (29). A positive correlation between the consumption of plasma fibrinogen and the use of batroxobin has also been reported (29). Zhou et al. further reported 7 patients who developed hypofibrinogenemia after batroxobin administration for bleeding prevention following colon polyp excision. Among them, 3 developed lower gastrointestinal bleeding; however, there was no decrease in plasma fibrinogen in 13 patients who did not use batroxobin (18). In a case report, a patient with hemoptysis who had been treated with long-term batroxobin also developed a bleeding tendency due to acquired hypofibrinogenemia (20). In this study, the development of acquired hypofibrinogenemia was associated with the total dose of batroxobin and duration of batroxobin use. The decrease rate of plasma fibrinogen levels according to the daily dose of batroxobin was not different between the acquired hypofibrinogenemia and non-hypofibrinogenemia groups. The risk of acquired hypofibrinogenemia after batroxobin administration was also high in patients with low baseline plasma fibrinogen levels. The odds of acquired hypofibrinogenemia were four times higher if the plasma fibrinogen level was less than 413 mg/dL, even in the normal range of plasma fibrinogen levels (P<0.000). Therefore, plasma fibrinogen levels should be measured before administration of batroxobin, and patients with low hypofibrinogen levels or on long-term or high-doses of batroxobin should have their plasma fibrinogen levels monitored closely. There has been a recent increase in the use of functional fibrinogen assays such as thromboelastography and rotational thromboelastometry, allowing for the identification of fibrinogen deficiency and transfusion more quickly in trauma and surgical bleeding cases (30). Further research is needed on the effectiveness of measuring baseline and follow-up fibrinogen levels with these functional fibrinogen assays in patients receiving batroxobin for hemoptysis.
This study found that the patients in the acquired hypofibrinogenemia group had significantly higher in-hospital mortality rates than the patients in the non-hypofibrinogenemia group. Acquired hypofibrinogenemia was also associated with increased 30-day mortality. Hypofibrinogenemia is a well-known prognostic factor for patients with trauma or those undergoing surgery (31-34). Plasma fibrinogen levels of less than 2 g/L are associated with an increased 3-month mortality rate in patients with traumatic brain injury (34). The minimum hemostatic fibrinogen level is 50 mg/dL at bleeding. For unprovoked hemorrhage, suggested fibrinogen concentrations are >100 mg/dL until hemostasis is normalized and >50 mg/dL until the bleeding surface is entirely restored (35). However, 30-day mortality was not correlated with the duration of batroxobin use or total doses of batroxobin. Therefore, patients administered batroxobin for hemoptysis should undergo monitoring for plasma fibrinogen levels and discontinue batroxobin if hypofibrinogenemia develops (12,20). In hypofibrinogenemic patients, thrombotic events can occur when thrombotic factor risks are present, so bleeding risk and thrombotic risk must be managed jointly (36).
This study had several limitations. First, this was a retrospective study conducted in a single center, which limits the generalizability of our findings. Second, we enrolled patients whose plasma fibrinogen levels were measured before the administration of intravenous batroxobin and at least once after receiving treatment. However, there is a possibility that some patients with hypofibrinogenemia were excluded as plasma fibrinogen was not measured every day. Third, the possibility of other factors affecting the reduction in plasma fibrinogen levels could not be excluded completely. Hemodilution and DIC are common causes of acquired hypofibrinogenemia. The medical charts in this study were thoroughly reviewed, and no hemodilution conditions were found. However, hemodilution could not be completely excluded from hydration because of the retrospective study design. The effect of DIC on hypofibrinogenemia and 30-day mortality was also evaluated in univariate and multivariate analyses. Fourth, because most hemoptysis patients in our institution receive batroxobin, we could not evaluate hypofibrinogenemia and prognosis based on batroxobin use. Additional studies are needed to investigate the hemostatic effect, hypofibrinogenemia occurrence, and prognosis of batroxobin in hemoptysis patients. Fifth, to avoid missing important variables in the multivariate analysis, we selected variables with a P value within 0.25 in the univariate analysis. However, the possibility of missing some important variables cannot be completely ruled out.
Conclusions
High-dose or long-term batroxobin use for hemoptysis is associated with the development of hypofibrinogenemia. Hypofibrinogenemia is associated with poorer outcomes and higher 30-day mortality in patients. Thus, the plasma fibrinogen levels of patients administered batroxobin for hemoptysis should be monitored, and batroxobin should be discontinued if hypofibrinogenemia occurs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-717/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-717/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-717/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-717/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board at Chonnam National University Hospital, Gwangju, Republic of Korea approved the study (ID: CNUH-2022-367). The requirement for informed consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Expert Panel on Thoracic I. ACR Appropriateness Criteria(R) Hemoptysis. J Am Coll Radiol 2020;17:S148-59. [Crossref] [PubMed]
- Lee BR, Yu JY, Ban HJ, et al. Analysis of patients with hemoptysis in a tertiary referral hospital. Tuberc Respir Dis (Seoul) 2012;73:107-14. [Crossref] [PubMed]
- Abdulmalak C, Cottenet J, Beltramo G, et al. Haemoptysis in adults: a 5-year study using the French nationwide hospital administrative database. Eur Respir J 2015;46:503-11. [Crossref] [PubMed]
- Tom LM, Palevsky HI, Holsclaw DS, et al. Recurrent Bleeding, Survival, and Longitudinal Pulmonary Function following Bronchial Artery Embolization for Hemoptysis in a U.S. Adult Population. J Vasc Interv Radiol 2015;26:1806-13.e1. [Crossref] [PubMed]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000;28:1642-7. [Crossref] [PubMed]
- Jin F, Li Q, Bai C, et al. Chinese Expert Recommendation for Diagnosis and Treatment of Massive Hemoptysis. Respiration 2020;99:83-92. [Crossref] [PubMed]
- Tziomalos K, Vakalopoulou S, Perifanis V, et al. Treatment of congenital fibrinogen deficiency: overview and recent findings. Vasc Health Risk Manag 2009;5:843-8. [Crossref] [PubMed]
- Pabinger I, Fries D, Schöchl H, et al. Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr 2017;129:303-16. [Crossref] [PubMed]
- Qiu M, Zhang X, Cai H, et al. The impact of hemocoagulase for improvement of coagulation and reduction of bleeding in fracture-related hip hemiarthroplasty geriatric patients: A prospective, single-blinded, randomized, controlled study. Injury 2017;48:914-9. [Crossref] [PubMed]
- Nagabhushan RM, Shetty AP, Dumpa SR, et al. Effectiveness and Safety of Batroxobin, Tranexamic Acid and a Combination in Reduction of Blood Loss in Lumbar Spinal Fusion Surgery. Spine (Phila Pa 1976) 2018;43:E267-73. [Crossref] [PubMed]
- Yao YT, Yuan X, Fang NX. Hemocoagulase reduces postoperative bleeding and blood transfusion in cardiac surgical patients: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18534. [Crossref] [PubMed]
- Zhang H. The Effects of Hemocoagulase on Coagulation Factors in an Elderly Patient with Upper Gastrointestinal Hemorrhage: A Case Report. Curr Drug Saf 2019;14:230-2. [Crossref] [PubMed]
- Stocker K, Barlow GH. The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol 1976;45:214-23. [Crossref] [PubMed]
- Itoh N, Tanaka N, Mihashi S, et al. Molecular cloning and sequence analysis of cDNA for batroxobin, a thrombin-like snake venom enzyme. J Biol Chem 1987;262:3132-5.
- Tsukamoto T, Sasaki H, Nakamura H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest 1989;96:473-6. [Crossref] [PubMed]
- Bhambure NM, Abhyankar NY, Gandhi Y, et al. Hemoptysis after batroxobin infusion using a fiberoptic bronchoscope. Chest 1991;99:1313.
- de Gracia J, de la Rosa D, Catalán E, et al. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respir Med 2003;97:790-5. [Crossref] [PubMed]
- Zhou HB. Hypofibrinogenemia Caused by Hemocoagulase After Colon Polyps Excision. Am J Case Rep 2017;18:291-3. [Crossref] [PubMed]
- Linglong X, Dijiong W. Prolonged Hemocoagulase Agkistrodon Halys Pallas Administration Induces Hypofibrinogenemia in Patients with Hematological Disorders: A Clinical Analysis of 11 Patients. Indian J Hematol Blood Transfus 2018;34:322-7. [Crossref] [PubMed]
- Kim TO, Kim MS, Kho BG, et al. Paradoxical pulmonary hemorrhage associated with hemocoagulase batroxobin in a patient with hemoptysis: A CARE-compliant case report. Medicine (Baltimore) 2021;100:e24040. [Crossref] [PubMed]
- Kawasugi K, Wada H, Honda G, et al. Hypofibrinogenemia is associated with a high degree of risk in infectious diseases: a post-hoc analysis of post-marketing surveillance of patients with disseminated intravascular coagulation treated with thrombomodulin alfa. Thromb J 2021;19:12. [Crossref] [PubMed]
- Naik BI, Tanaka K, Sudhagoni RG, et al. Prediction of hypofibrinogenemia and thrombocytopenia at the point of care with the Quantra® QPlus® System. Thromb Res 2021;197:88-93. [Crossref] [PubMed]
- Brunclikova M, Simurda T, Zolkova J, et al. Heterogeneity of Genotype-Phenotype in Congenital Hypofibrinogenemia-A Review of Case Reports Associated with Bleeding and Thrombosis. J Clin Med 2022;11:1083. [Crossref] [PubMed]
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008;32:1131-2. [Crossref] [PubMed]
- Boral BM, Williams DJ, Boral LI. Disseminated Intravascular Coagulation. Am J Clin Pathol 2016;146:670-80. [Crossref] [PubMed]
- Vu TT, Stafford AR, Leslie BA, et al. Batroxobin binds fibrin with higher affinity and promotes clot expansion to a greater extent than thrombin. J Biol Chem 2013;288:16862-71. [Crossref] [PubMed]
- Klöcking HP, Markwardt F, Güttner J. On the mechanism of batroxobin-induced fibrinolysis. Pharmazie 1989;44:504-5.
- Hu JW, Qin XS. Hypofibrinogenemia Caused by Hemocoagulase Injection: A Retrospective Study on Clinical Laboratory Findings. Chin Med Sci J 2020;35:151-6. [Crossref] [PubMed]
- Wei N, Jia Y, Wang X, et al. Risk Factors for Postoperative Fibrinogen Deficiency after Surgical Removal of Intracranial Tumors. PLoS One 2015;10:e0144551. [Crossref] [PubMed]
- Peng HT, Nascimento B, Beckett A. Thromboelastography and Thromboelastometry in Assessment of Fibrinogen Deficiency and Prediction for Transfusion Requirement: A Descriptive Review. Biomed Res Int 2018;2018:7020539. [Crossref] [PubMed]
- Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266-73. [Crossref] [PubMed]
- Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma 2008;64:S79-85; discussion S85. [Crossref] [PubMed]
- Karlsson M, Ternström L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost 2009;102:137-44. [Crossref] [PubMed]
- Lv K, Yuan Q, Fu P, et al. Impact of fibrinogen level on the prognosis of patients with traumatic brain injury: a single-center analysis of 2570 patients. World J Emerg Surg 2020;15:54. [Crossref] [PubMed]
- Simurda T, Asselta R, Zolkova J, et al. Congenital Afibrinogenemia and Hypofibrinogenemia: Laboratory and Genetic Testing in Rare Bleeding Disorders with Life-Threatening Clinical Manifestations and Challenging Management. Diagnostics (Basel) 2021.
- Simurda T, Casini A, Stasko J, et al. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb Res 2020;188:1-4. [Crossref] [PubMed]