
Comparison of oncological outcomes between trisegmentectomy and lobectomy for non-small cell lung cancer in the left upper division
Introduction
Segmentectomy has become increasingly popular for the resection of early-stage non-small cell lung cancer (NSCLC), given its ability to preserve pulmonary function compared with lobectomy (1-3). A phase 3 multicenter randomized controlled trial comparing segmentectomy and lobectomy for small-sized peripheral NSCLC has recently demonstrated the benefits of segmentectomy in terms of overall survival (OS) (4). However, the authors reported a significantly higher rate of local relapse in patients undergoing segmentectomy. These findings are in accordance with those of a 1995 randomized controlled study comparing lobectomy and sublobar resection for stage IA NSCLC, which documented higher rates of death and locoregional recurrence in patients undergoing sublobar resection (5). Such data highlight the need to consider the risk of locoregional recurrence in clinical decision-making regarding segmentectomy.
The left upper lobe is one of the largest lobes in the lungs and is divided into two anatomical units: the upper division (segments 1+2 and segment 3) and lingula (segments 4 and 5). This anatomical classification is similar to that used to classify the right upper and middle lobes. Although some studies have reported similar oncological outcomes for “split lobectomy” (i.e., lingulectomy and trisegmentectomy) and left upper lobectomy (6-11), most studies did not perform analyses based on tumor location.
Generally, securing the surgical margin is an important concern in patients undergoing limited resection procedures, such as wedge resection or segmentectomy. The National Comprehensive Cancer Network guidelines specify that a margin of >20 mm is acceptable (12), meaning that segmentectomy is not indicated in patients with tumors located close to the intersegmental plane. However, bilobectomy (upper and middle lobe lobectomy) is not recommended for tumors close to the interlobar plane in the right upper or middle lobe, even when the horizontal fissure of the right lung is dysplastic or absent. Therefore, we hypothesized that this indication is applicable to trisegmentectomy in the left upper lobe.
Three-dimensional (3D) computed tomography (CT) reconstruction software can be used to visualize intersegmental veins, making it easy to identify the intersegmental plane during segmentectomy. This cutting-edge software allows for accurate measurement of the distance between the tumor and intersegmental plane. However, no studies to date have included the distance from the intersegmental plane as a factor when comparing outcomes between trisegmentectomy and left upper lobectomy.
Therefore, to aid in establishing trisegmentectomy as a standard treatment for clinical N0 (cN0) NSCLC in the left upper lobe, we aimed to re-assess the feasibility of trisegmentectomy based on oncological outcomes according to tumor location. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-950/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Hyogo Cancer Center (No. G-91; approved on October 25, 2019), and written informed consent regarding the research use of their data was taken in the form of opt-out from all individual patients.
Study population
In this retrospective cohort study, we analyzed patients’ data obtained from the medical records of our institution. The study population comprised patients with cN0 NSCLC in the left upper division who had undergone either left upper lobectomy (L group) or trisegmentectomy (S group) between April 2006 and December 2020. We excluded patients with metachronous or recurrent NSCLC, those who had received induction therapy, and those who had undergone incomplete or extended resection (Figure 1). Patients with missing data for specific values were excluded from the analyses.

Analysis of 3D images
SYNAPSE VINCENT® (Fujifilm Corporation, Tokyo, Japan) 3D image analysis software was used to measure the predicted distance from the intersegmental plane. The 3D images were automatically integrated and further corrected manually. The process consisted of the following steps: (I) transferring the CT images to the 3D imaging system; (II) tracing the tumor, tracheobronchial tree, and lung parenchyma; (III) generating a 3D reconstruction from the traced CT images; (IV) defining the area of resection based on the bronchi-dominant region; and (V) calculating the distance from the intersegmental plane (Figure 2).
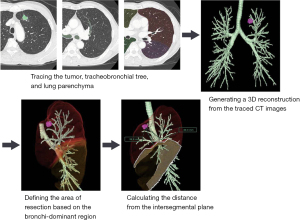
Operative procedure for trisegmentectomy
Initially, the segmental vein was exposed and resected, while the intersegmental vein (V3b) was preserved and detached toward the periphery. When the tumor was located close to the intersegmental plane, the V3b was sacrificed to secure the tumor margin. After ligation of the segmental artery, bifurcation of the bronchus was identified, and the bronchus was detached with a stapler to allow for dissection. The jet ventilation technique was used to identify the demarcation line using the inflation-deflation line as a reference (13); however, since 2018, systemic injections of indocyanine green (0.3 mg/kg) were used with or without the inflation-deflation method (14). Finally, electrocauterization was used to dissect along the demarcation line until mechanical stapling became feasible. Care was taken to minimize the number of junctional points along the mechanically stapled line to avoid the risk of postoperative fistula in the bronchioles. Generally, patients undergoing segmentectomy or lobectomy for cN0 NSCLC also underwent selective mediastinal lymphadenectomy. When a positive hilar lymph node was suspected based on intraoperative visualization, pathologic node assessments were performed using frozen sections. When findings from frozen sections were positive for lymph node metastasis, the procedure was converted from segmentectomy to lobectomy.
Surgical and oncological outcomes
Clinical and pathological tumor stages were classified according to the Union for International Cancer Control (UICC) 8th edition and the UICC 7th edition criteria, respectively. Complications requiring surgical treatment (Grades III–V according to the Clavien-Dindo classification) within 30 days were recorded. Prolonged air leakage was defined as air leakage lasting for >7 days or necessitating pleurodesis or invasive procedures (surgery or additional tube thoracostomy). Delayed air leakage was defined as air leakage necessitating tube thoracostomy after discharge. The change rate of forced expiratory volume in 1 s (FEV1.0) was defined as the change between preoperative and 6-month postoperative measurements.
Recurrence was classified as locoregional or distant. Locoregional recurrence was defined as recurrence in the ipsilateral lobes or ipsilateral hilar or mediastinal lymph nodes, while distant recurrence was defined as recurrence in the contralateral lobes, recurrence in the lymph nodes outside the hemithoracic organs, or dissemination in the pleural space. Recurrence-free survival (RFS) was defined as the period from the date of surgery to recurrence or death from any cause. OS was defined as the period from the date of surgery to death from any cause.
Follow-up
Perioperative data were collected from the hospital charts. For 2 years after surgical intervention, systemic and local examinations were performed every 6 months, including blood tests, chest and abdominal CT, head magnetic resonance imaging, and bone scintigraphy. These intensive examinations were also performed every year between 3 and 5 years postoperatively. Follow-up observations were performed for at least 5 years to check for tumor recurrence and evaluate survival.
Statistical analysis
To determine whether the indication for trisegmentectomy can be expanded to tumors in the left upper division regardless of distance from the intersegmental plane, we performed the following analyses (Figure 1). (I) To reduce bias when comparing clinical and oncological outcomes between trisegmentectomy and lobectomy, propensity score matching was performed based on age, sex, smoking history, histology, maximum standardized uptake value (SUVmax), clinical T factor, preoperative ratio of FEV1.0 to forced vital capacity (FEV1.0/FVC), and presence of ground-glass opacities (GGOs). Matching was performed at a 1:1 ratio using a caliper distance of 0.2. We compared RFS and OS between the patients undergoing trisegmentectomy (matched S group) and lobectomy (matched L group). (II) To verify whether trisegmentectomy was indicated independently of the tumor’s location, we compared RFS and OS between patients undergoing trisegmentectomy for tumors located ≤20 mm from the intersegmental plane (short-distance group) and those undergoing trisegmentectomy for tumors located >20 mm from the intersegmental plane (long-distance group). A multivariate Cox proportional hazards model was used to identify predictors of RFS in the trisegmentectomy cohort. Age, distance from the intersegmental plane, and SUVmax were used as covariates.
Several post hoc sensitivity analyses were performed to verify our findings. First, RFS and OS were compared between the unmatched lobectomy and trisegmentectomy groups. Second, univariate analysis of RFS was performed in the trisegmentectomy cohort without adjusting for other covariates.
RFS was estimated using the Kaplan-Meier method, and differences were analyzed using the log-rank test. Mann-Whitney U tests were used to compare continuous variables, while Fisher’s exact tests were used to compare nominal variables. Statistical analyses were performed using R software (The R Foundation for Statistical Computing, Vienna, Austria). A P value of <0.05 was considered statistically significant.
Results
Analysis 1 (overall cohort)
Patient and tumor characteristics
Table 1 summarizes the general characteristics and tumor data of the 252 patients included in the final analyses. Seventeen patients were excluded because of missing values (15 who did not undergo positron emission tomography CT, two without preoperative FEV1.0/FVC data, and one who underwent completion lobectomy due to lingula torsion after trisegmentectomy). Among the included patients, 190 underwent lobectomy, while 62 underwent trisegmentectomy (L and S groups). Age, sex distribution, smoking history, histology, clinical T factor, preoperative FEV1.0/FVC, and presence of GGOs were similar in both groups. The proportion of patients with an SUVmax of >2.5 was higher in the L group than in the S group (68.9% vs. 46.8%, P=0.002). Although not included in the propensity score matching procedure, the median consolidation size on CT was greater in the L group than in the S group (25.0 vs. 14.5 mm, P<0.001).
Table 1
| Characteristic | Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L group (n=190) | S group (n=62) | SMD | P value | Matched L group (n=46) |
Matched S group (n=46) |
SMD | P value | ||
| Age, years | 0.10 | 0.49 | 0 | 1.00 | |||||
| >75 | 41 (21.6) | 16 (25.8) | 12 (26.1) | 12 (26.1) | |||||
| ≤75 | 149 (78.4) | 46 (74.2) | 34 (73.9) | 34 (73.9) | |||||
| Sex | 0.06 | 0.76 | 0.05 | 1.00 | |||||
| Male | 123 (64.7) | 42 (67.7) | 32 (69.6) | 31 (67.4) | |||||
| Female | 67 (35.3) | 20 (32.3) | 14 (30.4) | 15 (32.6) | |||||
| Smoking history | 0.09 | 0.64 | 0 | 1.00 | |||||
| Ever | 124 (65.3) | 43 (69.4) | 32 (69.6) | 32 (69.6) | |||||
| Never | 66 (34.7) | 19 (30.6) | 14 (30.4) | 14 (30.4) | |||||
| Histology | 0.01 | 1.00 | 0.10 | 0.82 | |||||
| Adenocarcinoma | 140 (73.7) | 46 (74.2) | 34 (73.9) | 32 (69.6) | |||||
| Others | 50 (26.3) | 16 (25.8) | 12 (26.1) | 14 (30.4) | |||||
| SUVmax | 0.46 | 0.002 | 0.09 | 0.84 | |||||
| >2.5 | 131 (68.9) | 29 (46.8) | 23 (50.0) | 25 (54.3) | |||||
| ≤2.5 | 59 (31.1) | 33 (53.2) | 23 (50.0) | 21 (45.7) | |||||
| Clinical T factor (UICC 8th) | 1.12 | NA | 0.34 | 0.77 | |||||
| cTis | 0 (0.0) | 5 (8.1) | 0 (0.0) | 0 (0.0) | |||||
| cTmi | 1 (0.5) | 2 (3.2) | 1 (2.2) | 2 (4.3) | |||||
| cT1a | 11 (5.8) | 17 (27.4) | 10 (21.7) | 7 (15.2) | |||||
| cT1b | 52 (27.4) | 23 (37.1) | 18 (39.1) | 22 (47.8) | |||||
| cT1c | 66 (34.7) | 10 (16.1) | 13 (28.3) | 10 (21.7) | |||||
| cT2a | 38 (20.0) | 4 (6.5) | 2 (4.3) | 4 (8.7) | |||||
| cT2b | 11 (5.8) | 1 (1.6) | 2 (4.3) | 1 (2.2) | |||||
| cT3 | 11 (5.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Preoperative FEV1.0/FVC | 0.24 | 0.12 | 0.09 | 0.83 | |||||
| ≥70% | 135 (71.1) | 37 (59.7) | 31 (67.4) | 29 (63.0) | |||||
| <70% | 55 (28.9) | 25 (40.3) | 15 (32.6) | 17 (37.0) | |||||
| Presence of GGOs | 0.23 | 0.14 | 0.09 | 0.83 | |||||
| Yes | 95 (50.0) | 38 (61.3) | 26 (56.5) | 24 (52.2) | |||||
| No | 95 (50.0) | 24 (38.7) | 20 (43.5) | 22 (47.8) | |||||
Values are n (%) unless otherwise indicated. L group, lobectomy group; S group, trisegmentectomy group; SMD, standardized mean difference; SUVmax, maximum standardized uptake value; UICC, Union for International Cancer Control; FEV1.0, forced expiratory volume in 1 s; FEV1.0/FVC, FEV1.0 to forced vital capacity; GGO, ground-glass opacity.
After propensity score matching, 46 patients from each group were included in the matched analysis (matched L and S groups). The demographic and oncological characteristics of the patients are summarized in Table 1, which shows the similarities between the two groups. The median consolidation size on the CT image was also similar in both groups.
Surgical and oncological outcomes in the matched cohorts
Although the median operation time was shorter in the matched L group than in the matched S group (162 vs. 181 min, P=0.03), there were no significant differences in the median blood loss (85 vs. 90 mL, P=0.19), median hospital stay (16 vs. 13 days, P=0.06), or morbidity rate (15.2% vs. 6.5%, P=0.32) between the matched cohorts. Seven (15.2%) patients in the matched L group and three (6.5%) patients in the matched S group developed specific complications requiring surgical treatment (Grades III–V according to the Clavien-Dindo classification) within 30 days. All these 10 patients experienced prolonged or delayed air leakage. No postoperative deaths occurred within 30 days in either group. The change rate of FEV1.0 tended to be higher in the matched S group than in the matched L group, although the difference was not significant (88% vs. 90%, P=0.29). In terms of postoperative oncological outcomes, the extent of pleural invasion and pathological stage were similar between the groups (Table 2).
Table 2
| Parameters | Matched L group (n=46) | Matched S group (n=46) | P value |
|---|---|---|---|
| Median operation time, min [IQR] | 162 [128–192] | 181 [151–214] | 0.03 |
| Median blood loss, mL [IQR] | 85 [51–138] | 90 [20–140] | 0.19 |
| Median hospital stay, d [IQR] | 16 [12–21] | 13 [12–16] | 0.06 |
| Morbidity†, n (%) | 7 (15.2) | 3 (6.5) | 0.32 |
| Change rate of FEV1.0, % [IQR]‡,§ | 88 [81–91] | 90 [86–95] | 0.29 |
| Pathological stage (UICC 7th), n (%) | 0.48 | ||
| IA | 28 (60.9) | 31 (67.4) | |
| IB | 13 (28.3) | 14 (30.4) | |
| IIA | 3 (6.5) | 1 (2.2) | |
| IIB | 2 (4.3) | 0 (0.0) | |
| pl factor, n (%) | 0.32 | ||
| 0 | 39 (84.8) | 43 (93.5) | |
| 1 | 6 (13.0) | 3 (6.5) | |
| 2 | 1 (2.2) | 0 (0.0) | |
| Surgical procedure, n (%) | 0.002 | ||
| VATS | 10 (21.7) | 25 (54.3) | |
| Thoracotomy | 36 (78.3) | 21 (45.7) |
†, Clavien-Dindo ≥grade III; ‡, change rate from the preoperative data to the postoperative data (6 months after the surgery); §, missing values have been removed (postoperative respiratory examination was performed by 13 patients in L group and 28 patients in S group). L group, lobectomy group; S group, trisegmentectomy group; FEV1.0, forced expiratory volume in 1 s; IQR, interquartile range; pl, pleural invasion; UICC, Union for International Cancer Control; VATS, video-assisted thoracic surgery.
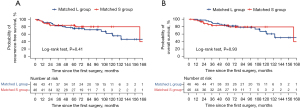
The median follow-up duration was 75.2 months in the matched L group and 61.2 months in the matched S group. The 5-year RFS rate did not significantly differ between the groups (75.5% vs. 84.0%, P=0.41, Figure 3A). The recurrence rate was 17.4% in the matched L group (five distant recurrences and three locoregional recurrences) and 8.7% in the matched S group (one distant recurrence and three both locoregional and distant recurrences). No significant differences were observed in the recurrence rate between the groups (P=0.35). Among patients with locoregional recurrence, mediastinal lymph node metastasis occurred in two patients, while multiple pulmonary metastases (including residual lobe metastasis) occurred in one patient. The 5-year OS rate did not significantly differ between the groups (82.0% vs. 83.3%, P=0.93, Figure 3B). In unmatched sensitivity analysis including all eligible patients, there were no significant differences between the L and S groups (Figure S1).
Analysis 2 (trisegmentectomy cohort)
The trisegmentectomy cohort comprised 15 cases in which the tumor was located ≤20 mm from the intersegmental plane on 3D-CT (short-distance group) and 47 cases in which it was located >20 mm from the intersegmental plane (long-distance group).
The 5-year RFS rate did not significantly differ between the short- and long-distance groups (79.4% vs. 81.2%, P=0.69, Figure 4A). The recurrence rate was 13.3% in the short-distance group (one case of distant recurrence and one case involving both locoregional and distant recurrence) and 8.5% in the long-distance group (two cases of distant recurrence and two cases involving both locoregional and distant recurrence). No significant difference was observed between the groups in terms of recurrence rate (P=0.63). Multivariate analysis revealed that the distance from the intersegmental plane was not a significant predictor of RFS (hazard ratio: 1.75, 95% confidence interval: 0.52–5.91, P=0.37, Table 3). The 5-year OS rate did not differ significantly between the short- and long-distance groups (79.0% vs. 80.2%, P=0.63, Figure 4B). Univariate sensitivity analysis yielded similar findings (hazard ratio: 0.79, 95% confidence interval: 0.24–2.56, P=0.69). Nevertheless, we should consider that these statistical analysis results might have been underpowered to reach statistical significance because of the small sample size.
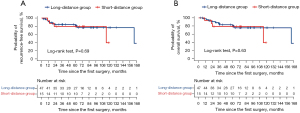
Table 3
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age >75 years | 2.49 | 0.76–8.15 | 0.13 |
| Distance from the intersegmental plane >20 mm | 1.75 | 0.52–5.91 | 0.37 |
| SUVmax >2.5 | 3.09 | 0.91–10.49 | 0.07 |
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value.
Discussion
The principal findings of this retrospective study were as follows: (I) trisegmentectomy for cN0 NSCLC in the left upper division was not significantly inferior to lobectomy in terms of oncological outcomes, and (II) multivariate analysis revealed that the distance from the intersegmental plane was not a significant predictor of RFS.
Although multi-institutional randomized clinical trials (JCOG0802/WJOG4607L and CALGB140503) have demonstrated the clinical value of segmentectomy, these trials were limited to patients with peripheral small-sized NSCLC (4,15). Given the anatomical similarity of the left and right upper lobes, the current study was designed to evaluate the benefit of trisegmentectomy for cN0 NSCLC in the left upper division based on oncological outcomes according to tumor location.
Some previous studies on NSCLC in the left upper division have reported the non-inferiority of trisegmentectomy to lobectomy (6-11). Among these studies, one of the largest studies was conducted by Zhou et al. (11), who performed propensity score matching, resulting in 273 pairs of patients undergoing video-assisted thoracoscopic left upper trisegmentectomy or lobectomy for stage I NSCLC. The authors reported no significant differences in clinical or oncological outcomes between the groups, in accordance with the findings of Analysis 1. Furthermore, the results of Analysis 2 suggest that the indication for trisegmentectomy can be expanded to include left upper division tumors ≤20 mm from the intersegmental plane. To the best of our knowledge, this is the first study to incorporate margin distance when analyzing trisegmentectomy outcomes.
One of the most important concerns when performing segmentectomy is the prevention of locoregional recurrence in the residual lobe. Previous studies have identified two lymphatic pathways in the lungs: the superficial subpleural route and the deep vessel route draining from the superficial system into the hilar segmental lymph nodes (16). Given that the intersegmental septum blocks superficial lymphatic spread to the adjacent segment, accurate intersegmental dissection during segmentectomy may allow removal of the tumor in the affected segment without infiltration of cancer cells into the neighboring segment. Moreover, for tumors located close to the intersegmental plane, our group aims to dissect the neighboring segment while sacrificing the intersegmental vein—a technique that has also been applied in similar scenarios in the right upper and middle lobes. Even when the minor fissure is incomplete and the tumor is close to the intersegmental plane, most thoracic surgeons spare the right middle or upper lobe without performing bilobectomy.
Accumulating evidence suggests that 3D-CT virtual simulation imaging can substantially improve preoperative assessments of the vasculature and bronchopulmonary trees and predictions of postoperative lung function (17-19). Such simulations can also be used to predict the tumor margin (17). In the current study, 3D-CT simulation was used to assess the distance of the tumor from the intersegmental plane. Currently, segmentectomy is not recommended for clinical T1 NSCLC when the distance from the intersegmental plane is ≤20 mm because of the difficulty in maintaining an adequate resection margin. However, Analysis 2 revealed no significant differences in RFS between patients with tumors ≤20 mm from the intersegmental plane and those with tumors >20 mm from the intersegmental plane. These results suggest that trisegmentectomy provides acceptable oncological outcomes, similar to those observed following lobectomy.
Our study has some limitations. First, this was a non-randomized, retrospective study conducted at a single institution. Despite the use of propensity score matching, the study design is associated with inherent biases in patient selection, among others. Second, the number of patients was relatively small. Therefore, further studies including a larger sample size are needed to clarify the clinical relevance of our findings. Finally, as surgical techniques have progressed, both open surgery and video-assisted thoracic surgery were included in the analysis. Consequently, future prospective studies are necessary to further reduce bias and validate the current results.
Conclusions
Our analysis suggests that oncological outcomes (i.e., RFS rates) following trisegmentectomy for cN0 NSCLC in the left upper division are not significantly inferior to those following lobectomy, even when the tumor is located close to the intersegmental plane.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-950/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-950/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-950/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-950/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Hyogo Cancer Center (No. G-91; approved October 25, 2019), and written informed consent regarding the research use of their data was taken in the form of opt-out from all individual patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McMurry TL, Shah PM, Samson P, Robinson CG, Kozower BD. Treatment of stage I non-small cell lung cancer: What’s trending? J Thorac Cardiovasc Surg 2017;154:1080-7. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Tane S, Nishio W, Nishioka Y, et al. Evaluation of the Residual Lung Function After Thoracoscopic Segmentectomy Compared With Lobectomy. Ann Thorac Surg 2019;108:1543-50. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Aprile V, Bertoglio P, Dini P, et al. Is left upper lobectomy always worthwhile for early stage lung cancer? A comparison between left upper lobectomy, trisegmentectomy, and lingulectomy. J Surg Oncol 2018;117:618-24. [Crossref] [PubMed]
- Iwasaki A, Hamanaka W, Hamada T, et al. Comparison between a case-matched analysis of left upper lobe trisegmentectomy and left upper lobectomy for small size lung cancer located in the upper division. Thorac Cardiovasc Surg 2007;55:454-7. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg 2012;144:S23-6. [Crossref] [PubMed]
- Witte B, Wolf M, Hillebrand H, et al. Split-lobe resections versus lobectomy for lung carcinoma of the left upper lobe: a pair-matched case-control study of clinical and oncological outcomes. Eur J Cardiothorac Surg 2014;45:1034-9. [Crossref] [PubMed]
- Zhou B, Xu X, Dai J, et al. Propensity-matched Comparison of VATS Left Upper Trisegmentectomy and Lobectomy. Ann Thorac Surg 2022;114:1007-14. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology–v. 2.2022: Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Altorki NK, Wang X, Kozono D, et al. Lobar or sub-lobar resection for peripheral clinical stage IA = 2 cm non-small cell lung cancer (NSCLC): results from an international randomized phase III trial (CALGB 140503 [Alliance]). Session information of the 2022 World Conference on Lung Cancer, PL03.06; 2022 Aug 6-9; Vienna, Austria. [cited 2022 Aug 19]. Available online: https://library.iaslc.org/conference-program?product_id=25
- Riquet M. Bronchial arteries and lymphatics of the lung. Thorac Surg Clin 2007;17:619-38. viii. [Crossref] [PubMed]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery†. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Kobayashi K, Saeki Y, Kitazawa S, et al. Three-dimensional computed tomographic volumetry precisely predicts the postoperative pulmonary function. Surg Today 2017;47:1303-11. [Crossref] [PubMed]


