Prevalence of metastasis in T1b esophageal squamous cell carcinoma: a retrospective analysis of 258 Chinese patients
Introduction
The 5-year overall survival (OS) of esophageal carcinoma ranges from 15% to 25% (1). To improve the OS, it is very important to focus on early esophageal cancer. At present, early esophageal cancer can be resected less invasively through endoscopy. There is a consensus that mucosal esophageal adenocarcinoma should undergo endoscopic therapy. However, in patients with submucosal cancer with low-risk characteristics, there is controversy over whether endoscopic treatment is a valid alternative to esophagectomy and no consensus is achieved (2,3). Some studies have also reported that LNM and mortality increased with an increase in the depth of submucosal invasion (4,5). T1 tumor is categorized into T1a (mucosal invasion) and T1b (submucosal invasion), and T1b is further subcategorized into inner one-third (sm1), middle one-third (sm2), and deep one-third (sm3) (6) (Figure 1). With regard to T1b, some studies have reported low rates of metastasis in sm1, whereas other studies have reported high rates of metastatic lymph nodes in sm1 comparable to sm2 and sm3 (7-9). The underlining reasons for the discrepancy may be the relatively small sample of sm1 and tumor heterogeneity (4,10,11). The present study included data from the largest single-center series of Chinese patients with T1b esophageal squamous cell carcinoma (ESCC) who underwent esophagectomy. It further analyzed the depth of invasion and other histopathological risk factors for LNM and OS.

Methods
Study population
From November 2009 to March 2014, 1731 patients underwent esophagectomy for cancer at First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China. Of these, 258 patients were pathologically diagnosed to have ESCC invading the submucosa (pT1b). The inclusion criteria were (I) presence of T1b esophageal cancer, regardless of regional or distant metastases and (II) ESCC. The exclusion criteria were (I) synchronous carcinomas; (II) preoperative chemo- and/or radiotherapy; (III) cervical esophagus; (IV) R1 resection; and (V) unidentified stratification (Figure 2). This retrospective study was approved by the Institutional Review Board of First Affiliated Hospital of Nanjing Medical University (ID: 2016-SR-050), and informed consent was waived.

Preoperative workup
The routine preoperative workup included clinical manifestations, physical examination, upper gastrointestinal tract endoscopy, and histologic confirmation of the carcinoma. Endoscopic ultrasound was used to observe the depth of tumor invasion and suspected metastatic lymph nodes when needed. Enhanced chest and abdominal computed tomography (CT), and cervical ultrasound were performed to evaluate the resectability of esophageal cancer. Positron emission tomography/computed tomography (PET/CT) was used when suspected metastatic lymph nodes were not clear.
Types of esophagectomy
All surgical procedures were performed by experienced surgeons. The McKeown procedure, Ivor-Lewis procedure, and Sweet procedure were the three most commonly used types of surgical procedures in China. Details about indications and surgical techniques were previously described (12). The main differences among these surgical procedures are the domain of lymph node resection and the incision approaches. In brief, total mediastinal lymphadenectomy was routinely performed through a right-sided approach (McKeown procedure for upper esophageal tumors and Ivor-Lewis procedure for middle and lower tumors), and on the left-sided using the Sweet procedure for lower esophageal tumors. As regard to the Sweet procedure, only the lymph nodes in the middle and lower mediastinum were removed because of the anatomic limitations (13). Stomach is the graft for esophageal replacement. Upper esophageal cancer requires cervical anastomosis, and middle or lower esophageal cancer requires intrathoracic anastomosis.
Histopathological assessment
Histopathological examination of the surgery resected specimens was performed at the Department of Pathology by Mingna Li and Zhihong Zhang who were blinded to previous pathological reports. The discordant cases were jointly discussed by a group of pathologists, which comprised two senior and two intermediate level pathologists with a work experience of more than six years.
Superficial esophageal cancers were classified into three main types: 0–I, superficial and protruding type; 0–II, superficial and flat type (0–IIa, slightly elevated type; 0–IIb, flat type; and 0–IIc, slightly depressed type); and 0–III, superficial and distinctly depressed type (14). In the present study, flat types were defined as 0–II; non-flat types were defined as 0–I and 0–III according to the previous literature (11).
A histopathological workup of the resected specimens and lymph nodes was performed using a standardized protocol. The specimens were pinned to cork mats and fixed with 10% formalin. After macroscopic assessment and photo-documentation with reflected light microscopy, the specimens were continuously cut into 2-mm wide slices. Then, after dehydration, the material was embedded in paraffin. Subsequently, about eight 4-µm thick serial sections were obtained from each 2-mm block, and they were deparaffinized and stained with hematoxylin and eosin (H & E) for microscopic observation. All sections of the tumors were evaluated. Then the histopathological grade, submucosal invasion depth, and lymphatic vessel invasions were evaluated. Lymphatic vessel invasions were considered when lymphatic endothelial cells were demonstrable. In questionable cases, an immunohistochemical test endothelial cell marker (monoclonal antibodies D2-40, MXB, Fuzhou, China) was applied. The tumor length was defined as the maximal tumor length under microscopy. The tumor width was defined as 2 mm multiply by the number of tumor sections. Tumor volume was calculated using (length × width2)/2 according to previous literature (15,16). As subdivision of submucosal esophageal cancer is not routinely performed at our center, the specimens were re-reviewed and subcategorized into three layers according to previous studies (4,17,18) (Figure 1). When a patient had synchronous esophageal cancers, histopathological factors of the maximum penetration depth were recorded.
Follow-up
After the surgery, the patients were followed up at the outpatient department every three months during the first year, and then six months after that using the same modalities. At each visit, clinical symptoms, physical examination, blood tumor markers, and results of the enhanced chest and upper abdominal CT scan were recorded. If the CT scan detected an enhanced focus measuring ≥10 mm or with a tendency to grow as compared with previous examinations, then recurrence was suspected, and a biopsy was performed to verify the recurrence.
Statistical analysis
With regard to the three layers of submucosal cancer, binary variables were compared using the Cochran-Armitage test, continuous variables were compared using the Jonckheere-Terpstra test, and ordinal variables were compared using the Cochran-Mantel-Haenszel correlation test. To obtain a cutoff of tumor length, tumor width, and tumor volume in predicting LNM, receiver operating characteristic (ROC) curves were used. With regard to the univariate and multivariate analysis for the predictors of LNM, a logistic regression was used, and odds ratio (OR) and 95% confidence interval (CI) were calculated. Categorical data were compared using Chi-square test or Fisher’s exact test when the expected cell values were too small. With regard to the univariate and multivariate analysis for the predictors of the OS, a cox regression was used, and hazard ratio and 95% CI were calculated. The OS was calculated using the Kaplan-Meier method, and the differences were statistically evaluated using the log-rank test. All tests were two-sided, and the significance level was set at P<0.05.
Results
Preoperative staging
A total of 212 patients completed preoperative staging, the results were: 6 T0N0 (3%), 125 T1N0 (60%), 5 T1N1 (2%), 66 T2N0 (31%), 7 T2N1 (3%) and 3T3N0 (1%).
Characteristics of patients, cancer, and surgery
Table 1 summarizes the basic characteristics of patients, cancer, and surgery. The distribution of submucosal invasion showed that 75 (29.1%), 73 (28.3%), and 110 (42.6%) patients had sm1, sm2, and sm3, respectively. Eighteen (7.1%) patients had a positive lymphovascular invasion (LVI). Overall, 5 (1.9%) patients had a good, 156 (60.5%) patients had a moderate, and 97 (37.6%) patients had a poor degree of differentiation. The median number of harvested lymph nodes was 10. Forty (15.5%) patients had positive lymph node metastasis (LNM); among whom 36 (14.0%) patients had pN1 disease and 4 (1.6%) patients had pN2 disease. Overall, 2 (0.8%) patients underwent ESR before esophagectomy.
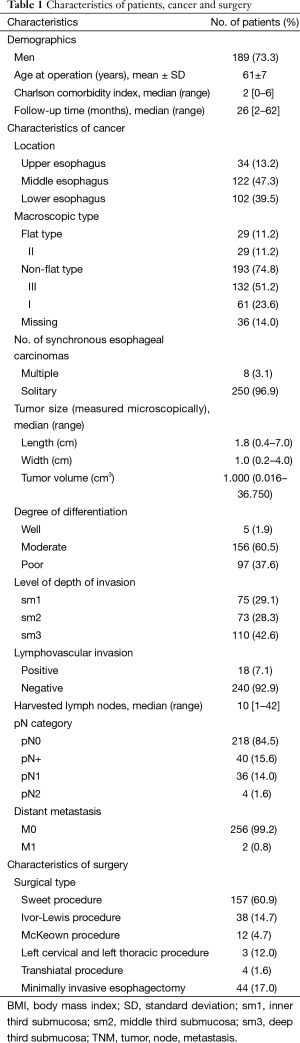
Full table
Cancer, surgical characteristics, and prognosis according to the depth of invasion
As submucosal invasion increased, the number of positive lymph nodes (P=0.082), and the pathologic classification (P=0.071) showed an increasing tendency. More synchronous esophageal cancers were correlated with less depth of submucosal invasion (P=0.029). The recurrence of tumor or death (P=0.047) increased with an increase in the depth of submucosal invasion. Total harvested lymph nodes (P=0.237), type of surgery (P=0.340), and macroscopic types (P=0.123) did not show an association with increasing depth of submucosal invasion. The LVI and distant lymph node involvement were not found to be associated with increasing depth of submucosal invasion (Table 2).
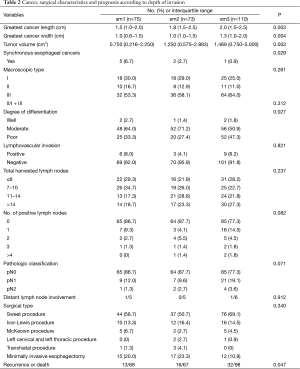
Full table
Risk factors for lymph node metastasis (LNM)
The prevalence of LNM was similar irrespective of sex, age at surgery, and type of surgery. Tumor length (P=0.015), tumor width (P=0.019), tumor volume (P=0.010), LVI (P<0.001), and sm3/(sm1 + sm2) (P=0.041) were associated with greater occurrence of LNM; poor/moderate degree of differentiation (P=0.073) was borderline significant (Table 3). Multivariate analysis identified tumor volume >1.856 cm3 (OR 2.349; 95% CI, 1.131–4.880) and positive LVI (OR, 4.956; 95% CI, 1.666–14.745) as independent risk factors. Neither the depth of invasion (sm3/sm1 + sm2) (OR, 1.749; 95% CI, 0.835–3.664) nor degrees of differentiation (poor/moderate) (OR, 1.450; 95% CI, 0.693–3.036) could predict the LNM (Table 4).
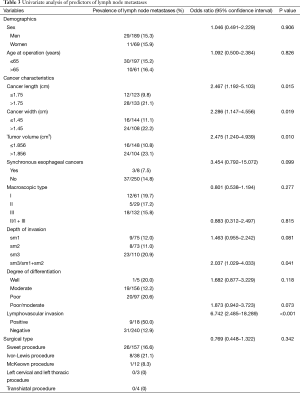
Full table
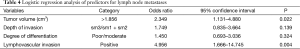
Full table
Distant metastases according to the depth of tumor invasion
With regard to distant metastases, no significant differences were observed for sm3 vs. sm1 (P=0.338) or sm3 vs. sm2 (P=0.353) (Table S1).

Full table
Prognostic factors for survival
There was no in-hospital mortality after surgery. The follow-up was complete in 242 (93.8%) patients. The rate of OS was 93.5%, 80.5%, and 64.7% at 1, 3, and 5 years after esophagectomy, respectively. Univariate cox regression analysis identified LNM to be associated with poor OS (P<0.001) and a positive LVI might affect the OS (P=0.092). Multivariate cox regression analysis identified only LNM as an independent risk factor for OS (Table 5). For patients with pN0 cancers, the rate of survival was 96.6%, 85.3%, and 66.2% at 1, 3, and 5 years, but 78.5%, 56%, and 34% for patients with pN1-2 cancers (P<0.001) at the same intervals (Figure 3). With regard to different depths of invasion, at 1, 3, and 5 years, the rate of OS was 95.6%, 84.6%, and 79.6% in sm1, 96.8%, 77.5%, and 68.9% in sm2, and 90.1%, 78.6%, and 49.2% in sm3 tumors, respectively (P=0.272) (Figure 4).
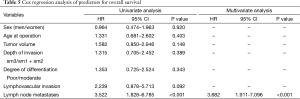
Full table


Risks of lymph node metastasis (LNM), recurrence, and death according to the depth of tumor, presence of lymphovascular invasion (LVI), and pathological grade
Next, the LNM, recurrence, and death were investigated according to the depth of tumor, presence of LVI, and pathological grade (Table S2).
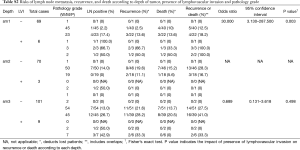
Full table
Comments
The principal findings are as follows: In T1b ESCC, depth of invasion (sm3) might be associated with regional LNM after univariate analysis; both tumor volume >1.856 cm3 and positive LVI predicted regional LNM after multivariate analysis; only regional LNM was associated with the OS after cox regression analysis.
In China, few studies have been conducted to investigate the submucosal esophageal cancer (13,19), but this is the first exhaustive study on the impact of depth of submucosal invasion on both LNM and OS in China. We believe the results of this study will provide evidence for gastroenterologists.
Compared to two previous studies by Hölscher et al. (20) and Ancona et al. (10) that included squamous cell cancer patients, the present study showed negative results. The possible reasons for the discrepancy are as follows. First, there was presence of a very small number of well-differentiated tumors (2%) in this series. Most samples resected were moderately or poorly differentiated, and their impact on LNM may mask the effects of depth of invasion. Second, the reliability and reproducibility of measuring the depth of submucosal invasion even though the reproducibility of submucosal classification was good concerning inter- or intra-observer variability on the prerequisite of an expert pathologist (21). Third, less than one-quarter of the patients had more than 14 lymph nodes harvested which could cause pathologic under-staging and hence the negative findings. Moreover, five-year survival rates in pN0 patients are lower than that reported by Hoelscher and Ancona. We speculate that nonperformance of a much-extended lymph node dissection rather than just the genetics of the Chinese tumors could be the possible cause of the low survival rate. Thus, we propose that an extended lymph node dissection should be performed for T1b esophageal cancers.
In comparison with the report of Akutsu et al. (5) with LNM rates of 21% in sm1, 30% in sm2, and 51% in sm3, the present study showed lower LNM rates, suggesting that there may be heterogeneity of surgical procedures in this series. It has been revealed that cervical-thoracic junction is the most frequent site of lymphatic involvement in T1b tumors. The extent of lymph node dissection, especially less optimal dissection via the Sweet approach (accounted for 60.9% in this series) may have a strong impact on the outcome of lymph node yield as well as patient survival. In the present study, the median number of lymph node dissection was 9 (IQR, 6–13) in the Sweet procedure, compared with 12 (IQR, 10–16) in the Ivor-Lewis procedure and 11.5 (IQR, 7.25–18.25) in the McKeown procedure (P<0.001). This could also explain why a median number of lymph node harvested (only 10) and the overall rate of lymph node involvement (only 15.5%) appeared to be lower than that reported in other series from the eastern countries (11,22).
The rate of positive lymphatic invasion in the present study (7.1%) was lower than that reported by Akutsu et al. (20.1%) (5) and Shimada et al. (74%) (11). We speculate that the discrepancy may be explained by the following two reasons. (I) The lower rate of positive lymphatic invasion may be associated with the experience of different pathologists, and sometimes lymphatic detection was performed with a certain degree of subjectivity. Dr. Hofstetter had mentioned in the discussion of the article by Raja et al. (4) that they upstaged to lymphatic invasion about 10% of the time when the slides were re-examined by another pathologist; (II) the standard for detection of lymphatic invasion in the present study was H & E rather than monoclonal antibody D2-40. Only the questionable slides were detected by a monoclonal antibody, and this may decrease the rate of detecting positive lymphatic invasion (23,24). However, both reasons were only speculations because the detailed pathological examination of lymphatic invasion was not fully explained in the articles by Akutsu et al. and Shimada et al.
To improve the OS, using sensitive methods to obtain positive lymph nodes is very important; however, up to now, there is no effective method for detecting LNM. Concerning early esophageal cancer, PET/CT is not useful in the evaluation of tumor depths and lymph nodes metastasis (25), and it is not always easy to distinguish sm1, sm2, and sm3 clearly with endoscopic ultrasonography (26). It is recommended that suspected T1 esophageal cancer, which is diagnosed using endoscopic ultrasound, should undergo diagnostic endoscopic mucosal resection or endoscopic mucosal dissection (EMR/ESD), and then the subsequent treatment strategy should be decided according to the pathological specimens. However, with EMR, it is unknown how much submucosa is removed. The result of the present study showed that tumor volume >1.856 cm3 and LVI predicted lymph node metastasis. This result will be useful for indication of surgery after diagnostic endoscopic resection (ER).
There is a criticism that division of submucosa into thirds is reasonable with an esophagectomy since the whole esophagus is removed, but with ER, it is unknown how much submucosa is removed. Therefore, division into thirds is not reliable or reproducible. Sm1 corresponds approximately to less than 200 µm in ESCC in the present study (27). Considering the ongoing debate between sm1 and sm2 + sm3, the inner division of esophagectomy could be applied to the endoscopic specimens.
This study has some limitations. First, as this is a retrospective analysis, the collected information may be biased. For example, patients lost to follow-up may affect the results of survival. Another bias may be that more synchronous lesions may be associated with less LNM. However, these cases were not excluded because they were not verified in a larger population (17). Second, the patient number may be small, although we detected a power of more than 81% for submucosal subgroup status to reach their impact on OS. Third, the domain of lymph node resection is greatly affected by the preference of the surgeon and surgical approaches. For instance, Sweet procedure might limit the resection number of the upper mediastinal lymph node. The three-field lymph node resection is not yet a standard procedure in China, and this may result in missing some positive cervical lymph nodes. In addition, the follow-up duration is relatively short with a median of 26 months, which may not reveal the differential impact of depth of invasion on mortality and a longer follow-up time is needed.
Conclusions
The present study indicates that submucosal ESCC had a substantial rate of LNM, even at sm1. In T1b ESCC, adjusting for possible covariates, depth of invasion does not predict LNM or OS.
Acknowledgements
We are very grateful to Qian Wang, Yuanyuan Wang, Shanshan Li for their work on follow-up.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [Crossref] [PubMed]
- Whiteman DC, Appleyard M, Bahin FF, et al. Australian clinical practice guidelines for the diagnosis and management of Barrett's esophagus and early esophageal adenocarcinoma. J Gastroenterol Hepatol 2015;30:804-20. [Crossref] [PubMed]
- Raja S, Rice TW, Goldblum JR, et al. Esophageal submucosa: the watershed for esophageal cancer. J Thorac Cardiovasc Surg 2011;142:1403-11.e1.
- Akutsu Y, Uesato M, Shuto K, et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. Ann Surg 2013;257:1032-8. [Crossref] [PubMed]
- Endo M, Yoshino K, Kawano T, et al. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus 2000;13:125-9. [Crossref] [PubMed]
- Rice TW, Zuccaro G Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998;65:787-92. [Crossref] [PubMed]
- Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005;446:497-504. [Crossref] [PubMed]
- Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703-10. [Crossref] [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [Crossref] [PubMed]
- Shimada H, Nabeya Y, Matsubara H, et al. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg 2006;191:250-4. [Crossref] [PubMed]
- Fujita H, Sueyoshi S, Tanaka T, et al. Optimal lymphadenectomy for squamous cell carcinoma in the thoracic esophagus: comparing the short- and long-term outcome among the four types of lymphadenectomy. World J Surg 2003;27:571-9. [Crossref] [PubMed]
- Li B, Chen H, Xiang J, et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2013;146:1198-203. [Crossref] [PubMed]
- Japanese Society for Esophageal Disease. Guideline for the clinical and pathologic studies on carcinoma of the esophagus. 8th ed. Kanahara, Tokyo, 1992:8-9.
- Euhus DM, Hudd C, LaRegina MC, et al. Tumor measurement in the nude mouse. J Surg Oncol 1986;31:229-34. [Crossref] [PubMed]
- Naito S, von Eschenbach AC, Giavazzi R, et al. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res 1986;46:4109-15. [PubMed]
- Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [Crossref] [PubMed]
- Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol 2010;8:248-53. [Crossref] [PubMed]
- Xue L, Ren L, Zou S, et al. Parameters predicting lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. Mod Pathol 2012;25:1364-77. [Crossref] [PubMed]
- Hölscher AH, Bollschweiler E, Schröder W, et al. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg 2011;254:802-7; discussion 807-8. [Crossref] [PubMed]
- Grotenhuis BA, van Heijl M, ten Kate FJ, et al. Inter- and intraobserver variation in the histopathological evaluation of early oesophageal adenocarcinoma. J Clin Pathol 2010;63:978-81. [Crossref] [PubMed]
- Eguchi T, Nakanishi Y, Shimoda T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol 2006;19:475-80. [Crossref] [PubMed]
- Kozłowski M, Naumnik W, Nikliński J, et al. Lymphatic vessel invasion detected by the endothelial lymphatic marker D2-40 (podoplanin) is predictive of regional lymph node status and an independent prognostic factor in patients with resected esophageal cancer. Folia Histochem Cytobiol 2011;49:90-7. [Crossref] [PubMed]
- Yonemura Y, Endou Y, Tabachi K, et al. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol 2006;37:1193-9. [Crossref] [PubMed]
- Cuellar SL, Carter BW, Macapinlac HA, et al. Clinical staging of patients with early esophageal adenocarcinoma: does FDG-PET/CT have a role? J Thorac Oncol 2014;9:1202-6. [Crossref] [PubMed]
- Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol 2010;8:1037-41. [Crossref] [PubMed]
- Takubo K, Aida J, Sawabe M, et al. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology 2007;51:733-42. [Crossref] [PubMed]


