Oligoprogression in non-small cell lung cancer: a narrative review
Introduction
Lung cancer is the most common non-cutaneous cancer worldwide and the second most common cancer in the United States. In 2022, there is an estimated 236,740 new cases, contributing to 12.4% of all new cancer cases and about 21.4% of all cancer deaths in the United States (1). Non-small cell lung cancer (NSCLC) accounts for about 80% of all lung cancers and portends a more favorable prognosis as compared to small cell lung cancers.
For NSCLC, the American Joint Commission on Cancer (AJCC) tumor-node-metastasis (TNM) staging system, which is developed by the International Association for the Study of Lung Cancer (IASLC), has limited definition of metastasis regarding prognostic indications but has been expanded from the 7th edition to include M1a to M1c in the most recent 8th edition (2). Traditionally, the standard of treatment for metastatic disease to prolong overall survival (OS) has been systemic therapy. However, within the realm of metastatic disease, oligometastatic disease (OMD) and oligoprogressive disease (OPD) have emerged as subsets that may portend improved survival, especially in the era of improved systemic therapy and improved technology including stereotactic body radiation therapy (SBRT)/stereotactic ablative radiotherapy (SABR) for delivering safe and high doses for better local control. We aim to clearly define and describe features of OPD in the context of OMD for NSCLC as well as discuss local ablative therapy (LAT) as an increasingly used management modality. We further aim to discuss current and future clinical trials based on literature review of scientific databases including PubMed as well as report several relevant current clinical trials underway as reported via Clinicaltrials.gov. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-536/rc).
Methods
Extensive literature review of oligoprogression in NSCLC was performed via Table 1. Several sources were used including peer-reviewed journal articles, textbooks, and relevant online databases as well as established literature. As the concept of oligometastasis emerged in the 1960s, our specified time frame begins in 1960 to the present day.
Table 1
| Items | Specification |
|---|---|
| Date of search | 02/02/2022–03/02/2022 |
| Databases and other sources searched | PubMed, ClinicalTrials.gov, EMBASE, GoogleScholar; textbooks |
| Search terms used | Non-Small Cell Lung Cancer (NSCLC), Oligoprogression, Oligometastases, Metastatic Disease, Stereotactic Body Radiation Therapy (SBRT), Stereotactic Ablative Radiation Therapy (SABR), Targeted Therapy, Tyrosine Kinase Inhibitor, Immunotherapy |
| Timeframe | 1960–2022 |
| Inclusion and exclusion criteria | Considered retrospective and prospective studies, phase II randomized studies, oligometastatic review articles |
| Selection process | Authors KTN and GS selected articles discussing local ablative therapy in NSCLC, and conferred with authors DPS, MTM, and HQ |
Historical perspective
Defining the oligometastatic state
To understand oligoprogression, we first need to understand the history of oligometastases. In the 1960s, Dr. Phillip Rubin and Dr. Jerold Green wrote a book entitled “Solitary Metastasis” summarizing data and exploring the concept of possible curative intent treatments in patients with one metastasis (seen on X-ray imaging) (3). In 1968, Rubin published an editorial questioning whether metastases are curable (4). In 1995, Hellman and Weichselbaum proposed the term “oligometastatic disease” as a distinct state between locally confined cancer and systemic metastatic disease. They also suggested that metastatic disease can exist along a spectrum of clinical states (5). Within this definition lies different clinical scenarios that can describe OMD; patients may present with a synchronous or de novo (OMD at time of diagnosis in which primary tumor and limited number of metastases are detected simultaneously) vs. metachronous disease (oligometastatic recurrence following primary therapy at least 3–6 months after initial diagnosis, also referred to as oligorecurrence).
The implication of this subset of patients beyond improved prognosis is that localized ablative treatment of these oligometastatic site(s) may impact survival.
From 2012–2016, Gomez et al. conducted a phase II randomized multicenter study of 49 patients with histologically confirmed oligometastatic NSCLC, having three or fewer oligometastatic sites after first line systemic therapy. Patients were randomized to either local consolidative therapy (LCT) and maintenance systemic therapy or maintenance therapy (or surveillance) alone. Consolidative therapy consisted of either radiation with chemotherapy or resection of all lesions with or without maintenance treatment. The initial reports were released in 2016 and final results in 2019. The trial was closed early due to significant progression free survival (PFS) benefit (11.9 months) in the LCT arm vs. the standard of care arm (3.9 months). In an updated analysis, with median follow-up of 38.8 months (range, 28.3–61.4 months) the difference remained significant with a median PFS of 14.2 months [95% confidence interval (CI): 7.4–23.1 months] with LCT vs. 4.4 months (95% CI: 2.2–8.3 months) with maintenance therapy/observation (P=0.022) (6,7).
In 2018, Iyengar et al. published a single institution randomized phase II trial evaluating whether intervening with non-invasive SBRT/SABR would lead to improvements in PFS for patients with oligometastatic NSCLC on chemotherapy. Patients without anaplastic lymphoma kinase/epidermal growth factor receptor (ALK/EGFR) mutations and those that did achieve partial response or stable disease were randomized to SBRT to all sites of gross disease with maintenance chemotherapy vs. maintenance chemotherapy alone. A total of 29 patients with NSCLC, 14 in the SBRT arm and 15 in the maintenance chemotherapy arm with up to five oligometastatic lesions were enrolled. It is worth emphasizing that in the SBRT arm, half (7/14) of the patients received hypofractionated (45 Gy/15 fractions) regimen and not true SBRT regimen with ablative doses given. The trial was stopped early due to significant improvements with the addition of SBRT (PFS 9.7 vs. 3.5 months, P=0.01). Toxicities were similar in both arms. Overall consolidative SBRT prior to maintenance chemotherapy tripled PFS with no observed difference in toxicity leading to conclusions that consolidative SBRT is beneficial in oligometastatic settings (8).
The landmark phase II SABR-COMET open-label randomized controlled trial (RCT) included 99 patients with oligometastases with up to five metastatic areas (93% had one to three areas and 42% with one single area) from any cancer (most commonly breast, colorectal and lung) to SBRT/SABR group (67%) or control group (33%). Of note, excluded were patients with femoral bone metastasis or the presence of one to three brain metastases with no disease elsewhere. Despite a higher rate of grade ≥2 toxicities in SABR (19 of 66 patients) vs. control (3 of 33 patients), median OS was higher in the SBRT group with 50 months in the SBRT arm compared to 28 months in the control. PFS was also doubled with SBRT (11.6 vs. 5.4 months) (9).
Defining oligoprogression
Oligoprogression is a relatively new concept, emerging as more effective systemic therapies become available. OPD is still not consistently defined in the literature, although it is generally considered to be defined as a few lesions (≤5) progressing in the background of otherwise stable OMD or stable polymetastatic disease (PMD) as seen on radiological imaging (10).
The European Society for Medical Oncology (ESMO) has sought to further identify and clarify terminology (11). In recent literature, the definition of OPD refers to up to 3–5 metastatic areas having progression (increased in size and/or avidity) while on/off systemic therapy, though current investigations are looking to expand the number of metastatic areas as defined to be oligoprogressive. The definition of “metastatic areas” is also not uniform but implies a limited number of sites of progression. Variations in definition include whether metastatic lesions present at initial diagnosis that resolve after systemic therapy should be included or if different lymph node stations should be defined separately or together.
The European Society for Radiotherapy and Oncology (ESTRO) and American Society for Radiation Oncology (ASTRO) convened a committee to establish consensus definition of OMD and define gaps in current evidence including sub-classifications such as OPD. They performed a systematic review of the literature that focused on radiotherapy for OMD. The consensus was that the oligometastatic state is independent of the primary tumor, metastatic location and the presence or length of disease-free interval. There was also consensus for extra-cranial OMD being limited to a maximum of 5 metastatic lesions off-protocol. OPD on systemic therapy is considered clinically from OMD with possibly worse prognosis but with a treatment goal that may be more focused on keeping patients on a current line of systemic therapy rather than ablation of metastasis. The group also supported the consensus that, due to the lack of validated biomarkers, the ability to deliver safe and clinically meaningful radiotherapy was a minimum requirement for OMD for RT planning (12).
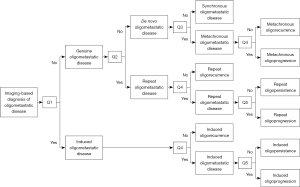
Guckenberger et al. proposed an OMD classification system that breaks down the oligometastatic state into eight subtypes. The two broad categories are a genuine oligometastatic state and an induced oligometastatic state. The genuine oligometastatic state is further divided into synchronous/de novo OMD, metachronous oligorecurrence and repeat oligorecurrence/persistence/progression. The induced state is subdivided into oligorecurrence, induced oligopersistence, or induced oligoprogression seen in Figure 1 (13). Overall, the goal with these eight subdivisions is to test these states of OMD in OligoCare prospective cohort [EORTC-ESTRO RADiotherapy InfrAstrucTure for Europe (E²-RADIatE)] to risk stratify the disease, and to further clarify which patients with OMD might benefit from treatment (14). We, instead, have proposed a model of defining metastatic disease to accurately characterize these states (Figure 2).
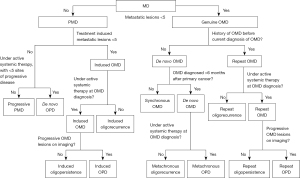
It is worthwhile to emphasize that OPD as a subset of OMD is a distinct entity compared to the de novo OPD (Figure 2). De novo OPD can be defined as less than 5 lesions progressing on imaging in a polymetastatic setting after systemic therapy. Unlike genuine OMD and OMD based OPD, de novo OPD is a very dynamic state as tumor kinetics and acquired mutations can progress at different rates at different tumor locations. It is also not known whether a transient window period will be responsive to LAT such as SABR/SBRT.
Patterns of progression
The frequency of reported OPD varies, though is estimated to be between 15–47% (15). OPD tends to be more common within the central nervous system (CNS), lungs, lymph nodes, bones as compared with adrenal or liver lesions. Oncogene mutations may increase likelihood of developing liver metastasis or pleural or pericardial metastasis (16).
Milano et al. in 2010 retrospectively looked at 77 patients with organ-confined oligometastasis (≤5 sites) and their pattern of oligoprogression following SABR; 73% of patients developed new metastasis, most frequently in same organ (occurring in 82% of first new metastases after SBRT and 89% of cumulative new metastases). For the lung, 73% of first recurrences occurred within the lung, then thoracic lymph nodes, then liver. A subset of patients develop progression at limited sites, amenable to additional courses of SBRT/SABR, with such treatment being associated with better survival. Oligoprogressive lesions following SBRT for organ confined disease often first appear in the organ confined site before progressing to distant extra organ sites (17). In the situation of EGFR+ NSCLC patients on tyrosine kinase inhibitor (TKI) therapy, most studies have indicated that the lung as the most common area of progression, with the CNS, and thoracic lymph nodes being the most common other site of progression (18). Other studies however have indicated the CNS as the most common site of oligoprogression for EGFR+ NSCLC (19).
Detection of oligoprogression
OPD is clinically diagnosed via imaging. Generally, to accurately stage NSCLC, both the ESMO and National Comprehensive Cancer Network (NCCN) recommend a positron emission tomography/computed tomography (PET/CT) scan and magnetic resonance imaging (MRI) of the brain due to the false negative rates of PET/CT scan in the brain (20).
Ng et al. in 2018 identified patients with metastatic EGFR+, ALK+, or ROS-1+ NSCLC on relevant TKI therapy from 2010–2016 and classified extracranial disease OPD (<4 lesions) by either 18F-FDG-PET/CT or CT. OPD was detected in 81.3% (26/32 patients) by PET/CT vs. 68.6% (24/35 patients) by CT (P=0.363). There was a non-significant trend to detect more extra-CNS disease with PET/CT. However, of these patients, there was a significant difference in the number that was treated with LAT 17/26 (65.4%) in 18F-FDG-PET/CT and 5/24 (20.8%) in CT group (P=0.004) (21).
Identifying OPD prior to diffuse dissemination to PMD is important as LAT with SBRT/SABR can lead to increased PFS and potentially OS. As a corollary the modality of imaging and the need for new radio-pharmaceuticals for PET based imaging becomes increasingly important in surveillance of these patients. This has been seen in other disease sites with increasing sensitivity, and specificity of prostate-specific membrane antigen (PSMA)-PET in prostate cancer diagnosis and treatment.
Treatment and management of oligoprogression
For limited metastatic disease, emerging data support locally ablative treatment (LAT) including resection (surgical metastasectomy) or radiotherapy such as SBRT/SABR, or stereotactic radiosurgery (SRS) in the case of brain metastasis (6,10,22). Yet, the optimal management of OPD remains controversial due to lack of prospective data. As increasing use of less invasive ablative techniques emerges which allows sparing of surrounding critical structures, radiotherapy has become a more commonly utilized option. The adoption of SBRT/SABR for OPD has been beneficial due to minimal interruption of systemic therapy, outpatient delivery of treatment, convenience of few fractions, and often with minimal adverse effects.
The biological basis of radiotherapy to eradicate oligoresistant disease lies in the fact that tumor cells are initially sensitive to systemic therapy and have had an initial complete or partial response to this therapy. This can be followed by development of resistance of certain tumor cells within discrete metastatic sites. Management of OPD consists of targeting treatment to alter the patterns of failure of those who progress on systemic therapy. In the case of NSCLC, molecular mutations and rearrangements give way to the development of targeted therapies that over time, through resistance and other molecular mechanisms, become less effective in controlling disease.
Treatment of OPD on systemic therapy
As an example of OPD management with NSCLC on systemic therapy, acquired resistance to TKI develops after a median of 8–13 months (23). SBRT added onto systemic treatment is thought to prolong response to systemic therapy by eradicating such non sensitive de-differentiated clones (24). In this sense, the goals of management of oligoprogression and LAT are to be able to continue systemic therapy and halt disease progression.
Recently, the Stereotactic Body Radiation Therapy in Newly Diagnosed Advanced Staged Lung Adenocarcinoma (SINDAS) trial evaluated first line TKI therapy for EGFR-mutated synchronous oligometastatic NSCLC and randomized patients to upfront radiation therapy (RT) vs. no RT; 133 patients with biopsy proved EGFR mutated adenocarcinoma with synchronous oligometastatic NSCLC without brain metastasis were enrolled from 2016–2019. They all received TKI therapy and were randomized to RT vs. no RT to all metastasis and primary tumor/involved regional lymphatics. Median follow-up was 23.6 months. Respective median PFS was 20.2 vs. 12.5 months (P<0.001) and median OS was 25.5 vs. 17.4 months (P<0.001). Based on the efficacy of results in the interim analysis, the trial was prematurely ended (25).
Modalities of LAT
Though most discussion thus far is centered on SBRT/SABR as LAT, there are other methods that may be used such as surgical excision and thermal ablative techniques such as radiofrequency ablation (RFA) and cryoablation. Surgical excision of metastasis has been demonstrated to improve outcomes in carefully selected patients as compared to outcomes of systemic therapy alone. Certain tumors are more easily resectable, such as lung, liver, adrenal, and brain (26).
Though these thermal ablative techniques are becoming more frequently used in lung cancer, the mechanism of their systemic immunomodulatory effects is still unclear. RFA is a modified electrocautery technique that is considered localized, minimally invasive in regards to tissue ablation. Clinically, it allows for local tumor destruction (27).
Metastasectomy or RFA may have the added benefit of obtaining tumor tissue which may allow for better classification of disease progression and mechanism of acquired resistance.
Randomized study of SBRT/SABR for OPD
Tsai et al. reported at the American Society of Radiation Oncology 2021 and 2022 meeting, the first randomized trial to evaluate the use of SBRT to treat oligoprogressive NSCLC and breast cancers with ≤5 extracranial oligoprogressive lesions. Of 106 enrolled patients, 59 had NSCLC and 47 had breast cancer. The median number of oligoprogressive lesions was 2, and total number of metastatic lesions was 5, with the maximum number being 10 lesions. Patients were randomized to receive standard of care treatment with vs. without SBRT/SABR to sites of OPD. For patients treated with SBRT, the median PFS was 44 weeks compared to 9 weeks for those who did not receive SBRT/SABR (P=0.001). Post SABR/SBRT, 40 of 56 patients further progressed, their sites of progression lung (35%), node (23%), and non-spine bone metastatic site (18%) and liver (11%) (28).
Brain metastasis and oligoprogression
It is thought that in patients with NSCLC who develop cerebral metastasis, certain characteristics such as EGFR+ NSCLC demonstrate a higher propensity for intracranial metastasis (29). Progression of disease in the brain may occur due to inadequate CNS penetration of systemic therapies that have emerged. Historically, brain metastasis has been associated with poor prognosis. However, in recent years with third generation TKI (i.e., osimertinib) and especially with the development of SRS, patients with brain metastasis may still have favorable disease with local control of primary tumor as one of the most important prognostic factors. The role of SRS for limited brain metastases is already well established.
Though not limited to NSCLC and not specific to oligoprogression, a 2014 study by Yamamoto et al. is a prospective observational study of patients with 1 to 10 new metastases (<3 cm) treated with SRS alone. Patients with 5–10 lesions were compared to patients with 2–4 tumors and patients with one tumor with primary end point of OS. Results showed that OS did not differ between the 5–10 lesion cohorts compared to the 2–4 lesion cohorts when treated with SRS, which indicates that local therapy may be suitable for patients with up to 10 metastasis (30).
Similarly, a recent review of patients with NSCLC and brain OMD with aggressive treatment with surgery to primary tumor and radiotherapy (with or without chemotherapy) showed a median survival of 15.5–31.8 months with 1 year survival of 50–71% and 2-year survival of 16–60%. Conclusions were that well-selected patients with NSCLC and exclusively oligometastatic cerebral disease represented a subgroup of patients in which aggressive local ablative treatment may help achieve long-term survival (31).
Targeted systemic therapy and oligoprogression
Local progression is the predominant pattern of failure for patients with advanced NSCLC treated with first line systemic therapy. The Norton-Simon hypothesis suggests a sigmoidal pattern of tumor growth and that the effect of systemic therapy is proportional to tumor growth. Ideas have emerged that LAT may move the tumor growth curve back to the exponential growth phase, allowing systemic therapies to remain effective and be used for longer durations (32).
Molecular studies: ALK+ and EGFR-mutations
A subset of NSCLC patients exhibit somatic EGFR mutation or ALK rearrangement, more commonly seen in never smokers, women, and patients of East Asian ethnicity. Less common mutations include MET, ROS1, BRAF and other mutations. In these patients, targeted TKI therapy results in higher response rates, improved PFS, and reduced side effects as compared to platinum-based chemotherapy. EGFR TKIs include third generation TKI osimertinib, second generation afatinib, third generation erlotinib and gefitinib. ALK rearrangements (found in about 5% of NSCLC) respond to TKIs such as crizotinib, alectinib, and ceritinib. ROS-1 mutations are seen in 1–2% of NSCLC and respond to crizotinib. BRAF V600E, MET, RET, and KRAS are emerging driver mutations that are thought to respond to vemurafenib, crizotinib, cabozantinib, and sotorasib respectively (10).
However, this same subset of patients tends to present with limited OPD (up to 50% estimated) as they quickly develop resistance. It is thought that radical local treatment to these oligoprogressive lesions may eradicate the de-differentiated clones and restore sensitivity of the metastatic disease. The literature in regard to radiation therapy is mainly based on reports of cases with ALK rearrangement and EGFR mutations as they appear to result in promising and favorable survival outcomes.
Selected prospective non-randomized studies of SBRT/SABR of NSCLC
Oligoprogression with EGFR or ALK mutation
In 2019, Weis et al. conducted a single arm prospective phase II study that enrolled 25 patients with EGFR mutant NSCLC on first generation TKI (erlotinib) who had 3 or fewer sites of extracranial progression treated with SABR/SBRT, subsequently continued on erlotinib. The median PFS post SBRT/SABR was 6 months (95% CI: 2.5–11.6 months) and OS was noted to be 29 months (95% CI: 21.7–36.3 months). The PFS in this prospective trial with erlotinib and SBRT/SABR is similar to that seen with second generation and third generation TKI such as dacomitinib and osimertinib (33).
Similarly, ATOM, a single arm phase II prospective study by Chan et al. in 2020, evaluated not strictly OPD but rather oliogopersistent disease; that is, patients on at least 3 months of TKI who on repeat PET/CT imaging have less than 4 PET-avid lesions. These patients were treated to all sites with SABR/SBRT. Unfortunately, due to poor accrual and competing drug trials, the study was closed early. A total of 18 patients were enrolled from 2014–2017, and 16 were analyzed. The median PFS was 15 months (18 months since randomization and being on first generation TKI), which is similar to second and third generation TKIs that would be started in a first line setting (34).
Selected retrospective non-randomized studies of SBRT/SABR of NSCLC oligoprogression with EGFR or ALK mutation
In a retrospective study of Memorial Sloan Kettering group of 184 patients, 18 EGFR-mutated patients with TKI therapy received local therapy (surgery, radiotherapy, or RFA) for extracranial OPD followed by continuation of TKI. Median OS was 41 months while median PFS was 10 months (35).
A single institution study by Weickhardt et al. investigated patients with ALK rearranged metastatic NSCLC (n=38) treated with crizotinib and EGFR-mutated NSCLC treated with erlotinib (n=27). A subset of patients with OPD suitable for LAT received either RT or surgery to these sites and were then continued on the same TKIs. Median PFS in ALK rearranged patients on crizotinib was 9 and 13.8 months for EGFR mutated patients on erlotinib (36).
Campo et al. conducted a literature review to identify publications regarding the use of RT in oligometastatic or oligoprogressive NSCLC. In several studies, SBRT allowed excellent local control and, in conjunction with TKI, was effective against disease in other anatomic sites, thereby prolonging PFS (37).
Several smaller scale studies have evaluated patients with apparent acquired resistance to TKIs (as seen by oligoprogression). Local therapies, in conjunction with continued systemic treatment, have been found to be well tolerated and associated with longer PFS and OS (38).
Oligoprogression under immune checkpoint inhibitors
There has been evidence that OPD that occurs with immune checkpoint inhibitors occurs later and with improved outcomes. This suggests improved treatment effects and more favorable biology of oligoprogressive NSCLC to immuno-oncology (IO) therapy. Rheinheimer et al. evaluated patients with OPD under treatment with PDL-1 inhibitors; 636 patients with stage IV NSCLC were evaluated. It was noted that OPD occurred later after the start of immunotherapy (median time to progression 9 vs. 2 months, P<0.0001) and was associated with longer OS than multifocal progression (36 vs. 16 months, P<0.001) (39).
Similarly, Wang et al. retrospectively reviewed patients with advanced NSCLC who received SBRT for OPD, defined as two or less sites of disease, after acquiring resistance to immune checkpoint inhibitors; 15 of 24 patients included were diagnosed with lung adenocarcinoma. After combining SBRT with checkpoint inhibitors, one- and two-year local control rates (LCRs) were 100% and 81.8% respectively. Median PFS and OS were 11 and 35 months respectively. This again, suggests a synergistic effect, perhaps by way of reshaping the immune microenvironment that over time, becomes resistant (40).
Tumor microenvironment (TME) as a contributing factor
At a molecular level, the classic “seed and soil” hypothesis introduced by Stephen Paget in 1889 recognized the interplay between “seed” tumor biology and “soil” which is encompassed by the complex micro and macro-environment that cancer cells maneuver to establish the metastatic state (41).
Favorable interactions must occur for metastasis to occur. The tumor seed is thought to undergo a series of sequential steps that must be taken prior to metastatic spread. This includes a loss of cell adhesion (detachment), migration and/or penetration, dedifferentiation, adhesion and invasion of circulatory vessels, extravasation and colonization of distant sites and growth. These processes are reliant on changes at the genetic, epigenetic level and soil interaction with micro and macro environments. This process contributes to tumor proliferation by “hiding” the tumor cells from the immune surveillance.
The microenvironment in NSCLC is characterized by rich angiogenesis (allowing for a rich tumor oxygen supply) as well as an immune environment composed by cytokines and immune cells usually related to chronic exposure of lung tissue to inhalant toxic agents (i.e., cigarette smoking). Immune response includes phagocytosis of inhaled pathogens and particles as well as epithelial cells modulating cytokines and chemokines.
The biological behavior and likely tumor heterogeneity contributes to OPD, as under the selective pressure of applied systemic treatment, there is promotion of development of one or more clones harboring intrinsic resistance mechanisms and the crosstalk between cancer cells and its surrounding TME (42).
Abscopal effect
There is literature to suggest that SBRT elicits abscopal effect, though the extent to which this occurs, and the clinical significance of such an effect remain controversial (43). There are various preclinical and clinical trials underway. Increasing number of reports of the abscopal effect have been made since the emergence of immunotherapy and immune checkpoint inhibitors, suggesting that there is, at the very least, a synergistic effect of combining RT with systemic therapy.While there has been emerging evidence of this effect, several pre-clinical and clinal models are still underway (44).
Importantly, SBRT concurrent or sequential with checkpoint inhibitors appears safe with response rate outside of irradiated field being modest (though unclear whether response was result of checkpoint inhibition alone or due to combination with SBRT). Weichselbaum has suggested that the synergistic effect is at most, modest, in clinical trials and that it is necessary to treat all sites of metastatic disease (45).
The phase II PEMBRO-RT study randomized patients with metastatic NSCLC to pembrolizumab +/− SBRT to a single site. Overall response was higher with SBRT (36% vs. 18%, P=0.07) but did not meet study’s criteria for clinical benefit though the PD-L1 negative subgroup seemed to benefit most from SBRT with evidence of an augmenting effect of SBRT on response to PD-1 blockade in metastatic NSCLC patients (46).
Less promising is a multi-center randomized open-label phase II trial from Germany presented at ESTRO. Patients with locally advanced or metastatic disease (not limited to NSCLC) were assigned to receive anti-PDL1 immune checkpoint therapy (standard of care) or combined SBRT (8 Gy × 3 fractions) prior to second or third immune checkpoint therapy cycle (up to a maximum of three sites). Of 99 patients randomized, 3 withdrew and 7 patients did not complete the study prescribed SBRT due to early disease progression or illness. Median follow-up of 8 (range, 0.7–33.1) vs. 11.2 (range, 0.7–35) months respectively with median PFS of 2.8 months in control arm vs. 4.4 months in experimental arm [P=0.7; hazard ratio (HR) =0.91; 95% CI: 0.55–1.49]. Objective response rate (ORR) did not differ significantly between both arms (22% vs. 27%; P=0.5), despite a LCR of 77% in irradiated patients. Treatment related toxicities were similar (47).
As there is no homogenous cohort, individualized treatment should be adapted to other factors such as mutational status, next-line systemic therapy options, number, and location of OPD, symptom management and other factors. Synergistic or abscopal effect cannot be relied on.
Though early in the pipelines, the implications of a synergistic or distant effect allows for a more promising future of certain subsets of metastatic disease.
Limitations and bias
We have detailed an overview of an approach to oligoprogression. In regards to treatment modalities, much focus was given to SBRT/SABR as LAT of choice as compared to surgery or RFA. RT is a relatively safe, effective and cost-effective approach and it is difficult to compare the different approaches directly. As such, more studies need to be performed to evaluate the efficacy of other modalities of local treatment.
In addition, optimal sequencing, dosing, and tolerability of different systemic agents alongside LAT remains under investigation. Many studies listed have variable acceptable protocols and small sample sizes, making conclusions difficult to attain. Selection bias plays a role in the OPD, as patient who undergo LAT may have more favorable outcomes though extent is unknown. Future randomized studies are needed. Additionally, as the definition of oligoprogression is an evolving one, with possible subclassifications such de novo, induced, and repeat OPD (see Figure 2), outcomes for each may be dramatically different in the coming years.
Conclusions and future directions
Recently, the American Radium Society published guidelines to define oligometastases and oligoprogression with recommendations of clinical trial enrollment. SBRT/SABR has been deemed to be both safe and effective in patients with limited metastatic disease though requiring more published data (48). Patients presenting with progression at a limited number of sites on a given line of systemic therapy may have favorable outcomes with aggressive LAT including SBRT/SABR. This is also reflected in current NCCN Guidelines (19). If SBRT/SABR is not feasible, other dose-intensive accelerated/hypofractionated conformal radiation therapy (CRT) regimens may be used. As much of the literature has been based on phase I/II trials, there are a limited number of randomized studies as well several phase III trials under way. Many phase I/II trials are also being evaluated for long term safety and efficacy (Table 2).
Table 2
| NCT number | Enrollment | Phase | Radiation dose | Immunotherapy |
|---|---|---|---|---|
| NCT02239900 (49) | 143 | 1 | 50 Gy/4 frac, 60 Gy/10 frac, 20 Gy/5 frac | Ipilimumab |
| NCT02492568 (50) | 92 | 2 | 24 Gy/3 frac | Pembrolizumab |
| NCT02608385 (51) | 117 | 1 | 3 or 5 frac (dose not specified) | Pembrolizumab |
| NCT03004183 (52) | 57 | 2 | 30 Gy/5 frac | Pembrolizumab |
| NCT03307759 (53) | 13 | 1 | Not specified | Pembrolizumab |
| NCT02444741 (54) | 104 | 1/2 | 50 Gy/4 frac, 45 Gy/15 frac | Pembrolizumab |
| NCT03511391 (55) | 99 | 2 | 24 Gy/3 frac | Nivolumab/pembrolizumab |
| NCT03313804 (56) | 57 | 2 | SBRT: BED >100 Gy, 3D-CRT: 30 Gy | Nivolumab/atezolizumab/pembrolizumab |
| NCT03223155 (57) | 78 | 1 | 3–5 frac (dose not specified) | Nivolumab/ipilimumab |
| NCT03391869 (58) | 360 | 3 | LCT: dose not specified | Nivolumab/ipilimumab |
| NCT03431948 (59) | 60 | 1 | 30–50 Gy | Nivolumab + urelumab/cabiralizumab |
| NCT02221739 (60) | 39 | 1/2 | 30 Gy/5 frac, 28.5 Gy/3 frac | Ipilimumab |
| NCT02639026 (61) | 30 | 1 | 24 Gy/3 frac, 17 Gy/1 frac | Durvalumab/tremelimumab |
| NCT03275597 (62) | 17 | 1b | 30–50 Gy/5 frac | Durvalumab + tremelimumab |
| NCT03212469 (63) | 55 | 1/2 | Not specified | Durvalumab + tremelimumab |
SABR/SBRT, stereotactic ablative radiotherapy/stereotactic body radiation therapy; NSCLC, non-small cell lung cancer; Gy, Gray; frac, fraction; BED, biologically effective dose; 3D-CRT, 3-dimensional conformal radiotherapy; LCT, local consolidative therapy.
Future studies specifically examining OPD include local therapy with or without dose intensified radiotHerapy for oligo-progressive disease in oncogene-Addicted Lung Tumors (HALT trial) which is a phase II/III trial looking at whether SBRT in patients with actionable mutations, currently on TKI and who develop OPD with less than or equal to five sites benefit from SBRT. Patients will be randomized in a 2:1 fashion to the SBRT arm and can continue on TKI therapy in the background (64).
Another trial examining OPD is the Stereotactic Radiation Therapy for Oligo-Progressive metastatic cancer (STOP trial). It is a multicenter phase II trial initially only examining NSCLC not limited to those with driver mutations; however, due to slow accrual it has been expanded to include all histologies. Patients are randomized in 2:1 fashion to the SBRT to a maximum of five oligoprogessive lesions vs. standard of care (65).
Further studies include a phase II trial of Osimertinib, Surgery, and Radiation Therapy in Treating Patients with Stage IIIB or IV Non-small Cell Lung Cancer With EGFR Mutations (NORTHSTAR trial). Currently osimertinib is standard of care for primary and first and secondary TKI resistant/progressive EGFR mutant metastatic NSCLC. However, the benefit of LAT with SABR/SBRT in patient’s progressing on first and second generation TKI with osimertinib is not known. Eligible patients include previously untreated patients with EGFR-mutant NSCLC (L858R or exon 19 deletion) or NSCLC patients with acquired EGFR T790M that was acquired following progression on first or second generation TKI (this subset of patients must have not received prior third generation TKI). This population includes patients who are progressing on first and second generation TKI and addressing whether SABR/SBRT with osimertinib improves primary outcome of PFS. Secondary outcomes include OS and PFS in oligometastatic subgroups (66).
Another study (NCT04767009) is an open-label, multicenter, phase II single arm trial of SBRT for Oligoprogressive NSCLC After Treatment With PD-1 Immune Checkpoint Inhibitors. The estimated accrual for the study is 59 participants, for patients without driver mutations who are progressing on primary therapy and continue to after PDL-1 therapy. The primary endpoints are safety and 1-year new lesion-free survival rate, and the secondary endpoints are PFS and OS (67).
Further phase III studies including Stereotactic Ablative Radiotherapy for Comprehensive Treatment (SABR-COMET) SABR-COMET 3 and SABR-COMET 10 are evaluating patients with up to three metastasis and 4–10 metastasis respectively which will help evaluate the impact of number of metastasis on survival in this subset of patients and possibly further define treatment recommendations for oligoprogression (68,69).
Overall, as SABR/SBRT continues to be shown to benefit patients with oligoprogression, additional studies and evaluation is needed to further delineate factors that may influence outcomes of treatment, including optimal radiation dose, burden and location of metastatic disease, evaluating the maximum number of metastases that can be included in LAT with continued benefit, and understanding further molecular characteristics and defining patient populations that will allow for most effective treatment. In this era of continually emerging targeted treatments and immunotherapy, especially in NSCLC, in which resistance will inevitably occur, it is important that we continue to advance our understanding of OPD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-536/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-536/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-536/coif). MTM serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. He reports royalties from Wolters Kluwer (UpToDate) and payment from Astra Zeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. Cham: Springer International Publishing, 2018.
- Rubin P, Green J. Solitary Metastases. Springfield, IL, USA: C.C. Thomas, 1968.
- Rubin P. Comment: are metastases curable? JAMA 1968;204:612-3.
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Sittenfeld SMC, Ward MC, Tendulkar RD, et al. editors. Essentials of clinical radiation oncology. 2nd edition. New York, NY, USA: Springer Publishing, 2021.
- ESMO. Oligometastatic disease classification. 2020. Available online: https://www.esmo.org/oncology-news/oligometastatic-disease-classification
- Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol 2020;148:157-66. [Crossref] [PubMed]
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. [Crossref] [PubMed]
- E2-RADIatE: EORTC-ESTRO RADiotherapy InfrAstrucTure for Europe. Available online: https://clinicaltrials.gov/ct2/show/NCT03818503
- Harada D, Takigawa N. Oligoprogression in Non-Small Cell Lung Cancer. Cancers (Basel) 2021;13:5823. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol 2010;33:157-63. [Crossref] [PubMed]
- Rossi A, Galetta D. Systemic Therapy for Oligoprogression in Patients with Metastatic NSCLC Harboring Activating EGFR Mutations. Cancers (Basel) 2022;14:832. [Crossref] [PubMed]
- Xu Q, Liu H, Jiang T, et al. P1.13-26 First-Line Continual EGFR-TKI Plus LAT Demonstrated Survival Benefit in Egfr-Mutant Nsclc Patients with Oligoprogressive Disease. J Thorac Oncol 2018;13:S592. [Crossref] [PubMed]
- NCCN. NCCN clinical practice guidelines in oncology. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- Ng TL, Morgan RL, Patil T, et al. Detection of oligoprogressive disease in oncogene-addicted non-small cell lung cancer using PET/CT versus CT in patients receiving a tyrosine kinase inhibitor. Lung Cancer 2018;126:112-8. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. Erratum in: N Engl J Med 2015;373:1582. [Crossref] [PubMed]
- Wang XS, Bai YF, Verma V, et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated NSCLC. J Natl Cancer Inst 2022; Epub ahead of print. [Crossref]
- Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer 2015;121:747-57. [Crossref] [PubMed]
- Friedman M, Mikityansky I, Kam A, et al. Radiofrequency ablation of cancer. Cardiovasc Intervent Radiol 2004;27:427-34. [Crossref] [PubMed]
- Tsai CJ, Yang JT, Guttmann DM, et al. Consolidative use of radiotherapy to block (CURB) oligoprogression: Interim analysis of the first randomized study of stereotactic body radiotherapy in patients with oligoprogressive metastatic cancer of the lung and breast. American Society For Radiation Oncology 2021 annual meeting. Chicago, IL USA:abstr LBA-3.
- Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med 2018;33:168-75. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Villarreal-Garza C, de la Mata D, Zavala DG, et al. Aggressive treatment of primary tumor in patients with non-small-cell lung cancer and exclusively brain metastases. Clin Lung Cancer 2013;14:6-13. [Crossref] [PubMed]
- Kim C, Hoang CD, Kesarwala AH, et al. Role of Local Ablative Therapy in Patients with Oligometastatic and Oligoprogressive Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:179-93. [Crossref] [PubMed]
- Weiss J, Kavanagh B, Deal A, et al. Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treat Res Commun 2019;19:100126. [Crossref] [PubMed]
- Chan OSH, Lam KC, Li JYC, et al. ATOM: A phase II study to assess efficacy of preemptive local ablative therapy to residual oligometastases of NSCLC after EGFR TKI. Lung Cancer 2020;142:41-6. [Crossref] [PubMed]
- Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346-51. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Campo M, Al-Halabi H, Khandekar M, et al. Integration of Stereotactic Body Radiation Therapy With Tyrosine Kinase Inhibitors in Stage IV Oncogene-Driven Lung Cancer. Oncologist 2016;21:964-73. [Crossref] [PubMed]
- Conforti F, Catania C, Toffalorio F, et al. EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung. Lung Cancer 2013;81:440-4. [Crossref] [PubMed]
- Rheinheimer S, Heussel CP, Mayer P, et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers (Basel) 2020;12:1046. [Crossref] [PubMed]
- Wang Z, Wei L, Li J, et al. Combing stereotactic body radiotherapy with checkpoint inhibitors after oligoprogression in advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:4368-79. Erratum in: Transl Lung Cancer Res 2022;11:504-5. [Crossref] [PubMed]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8:98-101.
- Belluomini L, Dodi A, Caldart A, et al. A narrative review on tumor microenvironment in oligometastatic and oligoprogressive non-small cell lung cancer: a lot remains to be done. Transl Lung Cancer Res 2021;10:3369-84. [Crossref] [PubMed]
- Shao C, Yang M, Pan Y, et al. Case Report: Abscopal Effect of Microwave Ablation in a Patient With Advanced Squamous NSCLC and Resistance to Immunotherapy. Front Immunol 2021;12:696749. [Crossref] [PubMed]
- Janopaul-Naylor JR, Shen Y, Qian DC, et al. The Abscopal Effect: A Review of Pre-Clinical and Clinical Advances. Int J Mol Sci 2021;22:11061. [Crossref] [PubMed]
- Weichselbaum RR. The 46th David A. Karnofsky Memorial Award Lecture: Oligometastasis-From Conception to Treatment. J Clin Oncol 2018;36:3240-50. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Spaas M, Sundahl N, Rottey S, et al. Immuno-radiotherapy in solid tumors: preliminary results of the randomized phase 2 CHEERS trial. ESTRO 2021.
- Amini A, Verma V, Simone CB 2nd, et al. American Radium Society Appropriate Use Criteria for Radiation Therapy in Oligometastatic or Oligoprogressive Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2022;112:361-75. [Crossref] [PubMed]
-
Ipilimumab and Stereotactic Body Radiation Therapy (SBRT) in Advanced Solid Tumors -
Pembrolizumab After SBRT Versus Pembrolizumab Alone in Advanced NSCLC (PEMBRO-RT) - Study of PD1 blockade by pembrolizumab with stereotactic body radiotherapy in advanced solid tumors. Available online: https://ClinicalTrials.gov/show/NCT02608385
- SBRT and oncolytic virus therapy before pembrolizumab for metastatic TNBC and NSCLC. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03004183
- Sequencing of stereotactic ablative body radiotherapy in combination with PD-1 blockade using pembrolizumab in metastatic non-small cell lung carcinoma (SABRseq). Available online: https://clinicaltrials.gov/ct2/show/NCT03307759
-
Pembrolizumab and Stereotactic Body Radiation Therapy or Non-Stereotactic Wide-Field Radiation Therapy in Treating Patients with Non-Small Cell Lung Cancer - Checkpoint Inhibition in Combination With an Immunoboost of External Beam Radiotherapy in Solid Tumors (CHEERS). Available online: https://clinicaltrials.gov/ct2/show/NCT03511391
-
Priming Immunotherapy in Advanced Disease with Radiation - Concurrent or sequential immunotherapy and radiation therapy in patients with metastatic lung cancer. Available online: https://ClinicalTrials.gov/show/NCT03223155
-
Nivolumab and Ipillimumab With or Without Local Consolidation Therapy in Treating Patients with Stage IV Non-Small Cell Lung Cancer - Stereotactic Body Radiotherapy (SBRT) Plus Immunotherapy for Cancer (C4-MOSART). Available online: https://clinicaltrials.gov/ct2/show/NCT03431948
-
Study of Combined Ionizing Radiation and Ipilimumab in Metastatic Non-Small Cell Lung Cancer (NSCLC) - Trial of hypofractionated radiotherapy in combination with MEDI4736 and tremelimumab for patients with metastatic melanoma and lung, breast and pancreatic cancers. Available online: https://ClinicalTrials.gov/show/NCT02639026
- Trial of Hypofractionated Radiotherapy in Combination with MEDI4736 and Tremelimumab for Patients with Metastatic Melanoma and Lung, Breast and Pancreatic Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT03275597
- A trial of durvalumab and tremelimumab in combination with SBRT in patients with metastatic cancer. Available online: https://ClinicalTrials.gov/show/NCT03212469
- Stereotactic body radiotherapy for the treatment of OPD (HALT). Available online: https://clinicaltrials.gov/ct2/show/NCT03256981
- Stereotactic radiotherapy for oligo-progressive metastatic cancer (The STOP Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT02756793
- Osimertinib, surgery, and radiation therapy in treating patients with stage IIIB or IV non-small cell lung cancer with EGFR mutations, NORTHSTAR study. Available online: https://clinicaltrials.gov/ct2/show/NCT03410043
- SBRT for oligoprogressive NSCLC after treatment with PD-1 immune checkpoint inhibitors. Available online: https://clinicaltrials.gov/ct2/show/NCT04767009
- Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic (1-3 metastases) cancer (SABR-COMET-3). Available online: https://clinicaltrials.gov/ct2/show/NCT03862911
- Stereotactic ablative radiotherapy for comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET 10). Available online: https://clinicaltrials.gov/ct2/show/NCT03721341