Improving lung cancer screening rates among patients with head and neck cancer in a radiation oncology clinic
Introduction
Lung cancer is the leading cause of death from cancer in the United States, accounting for close to 25% of all cancer deaths (1). The 5-year survival rate for lung cancer is only 21.7% because most patients have advanced disease at diagnosis (2). Without screening, patients seek care once they are symptomatic, unfortunately indicating more advanced disease. Evidence from randomized trials have shown a mortality benefit to screening using annual low-dose computed tomography (LDCT) scans in patients at high risk (3,4). Based on the National Lung Screening Trial (NLST) inclusion criteria, the United States Preventive Services Task Force (USPSTF) in 2013 recommended annual screening with LDCT in anyone aged 55–80 years old, with at least a 30 pack-year smoking history, who currently smokes or quit within the past 15 years (5). In March 2021, due in part to the broader inclusion criteria of the Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON), the USPSTF expanded the guidelines to include patients age 50–80 with at least a 20 pack-year smoking history, who currently smoke or quit within the past 15 years. Cancer Intervention and Surveillance Modeling Network (CISNET) model studies suggest that these new expanded lung cancer screening guidelines would be associated with lung cancer mortality reduced by 13% vs. 9.8%, with avoiding 503 vs. 381 lung cancer deaths per 100,000 persons in the population aged 45 to 90 over a lifetime of screening (6). Despite the strong evidence, the implementation of lung cancer screening guidelines has presented many challenges (7). Several studies have shown that the rate of lung cancer screening among eligible adults is abysmal, ranging from 3.8% to 14.4% (8-10).
Patients with head and neck cancer may be at increased risk of developing lung cancer since both malignancies can be associated with cigarette smoking (11). Even in the era of human papillomavirus (HPV)-associated head and neck cancer, many patients still have a smoking history. This portends a worse prognosis (12) and predisposes to secondary malignancies, including lung cancer. Although they were excluded from the NLST and NELSON trials, patients with prior malignancies have been included in many lung cancer screening programs. Currently, the National Comprehensive Cancer Network (NCCN) and the American Cancer Society head and neck cancer survivorship care guidelines do not recommend routine surveillance imaging for monitoring recurrence in the absence of signs and symptoms, however they do recommend that primary care clinicians should screen head and neck cancer survivors for lung cancer using LDCT when clinically indicating (13,14). The NCCN recommends lung cancer screening for patients with a prior malignancy (15), and the American Association of Thoracic Surgery guidelines state that “although this subgroup was ineligible for participation in previous screening trials, we believe antecedent cancer becomes an indication to start lung cancer screening at an earlier age and lesser tobacco exposure” (15,16).
There have been very few reports of lung cancer screening programs that target patients with head and neck cancer. Two general lung cancer screening programs reported that 5–6.4% of their patients with head and neck cancer were diagnosed with lung cancer through screening. Interviews of patients with head and neck cancer showed that most were not aware of LDCT screening but were very receptive to it (17). Interviews of clinicians brought up the lack of evidence for patients with prior malignancies, false positive rates, and risk over overdiagnosis and overtreatment. Some providers suggested that cancer specialists were better equipped to discuss lung cancer screening with the patients because they would have a better understanding of the survivor’s cancer history and recent health (18).
The objective of our study was to assess how the current USPSTF lung cancer screening guidelines are implemented in a radiation oncology clinic for patients with head and neck cancer and how we can improve lung cancer screening rates among these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-787/rc).
Methods
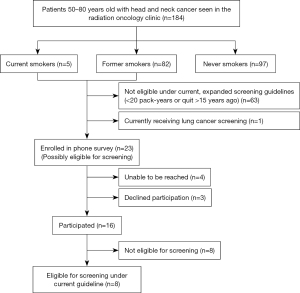
In this institutional review board approved study, we identified all patients with head and neck cancer seen in the radiation oncology clinic at our institution between June 1 and July 31, 2020 (prior to the implementation of the expanded USPSTF guidelines). We excluded patients <50 years old or >80 years old at the time of the visit (Figure 1). We included patients seen by a physician in person or by telemedicine; patients who had only phone calls or on-treatment visits (weekly radiation clinical evaluations) were excluded. Retrospective chart review was performed to collect demographics and other patient characteristics. Smoking history (smoking status, packs per day, years smoked, and quit date if applicable) was ascertained using the provider visit note and the electronic medical record documentation. History of lung cancer, previous lung cancer screening, and most recent chest computed tomography (CT) were recorded.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Stanford University (No. IRB00004947) and individual consent for this retrospective analysis was waived.
Phone survey
Patients thought to be eligible for lung cancer screening and those who were potentially eligible but had incomplete documentation were included in the phone survey portion of the study. The USPSTF expanded guidelines were used: patients 50–80 years old, with a smoking history ≥20 pack-years, who were either current smokers or quit ≤15 years ago. Patients who had had a chest CT in the past year were contacted in case they would become eligible for screening the following year.
For each patient, consent was obtained verbally at the beginning of the phone call, and a research information sheet was emailed or mailed to them afterwards. The phone survey included questions about their knowledge about lung cancer screening and willingness to be screened. Their smoking history was confirmed to assess for eligibility. For patients found to be eligible for screening, we offered to inform their radiation oncologist of their eligibility. If they agreed, their providers were contacted via email to inform them of their patients’ eligibility and options for lung cancer screening clinic at our institution. At least 3 attempts were made to contact each patient, and no incentive was provided for participation.
Statistical analysis
Continuous variables were described using mean and standard deviation, and categorical variables were described using percentages.
Results
Clinical characteristics
Of the 184 patients with head and neck cancer seen at our institution’s radiation oncology clinic (Table 1), the mean age on the day of the visit was 66.3 years (standard deviation, 7.7 years). The majority of patients were men (76%) and white (70%). The majority of the patients had cancer in the oropharynx (43%) or oral cavity (14%), and 4% of all patients had a prior diagnosis of lung cancer.
Table 1
| Characteristics | Values |
|---|---|
| Age (years), mean (standard deviation) | 66.3 (7.7) |
| Sex, n [%] | |
| Male | 139 [76] |
| Female | 45 [24] |
| Race, n [%] | |
| White | 129 [70] |
| Asian | 23 [13] |
| Other | 24 [13] |
| Unknown | 8 [4] |
| Location of head and neck cancer, n [%] | |
| Oropharynx | 79 [43] |
| Oral cavity | 26 [14] |
| Skin/cutaneous | 25 [14] |
| Salivary gland | 14 [8] |
| Nasopharynx | 10 [5] |
| Sinonasal | 10 [5] |
| Larynx | 8 [4] |
| Thyroid | 8 [4] |
| Ocular melanoma | 2 [1] |
| Unknown primary | 2 [1] |
| History of lung cancer, n [%] | |
| Yes | 8 [4] |
| No | 176 [96] |
| Smoking status, n [%] | |
| Current smoker | 5 [3] |
| Former smoker | 82 [45] |
| Never smoker | 97 [53] |
| Not documented | 0 |
| Pack years (out of 87 current or former smokers), n [%] | |
| <20 | 35 [40] |
| 20–29 | 12 [14] |
| ≥30 | 16 [18] |
| Not documented | 24 [28] |
| Quit date (out of 82 former smokers), n [%] | |
| ≤15 years | 21 [26] |
| >15 years | 53 [65] |
| Not documented | 8 [10] |
Values rounded up to whole percentages.
Smoking history (current, former, or never) was documented for all 184 (100%) patients. There were 5 (3%) patients who were current smokers, 82 (45%) former smokers, and 97 (53%) never smokers. Among the 87 current and former smokers, 16 (18%) patients had ≥30 pack years and 12 (14%) patients had 20–29 pack-years, making them potentially eligible for lung cancer screening under the expanded USPSTF guidelines. Pack-years was not documented for 24 (28%) patients. Among the 82 former smokers, 21 (26%) patients had quit smoking ≤15 years ago, making them potentially eligible for lung cancer screening. Quit date was not documented for 8 (10%) patients. Overall, there were 24 patients who met USPSTF criteria, or did not have adequate documentation to determine eligibility.
Phone survey
Of the 24 patients who were potentially eligible for lung cancer screening, 1 patient was currently receiving lung cancer screening. Of the 23 patients called for the phone survey, 4 patients were unable to be reached, 3 patients declined to participate and 16 patients participated in the phone survey (Table 2). There were 7 patients who were eligible for screening under the previous USPSTF guidelines and 1 patient who was eligible for screening under the expanded recommendations. Of those 8 patients, 3 patients had a chest CT scan within the past year, so their screening scan would be due the following year.
Table 2
| Phone survey questions | Response |
|---|---|
| Are you aware there is a way to screen for lung cancer? | |
| Yes | 4 |
| Yes, X-ray | 2 |
| No | 9 |
| No response | 1 |
| Would you be interested in lung cancer screening if you are eligible? | |
| Yes | 12 |
| Maybe | 1 |
| No | 1 |
| No response | 2 |
| For patients eligible for screening: Likelihood you will get screened (1–10), mean (standard deviation) | 9.4 (1.1) |
| Why? | “If I am eligible, it would be something I would like to know” |
| “It’s good to know” | |
| “Lung cancer is very dangerous disease, if we don’t find out through screening the death with be inevitable” | |
| “I want to” | |
| “My wife died of lung cancer 5 years ago because she never got screened. By the time she was diagnosed she was already stage 4. I want this project to work and I am excited for it to happen and save others” | |
| “I don’t know timing maybe?” | |
| “Because I have a long history of smoking” | |
| No response |
Overall, among 184 patients, there were 7 (4%) patients who are eligible for lung cancer screening under the previous USPSTF recommendations but not currently being screened. An additional patient was eligible for lung cancer screening under the previous USPSTF recommendations, but was currently being screened. With the expansion of the USPSTF lung cancer screening guidelines, a total of 9 (5%) patients are eligible for lung cancer screening (Figure 1).
Among the 16 patients who participated in the phone survey, only 6 (38%) patients were aware that there is a way to screen for lung cancer, but 2 of those patients thought screening was performed by X-ray. There were 12 (75%) patients who said they would be interested in lung cancer screening if they were found to be eligible. The patient who was not interested in lung cancer screening did not understand the question and thought they had to be screened at the very moment the phone survey was being conducted. The patients that were found to be eligible for screening were asked of the likelihood they would get screened from 1–10, 1 being least likely to 10 being very likely, and responses ranged from 7–10. Most patients acknowledged that it would be “good to know” (Table 2).
Discussion
In this study, among 184 patients with head and neck cancer seen in our radiation oncology clinic, 8 (4%) patients were eligible for lung cancer screening under the previous guidelines, and 1 additional patient would be eligible under the expanded guidelines. Among those, 3 patients had gotten a chest CT scan for other reasons, and only 1 patient was already being screened. All 184 patients had smoking history (current, former, or never) documented. However, among the 87 current or former smokers, there were 24 (28%) who did not have pack-years documented; among the 82 former smokers, there were 8 (10%) who did not have quit date documented.
The mortality benefit of lung cancer screening was clearly demonstrated in trials such as the NLST and NELSON (3,4), however the implementation of lung cancer screening guidelines has been challenging with rates of screening as low as 3.9% (19). An added challenge is that these large cohort studies have excluded patients with prior malignancies, and as a result, providers question the benefit of screening these patients. However, cancer survivors encompass a large percentage of our population, as of 2019 more than 16.9 million Americans had a history of cancer and we know that patients with head and neck cancer are at an increased risk of developing lung cancer (20). In one study of 139 patients with prior history of cancer (19% diagnosed with head and neck cancer) who were screened for lung cancer with LDCT, 5% of the patients were diagnosed with lung cancer, much higher than the 2.4% lung cancer diagnosis rate in the NLST which excluded patients with prior malignancies (21). A similar study of 543 patients with prior history of cancer (again 19% with head and neck cancer) showed a 6.4% rate lung cancer diagnosis with screening (22). The detection of metastatic disease could account for the increase in the lung cancer rates in this patient population, which some argue would complicate the implementation of lung cancer screening programs in part due to the prognostic ramifications of metastatic disease. However, often it can be very difficult to differentiate between a primary lung malignancy and metastatic disease at the time of diagnosis which is why a multidisciplinary approach to care is recommended (13).
With this in mind, many guidelines such as the NCCN and USPSTF recommend including patients with prior malignancies in lung cancer screening, after a shared decision-making discussion between provider and patient to assess comorbidities and life expectancy. In a previous study, interviews of clinicians brought up the lack of evidence for patients with prior malignancies, false positive rates, and risk over overdiagnosis and overtreatment. However, some providers suggested that cancer specialists were better equipped to discuss lung cancer screening with the patients because they would have a better understanding of the survivor’s cancer history and recent health (18). Several articles have proposed including lung cancer screening in formal head and neck cancer survivorship clinics (23,24). The NCCN and American Society of clinical oncology recommend lung cancer screening for head and neck cancer survivors when clinically indicated. Given the ongoing and long-term follow-up of these patients in survivorship clinic, it would be possible to implement a formal lung cancer screening program and ultimately identify both primary lung malignancies and metastatic disease (13,14). Our study highlighted a few ways we could increase lung cancer screening for these patients.
First, documentation of smoking history is essential in identifying patients who may be eligible for lung cancer screening. In our study, among the 87 current or former smokers, there were 24 (28%) who did not have pack-years documented; among the 82 former smokers, there were 8 (10%) who did not have quit date documented. Additional training for medical assistants to obtain this information and providers to review would be helpful.
Second, it is clear that patients knew little about screening but were interested in it. In the phone survey, over half of the patients were unaware that there is a way to screen for lung cancer highlighting the importance of patient education. We would expect that patients with a prior cancer diagnosis would be more likely to know about lung cancer screening, but that doesn’t seem to be the case. However, when patients were asked if they would be interested in lung cancer screening, the large majority of the patients were interested, suggesting that one of the limiting factors preventing patients from receiving lung cancer screen is a lack of patient education. These results matched previous findings in patient interview studies (17). Further research needs to be done see if community outreach projects focused on lung cancer screening education could increase overall lung cancer screening rates.
This study does have some limitations that need to be considered. This retrospective chart review study focused on only 2 months of clinic visit, which could be too short to see clear trends. Also, we are only looking at one clinic in a tertiary center with a lung cancer screening clinic, which is not available for all clinics, impeding the generalizability of these results. There were also several patients who were receiving active treatment for cancer or had undergone recent CT chest for their cancer, so it would be up to the provider to determine the appropriate time to screen these patients. Also, there were 4 patients that we were not able to get in contact with, therefore it is possible that the percentage of patients eligible for lung cancer screening may be even higher.
Nonetheless, this study highlights the complexities that have made the implementation of lung cancer screening challenging. If we aim to increase the rates of screening, we have to focus not only on increasing provider awareness, but also patient education at the community level.
Acknowledgments
Funding: This work was supported by Stanford Cancer Center Clinical Innovation Fund.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-787/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-787/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-787/coif). NSL serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2021 to August 2023. MFG has received grants from Varian Medical System and RefleXion Medical and hold stock from Roche, however these companies are not directly related to this work. QTL is a group chair of Radiation Therapy Oncology Group (RTOG). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Stanford University (No. IRB00004947) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- SEER. Cancer Statistics Review, 1975-2014. SEER Statistics. Available online: https://seer.cancer.gov/archive/csr/1975_2014/
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Moyer VAU.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Patel DC, Ramsey M, Phadke A, et al. Challenges in lung cancer screening: a review of implemented programs. Curr Chall Thorac Surg 2021;3:6.
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- Zahnd WE, Eberth JM. Lung Cancer Screening Utilization: A Behavioral Risk Factor Surveillance System Analysis. Am J Prev Med 2019;57:250-5. [Crossref] [PubMed]
- Zgodic A, Zahnd WE, Miller DP Jr, et al. Predictors of Lung Cancer Screening Utilization in a Population-Based Survey. J Am Coll Radiol 2020;17:1591-601. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [Crossref] [PubMed]
- Cramer JD, Grauer J, Sukari A, et al. Incidence of Second Primary Lung Cancer After Low-Dose Computed Tomography vs Chest Radiography Screening in Survivors of Head and Neck Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2021;147:1071-8. [Crossref] [PubMed]
- Nekhlyudov L, Lacchetti C, Davis NB, et al. Head and Neck Cancer Survivorship Care Guideline: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Cancer Society Guideline. J Clin Oncol 2017;35:1606-21. [Crossref] [PubMed]
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [Crossref] [PubMed]
- Seaman AT, Dukes K, Hoffman RM, et al. The complicated 'Yes': Decision-making processes and receptivity to lung cancer screening among head and neck cancer survivors. Patient Educ Couns 2018;101:1741-7. [Crossref] [PubMed]
- Dukes K, Seaman AT, Hoffman RM, et al. Attitudes of Clinicians about Screening Head and Neck Cancer Survivors for Lung Cancer Using Low-Dose Computed Tomography. Ann Otol Rhinol Laryngol 2020;129:23-31. [Crossref] [PubMed]
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2019;69:184-210. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2020.
- Halpenny DF, Cunningham JD, Long NM, et al. Patients with a Previous History of Malignancy Undergoing Lung Cancer Screening: Clinical Characteristics and Radiologic Findings. J Thorac Oncol 2016;11:1447-52. [Crossref] [PubMed]
- O'Dwyer E, Halpenny DF, Ginsberg MS. Lung cancer screening in patients with previous malignancy: Is this cohort at increased risk for malignancy? Eur Radiol 2021;31:458-67. [Crossref] [PubMed]
- Salz T, McCabe MS, Oeffinger KC, et al. A head and neck cancer intervention for use in survivorship clinics: a protocol for a feasibility study. Pilot Feasibility Stud 2016;2:23. [Crossref] [PubMed]
- Rivera MP, Henderson LM. Lung cancer screening and shared decision making in cancer survivors: the long and winding road. Transl Lung Cancer Res 2019;8:119-23. [Crossref] [PubMed]