
Treatment patterns and outcomes in patients with Pancoast tumors: a national cancer database analysis
Highlight box
Key findings
• Patients with Pancoast tumors receive chemoradiation ahead of surgery in only 25% of cases.
What is known and what is new?
• Neoadjuvant chemoradiation is standard of care for patients with Pancoast tumors.
• Most patients do not get neoadjuvant treatment. Patients who get neoadjuvant treatment had improved survival compared to patients who received upfront surgery. When surgery is done first, patients who received adjuvant treatment experienced improved survival.
What is the implication, and what should change now?
• More standardization toward neoadjuvant treatment would benefit patients. Future studies can assess whether there is an increased rate of neoadjuvant treatment for patients with Pancoast tumors.
Introduction
Pancoast tumors account for nearly 5% of lung tumors encompassing the apex of the lung. These tumors commonly involve the ribs, periosteum, brachial plexus or subclavian vessels (1). Also known as superior pulmonary sulcus tumors, most of these tumors are non-small-cell lung cancers (NSCLCs) (2). Surgery can be technically challenging at times, with mortality and complication rate higher than standard lobectomy (3-5).
Over the last several decades, advancements in treatment options have improved the outcomes in patients with Pancoast tumors (6). Resection with negative margins and complete pathologic response are positive prognostic indicators (7). Positive lymph node status, brachial plexus invasion and great vessel involvement worsen overall prognosis (8-10). Neoadjuvant chemoradiation treatment followed by surgery has emerged as the standard of care in patients without lymph node involvement (11-15). With tri-modality treatment, five-year survival ranges from 55% to 70% (12).
Although tri-modality therapy is standard of care, many patients are offered alternative treatment plans for various reasons. Previous literature has not focused on the percentage of patients who receive standard of care compared to other approaches. As such, our goals were to identify treatment patterns and outcomes in patients with node-negative Pancoast tumors. The National Cancer Database (NCDB) was utilized over a 13-year period to perform this study. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1077/rc).
Methods
Patients
The NCDB was developed as a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society as a comprehensive database of cancer cases in the United States (16). The NCDB is a clinical oncology database which acquires hospital registry data from over 15,000 Commission on Cancer-accredited facilities. The NCDB contains over 70% of all newly diagnosed cancer cases and has more than 34 million records overall. The NCDB was queried for all cases of Pancoast tumors from 2004 to 2017. Although there is no strict delineation for Pancoast tumor in the NCDB, all patients with upper lobe tumors who underwent chest wall resection were included in the analysis. This is consistent with the definition of Pancoast tumors being those of the apex of the lung invading surrounding structures including nerve roots, rib bone and periosteum, subclavian vessels, and chest wall (17). Only patients with node-negative disease were included in the analysis to reduce confounding from more advanced disease. The data used in the study were derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Texas Medical Branch institutional review board (IRB# 19-0168) and individual consent for this retrospective analysis was waived as the database we received was de-identified prior to us receiving it.
Variables
The following variables were collected for each patient: age, gender, race/ethnicity, medical insurance status, income level, Charlson-Deyo comorbidity score, distance from the patient’s residence to the treating institution, urban vs. rural location of patient residence, year of diagnosis, clinical stage, pathologic stage, type of treating institution, high-volume vs. low-volume lobectomy institution, histology, type of treatment given (surgery, chemotherapy, radiation), time from diagnosis to start of treatment and to mortality. Income was measured as the median income of the zip code in which the patient lived. A center was considered to be high-volume if it performed more than an average of 40 or more lobectomies per year, as 40 was the guideline by the Leapfrog Group during the midpoint of the study (18). To calculate yearly volume, we divided the total number of cases at each institution by the number of years of the study. The primary endpoint was the receipt of neoadjuvant chemoradiation. The secondary endpoints were overall survival in both the neoadjuvant and upfront surgery groups.
Statistical methods
Demographics, cancer characteristics, facility characteristics and treatment patterns were presented as means, standard deviation, median, and quartile 1 and 3 for continuous variables, and as frequencies and percentage for categorical variables. A logistic regression model was used to compare treatment patterns and outcomes. The logistic regression model was adjusted for demographics and facility characteristics. To examine the effect of treatment pattern on survival, unadjusted survival rates were estimated by using Kaplan-Meier method and compared with the log-rank test. Multivariate Cox proportional hazard models were used to estimate hazard ratios adjusted for demographics and facility characteristics. All patients were censored at the last contact date or at the end of study (December 31, 2019). The proportional hazard assumption was evaluated first by visual inspection of the Kaplan-Meier curve and then by adding the interaction between race and log (time) to the model and checking for significance. Race was reported as Caucasian, African American and “other” since the “other” category was only 2.9% of the cohort. The “other” category consisted of Hispanic, Asian, Pacific Islander and Native American. In both the Kaplan-Meier analysis and the Cox proportional hazards model, patients diagnosed in 2017 were excluded due to not having follow-up information. All analyses were conducted using SAS 9.4 (Cary, NC, USA). Patients who received (I) chemotherapy only or (II) radiation treatment only before surgery were excluded from the analysis. When subdividing the upfront surgery group, a patient was considered to have received adjuvant treatment if it was received within 3 months of the date of surgery.
Results
Demographics
After exclusion of patients with missing data and those that did not fit the above criteria, a total of 2,910 patients were identified to have Pancoast tumors and no positive lymph nodes. Demographic data for this cohort is displayed in Table 1. Mean age was 63.7±10.5 years. Approximately 34% of the cohort was between the ages of 60 and 70. The majority of the cohort was Caucasian, male and lived in metropolitan areas. In the cohort there were 25% (717/2,910) of patients who underwent neoadjuvant chemoradiation prior to surgery. Table 2 shows patients who underwent neoadjuvant chemoradiation treatment compared to the rest of the cohort. When comparing patients who had neoadjuvant chemoradiation treatment versus the rest of the cohort, patients who received surgery first were older, less likely to have private insurance and were more likely to be treated at low-volume centers. There were no significant differences in racial distribution, income or distance from home to the treating facility. Table S1 shows the multivariate logistic regression analyses of these results. In this supplemental table we have included the results of the multivariate analyses with corresponding 95% confidence intervals.
Table 1
| Variables | Number (%) |
|---|---|
| All | 2,910 |
| Age at diagnosis, years | |
| Mean ± SD | 63.7±10.5 |
| Median, Mean [Q1–Q3] | 65 [55–72] |
| <50 | 308 (10.6) |
| 50–59 | 708 (24.3) |
| 60–69 | 983 (33.8) |
| 70–79 | 911 (31.3) |
| Gender | |
| Male | 1,753 (60.2) |
| Female | 1,157 (39.8) |
| Race | |
| Caucasian | 2,561 (88.0) |
| African American | 266 (9.1) |
| Other | 83 (2.9) |
| Metropolitan/rural | |
| Metropolitan | 2,307 (79.3) |
| Urban | 603 (20.7) |
| Primary payor | |
| Not insured | 114 (3.9) |
| Private | 1,176 (40.4) |
| Medicaid | 227 (7.8) |
| Medicare | 1,393 (47.9) |
| Income | |
| Q1 | 523 (18.0) |
| Q2 | 687 (23.6) |
| Q3 | 815 (28.0) |
| Q4 | 885 (30.4) |
| Charlson-Deyo Score | |
| 0 | 1,549 (53.2) |
| 1 | 983 (33.8) |
| 2+ | 378 (13.0) |
| Region | |
| Midwest | 859 (29.5) |
| Northeast | 582 (20) |
| South | 1,137 (39.1) |
| West | 332 (11.4) |
| Distance from home to facility (miles) | |
| Median, Mean [Q1–Q3] | 13 [5.5–31.9] |
| Facility type | |
| Community Cancer Program | 203 (7.0) |
| Comprehensive Community Cancer Program | 1,205 (41.4) |
| Academic/Research | 1,106 (38.0) |
| Integrated Network Cancer Program | 396 (13.6) |
| Facility volume (surgeries per year) | |
| <40 per year | 1,287 (44.2) |
| 40+ per year | 1,623 (55.8) |
| Histology | |
| Squamous cell carcinoma | 1,548 (53.2) |
| Adenocarcinoma | 937 (32.2) |
| Other | 425 (14.6) |
| T category | |
| T3 | 2,306 (79.2) |
| T4 | 604 (20.8) |
| Vital status | |
| Dead | 1047 (36.0) |
| Alive | 1863 (64.0) |
| 30-day mortality | |
| Alive | 2,822 (97.0) |
| Dead | 88 (3.0) |
| 90-day mortality | |
| Alive | 2,705 (93.0) |
| Dead | 205 (7.0) |
SD, standard deviation; Q1, first quarter; Q2, second quarter; Q3, third quarter; Q4, fourth quarter.
Table 2
| Variables | Upfront surgery | Neoadjuvant chemoradiation | P value |
|---|---|---|---|
| N | 2,193 | 717 | |
| Age at diagnosis, years | 65.0 | 59.0 | <0.001 |
| Male | 60.2% (1,320/2,193) | 60.4% (433/717) | 0.925 |
| Race | 0.330 | ||
| White | 88.5% (1,941/2,193) | 86.5% (620/717) | |
| Black | 8.7% (191/2,193) | 10.5% (75/717) | |
| Other | 2.8% (61/2,193) | 3.1% (22/717) | |
| Metropolitan | 78.4% (1,720/2,193) | 81.9% (587/717) | 0.049 |
| Primary payor | <0.001 | ||
| Uninsured | 3.4% (74/2,193) | 5.6% (40/717) | |
| Private | 37.2 (816/2,193) | 50.2% (360/717) | |
| Medicaid | 7.4% (162/2,193) | 9.1% (65/717) | |
| Medicare | 52.0% (1,141/2,193) | 35.2% (252/717) | |
| Income | 0.116 | ||
| Q1 | 18.9% (415/2,193) | 15.1% (108/717) | |
| Q2 | 23.1% (507/2,193) | 25.1% (180/717) | |
| Q3 | 27.6% (605/2,193) | 29.3% (210/717) | |
| Q4 | 30.4% (666/2,193) | 30.5% (219/717) | |
| Charlson-Deyo Score | <0.001 | ||
| 0 | 50.0% (1,097/2,193) | 63.0% (452/717) | |
| 1 | 354 (777/2,193) | 28.7% (206/717) | |
| 2+ | 14.6% (319/2,193) | 8.2% (59/717) | |
| Region | 0.025 | ||
| Midwest | 28.6% (626/2,193) | 32.5% (233/717) | |
| Northeast | 19.4% (425/2,193) | 21.9% (157/717) | |
| South | 40.1% (880/2,193) | 35.8% (257/717) | |
| West | 12.0% (262/2,193) | 9.8% (70/717) | |
| Great circle distance, mean (miles) | 29.3 | 34.6 | 0.370 |
| Facility type | 0.002 | ||
| Community Cancer Program | 7.3% (161/2,193) | 5.9% (42/717) | |
| Comprehensive Community Cancer Program | 42.5% (932/2,193) | 38.1% (273/717) | |
| Academic/Research | 37.3% (817/2,193) | 40.3% (289/717) | |
| Integrated Network Cancer Program | 12.9% (283/2,193) | 15.8% (113/717) | |
| Facility volume (surgeries per year) | <0.001 | ||
| <40 per year | 45.8% (1,005/2,193) | 39.3% (282/717) | |
| 40+ per year | 54.2% (1,188/2,193) | 60.7% (435/717) |
Q1, first quarter; Q2, second quarter; Q3, third quarter; Q4, fourth quarter.
Survival
Overall 30-day mortality for the cohort was 3.3%, while overall 90-day mortality was 7.5%. When analyzing the entire group, the only variable which was associated with improved survival was facility volume, as high-volume centers had an improved overall survival both at 30 days (hazard ratio 0.57, 95% CI: 0.35–0.93) and 90 days (hazard ratio 0.71, 95% CI: 0.52–0.98).
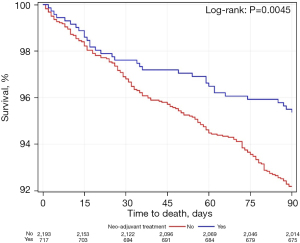
Figures 1-3 shows Kaplan-Meier analyses for patients who underwent neoadjuvant chemoradiation treatment versus upfront surgery at 30 days, 90 days and 14 years. Patients who received neoadjuvant chemoradiation had similar survival when compared to the upfront surgery group at 30 days (P=0.27), but experienced a significantly improved survival at 90 days (P<0.01). At the 14-year period, survival estimates were worse for the upfront surgery group compared to the neoadjuvant group (P<0.01).


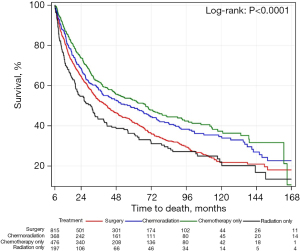
Survival in upfront surgery group
Figure 4 demonstrates overall survival in the upfront surgery group only. Patients in this group were subdivided into surgery only, surgery followed by chemoradiation treatment, surgery followed by chemotherapy only and surgery followed by radiation only. There was a statistically significant difference in survival among these 4 groups. Patients in the upfront surgery group who underwent adjuvant chemoradiation had the best survival, followed by patients who underwent adjuvant chemotherapy alone. Patients who underwent adjuvant radiation alone and patients who received no adjuvant treatment had similar survival rates and the worst rates in the upfront surgery group.
Discussion
In our study we examined trends in treatment patterns for Pancoast tumors over a 13-year period using the NCDB. Standard of care treatment for Pancoast tumors has changed from upfront surgical resection followed by adjuvant therapy to a trimodality approach with neoadjuvant chemoradiation followed by surgical resection (1,19). This trimodality approach has been associated with improved outcomes and longer disease free and overall survival (20-22). Given that trimodality treatment is considered standard of care for Pancoast tumors, we wanted to investigate how often this is being applied to patients nationally. We queried the NCDB starting in 2004 when this trimodality approach was accepted as standard of care. The NCDB does not specifically identify tumors as Pancoast tumors. As such, we used upper lobe tumors which required chest wall resections as a surrogate for Pancoast tumors. Due to this limitation, we may have not captured every patient with a Pancoast tumor during this study time, and additionally may have included some upper lobe tumors that were not Pancoast tumors. But the fact that (I) the majority of tumors were squamous cell cancer and (II) there was overall improvement in survival with neoadjuvant treatment suggests that the majority of patients did have Pancoast tumors. Furthermore, the categorization of Pancoast tumors is relatively subjective in clinical practice. Although these tumors are labeled as superior sulcus tumors, most clinicians categorize these tumors based on their initial surgical resectability and distance from critical structures such as the brachial plexus and the great vessels (23,24). But there is no absolute distance that can be measured or recorded which will define a Pancoast tumor absolutely. As such, we feel that this study and use of the NCDB can have useful results to guide treating providers.
When examining the treatment patterns in the United States over our study period, we observed that patients who received neoadjuvant chemoradiation followed by surgical resection had the best survival. This supports the body of literature that has demonstrated that neoadjuvant therapy contributes to improved survival in patients with Pancoast tumors (25). We were surprised, however, with the low adherence to this standard of care treatment in our cohort. Of the 2,910 unique patients in the cohort, less than 25% received neoadjuvant chemoradiation. Although this trend suggests that the majority of patients are receiving surgery upfront, further multi-institutional studies should be performed prospectively to get a more precise idea about the percentage of patients with Pancoast tumors who receive neoadjuvant chemoradiation treatment.
Our study also revealed that high-volume institutions were more likely to offer neoadjuvant treatment compared to low-volume institutions. We chose 40 pulmonary resections as the cutoff because this number was adopted by the Leapfrog Group (18). We hypothesize that high-volume institutions may be more accustomed to utilizing multi-modality treatment plans for patients and thus may be more likely to offer neoadjuvant treatment ahead of surgery.
When examining the upfront surgery group only, patients who received adjuvant chemoradiation had the best survival overall when compared to those who received chemotherapy alone, radiation alone, or no adjuvant therapy. This pattern suggests that a multidisciplinary treatment plan is beneficial even if surgery is performed upfront. Our study did progress over a long time period. During this time, there were significant advancements in neoadjuvant regimens and surgical conduct (26). We feel that this study may prompt future studies to examine whether practice patterns have changed in the last few years as the database is updated.
In the future, our group would like to further understand why certain patients are offered standard of care treatment for Pancoast tumors while the majority in our study were offered treatment below the standard of care. We would like to obtain information directly from cancer centers as to their practice patterns for treatment of Pancoast tumors in the node negative patient population. We hope these future studies will help us determine how to better serve patients and promote standard of care treatment for all node negative Pancoast tumor patients.
Conclusions
In a query of the NCDB over a period of 13 years, we observed that only 24% of patients with Pancoast tumors are offered standard of care treatment with a trimodal treatment approach. Patients who received standard of care treatment had improved overall survival compared to those who received other treatment patterns. In patients who received upfront surgical resection, adjuvant chemoradiation was associated with improved survival compared to adjuvant chemotherapy, radiation therapy or no adjuvant therapy. Future studies to examine why certain patients are offered standard of care treatment while most are not needed to understand how to promote widespread acceptance of standard of care for all Pancoast tumor patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1077/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1077/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1077/coif). ICO serves as an unpaid editorial board member of Journal of Thoracic Disease from February 2021 to January 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Texas Medical Branch institutional review board (IRB# 19-0168) and individual consent for this retrospective analysis was waived as the database we received was de-identified prior to us receiving it.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zarogoulidis K, Porpodis K, Domvri K, et al. Diagnosing and treating pancoast tumors. Expert Rev Respir Med 2016;10:1255-8. [Crossref] [PubMed]
- Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J Thorac Dis 2013;5:S342-58. [Crossref] [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6:S108-15. [Crossref] [PubMed]
- Shimada Y, Kudo Y, Maehara S, et al. Significant prognostic determinants in lung cancers of the superior sulcus: comparable analysis of resected and unresected cases. Gen Thorac Cardiovasc Surg 2020;68:801-11. [Crossref] [PubMed]
- Bao F, Yu F, Hao X, et al. Surgical resection of superior pulmonary sulcus tumor after neoadjuvant chemoradiation via the anterior transmanubrial approach: a case report. Ann Transl Med 2021;9:1603. [Crossref] [PubMed]
- Marulli G, Battistella L, Mammana M, et al. Superior sulcus tumors (Pancoast tumors). Ann Transl Med 2016;4:239. [Crossref] [PubMed]
- Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 2005;129:1250-7. [Crossref] [PubMed]
- Marulli G, Battistella L, Perissinotto E, et al. Results of surgical resection after induction chemoradiation for Pancoast tumours †. Interact Cardiovasc Thorac Surg 2015;20:805-11; discussion 811-2. [Crossref] [PubMed]
- Okubo K, Wada H, Fukuse T, et al. Treatment of Pancoast tumors. Combined irradiation and radical resection. Thorac Cardiovasc Surg 1995;43:284-6. [Crossref] [PubMed]
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [Crossref] [PubMed]
- Marra A, Eberhardt W, Pöttgen C, et al. Induction chemotherapy, concurrent chemoradiation and surgery for Pancoast tumour. Eur Respir J 2007;29:117-26. [Crossref] [PubMed]
- Lin TY, Atrchian S, Humer M, et al. Clinical outcomes of pancoast tumors treated with trimodality therapy. J Thorac Dis 2021;13:3529-38. [Crossref] [PubMed]
- Wright CD, Menard MT, Wain JC, et al. Induction chemoradiation compared with induction radiation for lung cancer involving the superior sulcus. Ann Thorac Surg 2002;73:1541-4. [Crossref] [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6:S180-93. [Crossref] [PubMed]
- Jeannin G, Merle P, Janicot H, et al. Combined treatment modalities in Pancoast tumor: results of a monocentric retrospective study. Chin Clin Oncol 2015;4:39. [Crossref] [PubMed]
- Winchester DP, Stewart AK, Bura C, et al. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol 2004;85:1-3. [Crossref] [PubMed]
- Majeed FA, Ali A, Chatha SS, et al. Management of Malignant Chest Wall Tumors. J Coll Physicians Surg Pak 2021;30:833-6. [Crossref] [PubMed]
- Clark JM, Cooke DT, Chin DL, et al. Does one size fit all? An evaluation of the 2018 Leapfrog Group minimal hospital and surgeon volume thresholds for lung surgery. J Thorac Cardiovasc Surg 2020;159:2071-2079.e2. [Crossref] [PubMed]
- Robinson LA, Tanvetyanon T, Grubbs D, et al. Induction chemoradiotherapy versus chemotherapy alone for superior sulcus lung cancer. Lung Cancer 2018;122:206-13. [Crossref] [PubMed]
- Blaauwgeers JL, Kappers I, Klomp HM, et al. Complete pathological response is predictive for clinical outcome after tri-modality therapy for carcinomas of the superior pulmonary sulcus. Virchows Arch 2013;462:547-56. [Crossref] [PubMed]
- Buderi SI, Shackcloth M, Woolley S. Does induction chemoradiotherapy increase survival in patients with Pancoast tumour? Interact Cardiovasc Thorac Surg 2016;23:821-5. [Crossref] [PubMed]
- Palumbo VD, Fazzotta S, Fatica F, et al. Pancoast tumour: current therapeutic options. Clin Ter 2019;170:e291-4. [Crossref] [PubMed]
- Waseda R, Klikovits T, Hoda MA, et al. Trimodality therapy for Pancoast tumors: T4 is not a contraindication to radical surgery. J Surg Oncol 2017;116:227-35. [Crossref] [PubMed]
- Parissis H, Young V. Treatment of pancoast tumors from the surgeons prospective: re-appraisal of the anterior-manubrial sternal approach. J Cardiothorac Surg 2010;5:102. [Crossref] [PubMed]
- Peedell C, Dunning J, Bapusamy A. Is there a standard of care for the radical management of non-small cell lung cancer involving the apical chest wall (Pancoast tumours)? Clin Oncol (R Coll Radiol) 2010;22:334-46. [Crossref] [PubMed]
- Aokage K, Tsuboi M, Zenke Y, et al. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Jpn J Clin Oncol 2022;52:383-7. [Crossref] [PubMed]


