
The short-term outcomes of nonintubated anesthesia compared with intubated anesthesia in single-port video-assisted lung surgery in enhanced recovery after thoracic surgery: results from a single-center retrospective study
Highlight box
Key findings
• NI-SP-VALS promoted patient recovery and shortened the length of hospital stay and catheter stay; NI-SP-VALS reduced postoperative inflammatory and pain.
What is known and what is new?
• The applications of ERAS in lung surgery have been widely reported.
• We report on non-intubation anesthesia combined with ERAS in the perioperative period of thoracic surgery, which has rarely been reported in previous studies.
What is the implication, and what should change now?
• NI-SP-VALS promotes rapid patient recovery and may be an effective choice for clinical surgeons in the future. Further prospective randomized studies are needed.
Introduction
Since video-assisted thoracoscopic surgery (VATS) was first applied in lung surgery in the 1990s, VATS has gradually become the first choice to replace traditional open thoracic surgery. Early thoracoscopic surgery usually used 2, 3, or even 4 operating ports. In 2011, single-port VATS was first applied and reported in lung surgery (1). In recent years, single-port VATS has become more widely used in thoracic surgery as surgeons become more experienced. Some researchers have shown that the postoperative effects of single-port VATS are better than those of multiple-port VATS in terms of ensuring safety and tumor-free principles (2). In some large general hospitals, thoracic surgeons have performed complex lung surgeries with single-port VATS, including sleeve resection and reconstruction of the trachea and carina (3,4).
The development of VATS, particularly single-port VATS, has greatly promoted the application of enhanced recovery after surgery (ERAS). The concept of ERAS, also called fast-track surgery (FTS), was proposed by Kehlet, a famous thoracic surgeon from Denmark (3). ERAS is an interprofessional, goal-directed program that begins from preoperative preparation to postoperative recovery. The purpose of ERAS is to reduce perioperative pressure, improve pain and capacity for activities, and reduce postoperative complications. ERAS is a multidisciplinary process that requires the collaboration of surgeons, nurses, anesthesiologists, physiotherapists, and dietitians (4). Although the perioperative management of ERAS has been implemented in many thoracic medical centers, there is no standard process to date. Combining practices of Chinese ERAS with those from other countries and according to the clinical situation of our thoracic center, we have independently formulated ERAS programs. These programs have been implemented, and some initial results have been obtained.
Anesthetic methods for thoracic surgery have changed with the trend toward minimizing trauma. The traditional anesthetic method in thoracic surgery is general anesthesia with tracheal intubation, which can lead to many postoperative adverse reactions, such as throat discomfort, irritable cough, nausea and vomiting, ventilator-related complications, and impaired lung function (5,6). Recently, nonintubated anesthesia has been used in thoracic surgery. A study has shown that thoracoscopic surgery with nonintubated anesthesia not only ensures operational safety but also reduces the incidence of anesthesia-related complications, such as airway damage, lung function impairment, and postoperative vomiting (6). Nonintubated anesthesia was initially used in common surgeries, such as wedge pneumonectomy, anatomic segmental pneumonectomy, and lobectomy. In recent years, it has also been used in complicated thoracic surgeries, including bronchotomy and sleeve lobectomy (7-10). Nonintubated anesthesia shares similarities with intubated anesthesia in perioperative procedures and reduces anesthesia-related complications. A study has shown that nonintubated anesthesia may affect surgical operations and influence postoperative recovery (11). Thus, more studies are necessary to ensure the safety and availability of nonintubated anesthesia, especially for some complicated thoracic surgeries, and the surgeon’s experience should also be considered.
The application of nonintubated anesthesia in thoracic surgery has been proven to provide advantages in rehabilitation; however, nonintubated anesthesia that retains spontaneous breathing has the potential to increase the risk of surgery, such as reflux aspiration during the operation, high breathing activity, etc. The feasibility of its application remains controversial. Moreover, no reports exist concerning the application of nonintubated anesthesia under ERAS management in single-port thoracoscopic lung surgery, and the feasibility of its application in single-port thoracoscopic lung surgery should be evaluated. Based on the ERAS perioperative management concept, our thoracic center has implemented nonintubated anesthesia single-port VATS for years. The results of 272 patients who underwent single-port VATS under whole-course management of ERAS are reported and demonstrate the feasibility of nonintubated anesthesia in minimally invasive thoracic surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1689/rc).
Methods
General materials
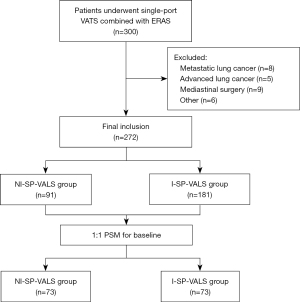
Patients who underwent ERAS procedures and single-port VATS in our hospital (July 1, 2021, to March 31, 2022) were analyzed retrospectively. The algorithm for patient’s selection are shown in Figure 1. Inclusion criteria were as follows: (I) patients diagnosed with lung nodules; (II) patients who underwent single-port VATS; and (III) patients who agreed to participate in ERAS procedures. Exclusion criteria were as follows: (I) patients who underwent double-port or multiple-port VATS or underwent other surgeries at the same time; (II) patients who underwent mediastinal surgery; (III) patients who had a history of previous lung surgery or malignancy; (IV) patients with a body mass index (BMI) >30 kg/m2; (V) patients with anesthetic contraindications or difficult airways; and (VI) those with incomplete patient data. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This study was approved by our ethics committee of Huadong Hospital Affiliated to Fudan University (No. 2021K010). Informed consent was obtained from all patients.
ERAS procedures
Preoperative stage
Patients who agreed to participate in the ERAS procedures were asked to quit smoking and drinking alcohol and begin pulmonary function exercises 2 weeks before surgery. Individual psychological counseling from medical social workers was provided for patients who felt anxious and nervous about the operation. Nutritionists conducted nutritional assessments of the patients and instructed them to take a clear drink containing carbohydrates 2–3 hours before anesthesia.
Intraoperative stage
Anesthesiologists used antibiotics proactively during surgery and complete infusion 30 min before skin excision. For this study, the observation group underwent general anesthesia with spontaneous breathing combined with thoracic paravertebral, intercostal nerve, and vagus nerve blocks. The control group underwent general anesthesia with endotracheal intubation. All the patients underwent single-port VATS. The surgeons decided whether to indwell the catheter at the end of the procedure. Chest drains (12 mm arrow tube or 18 mm conventional chest drains) were routinely indwelled.
Postoperative stage
A patient-controlled analgesia pump was used for postoperative analgesia, and analgesics were mainly nonsteroidal anti-inflammatory drugs (NSAIDs) rather than opioids. Fluid intake was available 4 hours after surgery. We encouraged patients to start off-bed activity and pulmonary function exercises under the introduction of physiatrists on the first day after surgery. For patients with an indwelling catheter, the catheter was removed on the first postoperative day. Radiographic examination was performed 1–2 days after the operation to evaluate lung recruitment, and whether to remove the thoracic drainage tube was decided according to the volume of chest drains. In addition, the social work and nursing departments attended to the care and psychological needs of patients after surgery.
Discharge standard
The standards for patient discharge were as follows: (I) the patient returned to a solid diet without fluid therapy; (II) oral analgesics could provide good analgesia; (III) the wound had healed well without infection; and (IV) the patient recovered physical strength and could move freely.
Anesthesia and surgical methods
Nonintubated anesthesia single-port video-assisted lung surgery (NI-SP-VALS) group
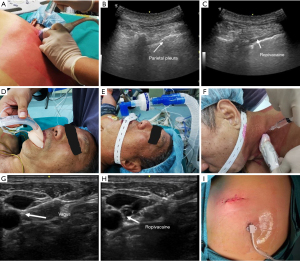
Before general anesthesia, the patient was placed in the prone or lateral decubitus position and injected with ropivacaine in the thoracic paravertebral space at T2, T4, and T6 under ultrasound guidance for the thoracic paravertebral block. Dexmedetomidine, propofol, sufentanil, and etomidate were administered intravenously (IV). After the patient lost consciousness, a laryngeal mask was placed, and a ventilator tube was connected to provide oxygen and monitor the end-expiratory carbon dioxide concentration. After the patient was turned over, a cervical vagus nerve block was performed with 2% lidocaine under ultrasound guidance. Propofol, remifentanil, and dexmedetomidine were used to intraoperatively maintain the depth of anesthesia. The patient was placed in a healthy lateral decubitus position, and a small incision was made at the junction of the fourth or fifth intercostal and posterior axillary lines. After smooth access to the thorax, the thoracic surgeon performed the fourth and fifth intercostal nerve blocks using 2% lidocaine. Subsequently, different surgical methods were selected according to preoperative evaluation and intraoperative conditions. Mediastinal lymph node dissection or sampling for patients undergoing wedge resection was not routinely performed, whereas patients undergoing segmentectomy and lobectomy had their mediastinal lymph nodes routinely dissected or sampled. At the end of the procedure, a chest tube (12 mm arrow tube or 18 mm conventional chest drain) was placed after the chest cavity had been rinsed and the absence of active bleeding or air leakage had been confirmed. Then, the chest wall was sutured layer by layer. After emergence from anesthesia, the patient was transferred to the ward for observation of vital signs and general condition (Figure 2).
Intubated anesthesia single-port video-assisted lung surgery group
Dexmedetomidine, propofol, sufentanil, etomidate, and rocuronium were administered IV. After the patient lost consciousness, a double-lumen endotracheal tube was placed under the video laryngoscope, and the anesthesia machine adopted a unilateral lung mechanical ventilation volume control mode. Propofol, remifentanil, and dexmedetomidine were used to maintain the depth of anesthesia in intraoperation. The surgical method was the same as that for the NI-SP-VALS group. After emergence from anesthesia, the patient was transferred to the ward for observation of vital signs and general condition.
Observation indexes
Intraoperative data, including anesthesia time, lowest SpO2, operation time, lymph node resection, and intraoperative fluid volume, were collected. Postoperative clinical indicators, including drug use and blood routine [white blood cell count (WBC), neutrophilic granulocyte percentage (NEUT%), percentage of lymphocytes (LYM%), platelet count (PLT), hemoglobin level (HGB), and albumin level (ALB)], duration of tube (chest drain and catheter) indwelling, total drainage volume of chest tube, duration of analgesic and antibiotic application, postoperative pain score [visual analog scale (VAS)], postoperative satisfaction (hundred-mark system), the time of first postoperative food intake and ambulation, postoperative vomiting and throat discomfort, and postoperative complications were collected. The length of stay and cost of hospitalization (total, anesthesia, and operation costs) were also collected. In addition, the patient was followed up for 3 months after discharge to assess physical performance, pain, cough [numeric rating scale (NRS)], anxiety, readmission, and compliance with follow-up.
Statistical analysis
SPSS software (version 26.0; IBM Corp., Armonk, NY, USA) was used to analyze the data. Categorical variables are described as absolute and relative frequencies, while quantitative variables are described as mean ± standard deviation (SD) or median (range), when applicable. To compare quantitative variables, we used Student’s t-test or Mann-Whitney test, wherever applicable. Categorical variables were compared between the two groups using the chi-square test and Fisher exact test. The inspection level was set at two-sides α=0.05. There was no missing patient data in our study.
Results
A total of 300 patients who underwent ERAS procedures combined with single-port VALS from July 2021 to March 2022 were included; of these, 28 were excluded because of exclusion criteria, and 272 patients (91 in the NI-SP-VALS group and 181 in the I-SP-VALS group) were enrolled in the study. After propensity score matching (PSM) was completed for the baseline indicators, 73 patient pairs were included in the final outcome analysis (Figure 1).
Clinical characteristics
Baseline data, including age, sex, BMI, smoking history, medical history, pulmonary function test [forced vital capacity % (FVC%), forced expiratory volume in 1 second % (FEV1%), carbon monoxide diffusing capacity % (DLCO%)], preoperative blood routine (WBC, NEUT%, LYM%, HGB, PLT, ALB), American Society Anesthesiologists (ASA) class, nodule location, histologic types, pathological TNM stage, operating methods, and inpatient ward of the patients, were statistically analyzed (Table 1). There were statistically significant differences in age, sex, FVC%, FEV1%, histologic types, operating methods, and inpatient wards (P<0.05). To eliminate baseline differences, the PSM method was used to perform 1:1 matching on baseline data, and there was no statistical difference in any of the baseline data after matching (Table 1).
Table 1
| Variablea | Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NI-SP-VALS group (n=91) | I-SP-VALS group (n=181) | χ2/t/Z/U | P | NI-SP-VALS group (n=73) | I-SP-VALS group (n=73) | χ2/t/Z/U | P | ||
| Age | 54.90±13.65 | 62.00 (51.00, 67.00) | 2.094 | 0.036 | 55.96±13.48 | 58.00 (45.50, 64.50) | −0.188 | 0.851 | |
| Gender | 6.863 | 0.009 | 1.337 | 0.856 | |||||
| Female | 68 (74.7) | 106 (58.6) | 51 (69.9) | 52 (71.2) | |||||
| Male | 23 (25.3) | 75 (41.4) | 22 (31.1) | 21 (28.8) | |||||
| BMI | 22.43 (21.00, 24.53) | 23.35±3.12 | −1.615 | 0.106 | 22.37 (20.98, 24.21) | 23.24±3.48 | −1.090 | 0.181 | |
| Current or former smokers | 0.058 | 0.809 | 0.056 | 0.814 | |||||
| No | 75 (82.4) | 147 (81.2) | 62 (84.9) | 63 (86.3) | |||||
| Yes | 16 (17.6) | 34 (18.8) | 11 (15.1) | 10 (13.7) | |||||
| Medical history | 4.229 | 0.367 | 5.046 | 0.244 | |||||
| No | 61 (67.0) | 109 (60.2) | 54 (74.0) | 47 (64.4) | |||||
| Hypertension | 17 (18.7) | 28 (15.5) | 11 (15.1) | 10 (13.7) | |||||
| Diabetes | 1 (1.1) | 7 (3.9) | 1 (1.4) | 2 (2.7) | |||||
| Coronary heart disease | 1 (1.1) | 2 (1.1) | 1 (1.4) | 0 (0.0) | |||||
| Other | 11 (12.1) | 35 (19.3) | 6 (8.2) | 14 (19.2) | |||||
| Pulmonary function test, % | |||||||||
| FVC pred | 98.79±12.58 | 94.00 (85.75, 101.70) | 2.947 | 0.020 | 96.51±11.88 | 97.22±14.08 | −0.329 | 0.743 | |
| FEV1 pred | 107.51 (97.80, 117.20) | 101.63±15.77 | 2.819 | 0.048 | 103.60 (96.25, 112.40) | 106.31±15.14 | 0.922 | 0.357 | |
| DLCO pred | 95.20 (85.60, 106.60) | 100.90 (86.18, 116.25) | −1.334 | 0.771 | 95.19 (85.50, 106.55) | 103.73±23.80 | 1.840 | 0.066 | |
| Preoperative blood routine | |||||||||
| WBC | 6.00 (5.20, 6.90) | 5.90 (5.00, 6.70) | −0.769 | 0.442 | 6.00 (5.00, 7.00) | 6.02±1.36 | −0.386 | 0.700 | |
| NEUT, % | 64.76±8.79 | 64.01±8.88 | 0.665 | 0.507 | 65.06±9.04 | 64.19±8.24 | 0.310 | 0.543 | |
| LYM, % | 27.69±7.61 | 27.65±7.72 | 0.040 | 0.968 | 27.42±7.84 | 27.40 (24.00, 31.50) | 0.632 | 0.527 | |
| HGB, g/L | 135.50 (129.00, 143.00) | 138.14±14.97 | 1.021 | 0.307 | 136.00 (129.00, 143.50) | 136.57±16.34 | 0.025 | 0.980 | |
| PLT, 10^9/L | 214.00 (188.00, 256.00) | 219.00 (179.00, 263.00) | −0.032 | 0.975 | 212.00 (184.00, 255.00) | 220.00 (190.50, 258.45) | 0.513 | 0.608 | |
| ALB, g/L | 46.99±2.75 | 46.46±3.22 | 1.357 | 0.176 | 47.02±2.77 | 46.74±2.68 | 0.636 | 0.526 | |
| ASA class | 1.715 | 0.424 | 0.274 | 0.919 | |||||
| I | 26 (28.6) | 40 (22.1) | 21 (28.8) | 20 (27.4) | |||||
| II | 61 (67.0) | 129 (71.3) | 48 (65.8) | 50 (68.5) | |||||
| III | 4 (4.4) | 12 (5.9) | 4 (5.5) | 3 (4.1) | |||||
| Nodule location (lobe) | 6.747 | 0.150 | 2.989 | 0.577 | |||||
| LUL | 28 (30.8) | 35 (19.3) | 21 (28.8) | 14 (19.2) | |||||
| LLL | 16 (17.6) | 32 (17.7) | 14 (19.2) | 16 (21.9) | |||||
| RUL | 27 (29.7) | 52 (28.7) | 23 (31.5) | 21 (28.8) | |||||
| RML | 3 (3.3) | 13 (7.2) | 3 (4.1) | 5 (6.8) | |||||
| RLL | 17 (18.6) | 49 (27.1) | 12 (16.4) | 17 (23.3) | |||||
| Histologic types | 6.177 | 0.046 | 0.052 | 0.820 | |||||
| Adenocarcinoma | 80 (87.9) | 137 (75.7) | 62 (84.9) | 61 (83.6) | |||||
| Squamous carcinoma | 0 (0.0) | 3 (1.7) | 0 (0.0) | 0 (0.0) | |||||
| Other | 11 (12.1) | 41 (22.7) | 11 (15.1) | 12 (16.4) | |||||
| pTNM stage | 0.178 | 0.999 | 1.000 | ||||||
| Stage I | 79 (86.8) | 137 (75.7) | 61 (83.6) | 61 (83.6) | |||||
| Stage II | 1 (1.1) | 4 (2.2) | 1 (1.4) | 0 (0.0) | |||||
| Stage III | 0 (0.0) | 2 (1.3) | 0 (0.0) | 0 (0.0) | |||||
| Other | 11 (12.1) | 38 (21.0) | 11 (15.1) | 12 (16.4) | |||||
| Operating methods | 23.946 | 0.000 | 0.303 | 0.859 | |||||
| Wedge resection | 71 (78.0) | 88 (48.6) | 53 (72.6) | 50 (68.5) | |||||
| Segmentectomy | 15 (16.5) | 48 (26.5) | 15 (20.5) | 17 (23.3) | |||||
| Lobectomy | 5 (5.5) | 45 (24.9) | 5 (6.8) | 6 (8.2) | |||||
| Inpatient ward | 17.477 | 0.000 | 0.833 | 0.659 | |||||
| General ward | 39 (42.9) | 120 (66.3) | 37 (50.7) | 34 (46.6) | |||||
| Special ward | 27 (29.7) | 42 (23.2) | 19 (26.0) | 24 (32.9) | |||||
| Daytime ward | 25 (27.5) | 19 (10.5) | 17 (23.3) | 15 (20.5) | |||||
a, continuous data are shown as mean ± standard deviation or median (range), and categorical variables as the number (%). Data are shown as numbers (percentages), mean ± standard deviation, or median (range). PSM, propensity score matching; NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, carbon monoxide diffusing capacity; WBC, white blood cell count; NEUT, neutrophilic granulocyte; LYM, lymphocyte; HGB, hemoglobin; PLT, platelet; ALB, albumin; ASA, American Society of Anesthesiologists; LUL, left-upper lobe; LLL, left-lower lobe; RUL, right-upper lobe; RML, right-middle lobe; RLL, right-lower lobe; pTNM, pathological tumor, node, and metastasis classification.
Perioperative situation
Intraoperative situation
All patients successfully completed the operation as planned, and none of the patients in the NI-SP-VALS group was converted to intubated anesthesia during the operation. There were no significant differences in the operation time, anesthesia time, intraoperative fluid input, or lymph node dissection between the two groups. However, the lowest SpO2 level in the NI-SP-VALS group was lower than that in the I-SP-VALS group [96.00% (94.00–97.50%) vs. 97.00% (95.00–98.50%); P=0.035; Table 2].
Table 2
| Variablea | NI-SP-VALS group (n=73) | I-SP-VALS group (n=73) | χ2/t/Z/U | P |
|---|---|---|---|---|
| Operation time, min | 60.00 (48.00, 86.50) | 65.00 (51.50, 90.00) | 1.362 | 0.173 |
| Lymph nodes | 0.00 (0.00, 3.00) | 0.00 (0.00, 3.00) | 0.569 | 0.569 |
| Anesthesia time, min | 112.00 (80.00, 140.50) | 106.00 (83.00, 129.50) | −0.476 | 0.634 |
| Total fluid, mL | 1,100.00 (1,000.00, 1,100.00) | 1,100.00 (1,000.00, 1,100.00) | −0.185 | 0.853 |
| Lowest SpO2, % | 96.00 (94.00, 97.50) | 97.00 (95.00, 98.50) | 2.108 | 0.035 |
| Transit intubation | 0 |
a, continuous data are shown as median (range). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery; SpO2, peripheral capillary oxygen saturation.
Postoperative situation
Routine blood and biochemical indices were tested for all patients on the first day after the operation. The WBC count in the NI-SP-VALS group was lower than that in the I-SP-VALS group, and the difference was statistically significant [9.45 (8.08–11.30) vs. 11 (8.50–12.80)/L; P=0.009]. There were no significant differences in NEUT%, LYM%, HGB, PLT, or ALB. No statistical difference was found between the two groups in terms of the duration of postoperative antibiotic and analgesic use (Table 3).
Table 3
| Variablea | NI-SP-VALS group (n=73) |
I-SP-VALS group (n=73) |
χ2/t/Z/U | P |
|---|---|---|---|---|
| Analgesic use, day | 2.00 (2.00, 3.00) | 2.00 (2.00, 3.00) | 0.031 | 0.975 |
| Antibiotic use, day | 2.00 (2.00, 3.00) | 2.00 (2.00, 3.00) | −0.141 | 0.888 |
| Postoperative blood routine | ||||
| WBC | 9.45 (8.08, 11.30) | 11 (8.50, 12.80) | 2.611 | 0.009 |
| NEUT, % | 81.15 (77.78, 85.08) | 83.10 (79.20, 85.80) | 1.178 | 0.239 |
| LYM, % | 12.55 (9.75, 15.32) | 11.20 (8.95, 14.00) | −1.307 | 0.191 |
| HGB, g/L | 126.00 (119.00, 134.25) | 126.00 (115.50, 149.00) | −0.094 | 0.925 |
| PLT, 109/L | 192.50 (163.75, 232.50) | 206.00 (166.00, 235.50) | 1.442 | 0.149 |
| ALB, g/L | 38.42±3.38 | 38.64±2.92 | −0.412 | 0.681 |
a, continuous data are shown as mean ± standard deviation or median (range). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery; WBC, white blood cell count; NEUT, neutrophilic granulocyte; LYM, lymphocyte; HGB, hemoglobin; PLT, platelet; ALB, albumin.
The duration of postoperative catheter stay in the NI-SP-VALS group was significantly shorter than that in the I-SP-VALS group [0.50 (0.20–2.00) vs. 2.00 (2.00–2.00) d, P<0.001]. The total drainage volume and complications were lower in the NI-SP-VALS group than in the I-SP-VALS group, but the differences were not statistically significant. There was no significant difference in the indwelling time of the chest drain or pain score 2 days after the operation (Table 4). There was no significant difference in degree of postoperative satisfaction, first time of oral intake, first time of out of bed activity, vomiting, or throat discomfort between the two groups (Table 5).
Table 4
| Variablea | NI-SP-VALS group (n=73) |
I-SP-VALS group (n=73) |
χ2/t/Z/U | P |
|---|---|---|---|---|
| Thoracic drainage, day | 2.00 (2.00, 2.00) | 2.00 (2.00, 2.00) | 1.748 | 0.080 |
| Total drainage volume, mL | 90.00 (40.00, 200.00) | 102.50 (46.25, 228.25) | 0.671 | 0.503 |
| Catheter stay, day | 0.50 (0.20, 2.00) | 2.00 (2.00, 2.00) | 5.658 | 0.000 |
| Postoperative pain, VAS | ||||
| Day 1 | 1.009 | 0.313 | ||
| Day 2 | 1.637 | 0.102 | ||
| Complications | 4.322 | 0.479 | ||
| No | 71 (97.3) | 66 (90.4) | ||
| Pneumonia | 0 (0.0) | 2 (2.7) | ||
| Air leakage >3 days | 1 (1.4) | 2 (2.7) | ||
| Hemothorax | 0 (0.0) | 1 (1.4) | ||
| Chylothorax | 0 (0.0) | 0 (0.0) | ||
| Atrial fibrillation | 0 (0.0) | 1 (1.4) | ||
| Other | 1 (1.4) | 0 (0.0) |
a, continuous data are shown as median (range) and categorical variables as the number (%). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery; VAS, visual analog scale.
Table 5
| Variablea | NI-SP-VALS group (n=73) |
I-SP-VALS group (n=73) |
χ2/t/Z/U | P |
|---|---|---|---|---|
| Postoperative satisfaction, % | 100.00 (95.00, 100.00) | 100.00 (91.25, 100.00) | 0.164 | 0.685 |
| Postoperative oral intake of food, hour | 12.00 (12.00, 12.00) | 12.00 (12.00, 12.00) | 1.884 | 0.170 |
| Out of bed activity, hour | 12.00 (12.00, 72.00) | 12.00 (12.00, 72.00) | 0.319 | 0.572 |
| Postoperative vomiting | 0 | 1 | ||
| No | 70 (95.9) | 70 (95.9) | ||
| Yes | 3 (4.1) | 3 (4.1) | ||
| Postoperative throat discomfort | 0.669 | 0.414 | ||
| No | 64 (87.7) | 67 (91.8) | ||
| Yes | 9 (12.3) | 6 (8.2) |
a, continuous data are shown as median (range) and categorical variables as the number (%). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery.
The total length of hospital stay in the NI-SP-VALS group was shorter than that in the I-SP-VALS group, and the difference was statistically significant [4.00 (4.00–5.00) vs. 4.50 (4.00–5.75) d; P=0.029]. Although hospitalization, surgery, and anesthetic costs were lower in the NI-SP-VALS group, no statistically significant difference was found between the two groups (Table 6).
Table 6
| Variablea | NI-SP-VALS group (n=73) | I-SP-VALS group (n=73) | χ2/t/Z/U | P |
|---|---|---|---|---|
| Hospital stays, day | 4.00 (4.00, 5.00) | 4.50 (4.00, 5.75) | 2.187 | 0.029 |
| Hospitalization cost, CNY | 56,007.09 (49,420.09, 65,156.08) | 60,738.28 (48,851.78, 78,006.96) | 1.481 | 0.138 |
| Surgery cost, CNY | 7,007.00 (6,290.00, 8,981.06) | 7,122.00 (6,290.00, 9,092.25) | 0.479 | 0.632 |
| Anesthetic cost, CNY | 2,092.50 (2,082.50, 2,152.50) | 2,137.50 (2,087.50, 2,152.50) | 1.946 | 0.052 |
a, continuous data are shown as or median (range). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery.
Follow-up after discharge
Patients were followed up for 3 months after discharge, and the pain scores of the NI-SP-VALS group at 1 and 3 months after discharge were significantly higher than those of the I-SP-VALS group [1 month: 0.00 (0.00–3.00) vs. 0.00 (0.00–1.75), P=0.048; 3 months: 0.00 (0.00–2.00) vs. 0.00 (0.00–0.00), P=0.028]. There was no significant difference in the readmission rate 1 month after discharge, cough score, or follow-up compliance (Table 7). Multiple emotional assessments of the two groups (before surgery, during hospitalization, 1 month after surgery, and 3 months after surgery) showed no significant differences in anxiety between the two groups (Table 8).
Table 7
| Variablesa | NI-SP-VALS group (n=73) | I-SP-VALS group (n=73) | χ2/t/Z/U | P |
|---|---|---|---|---|
| 1 month | ||||
| Physical performance | 7.00 (6.00, 8.00) | 8.00 (7.00, 8.00) | 1.709 | 0.191 |
| Pain | 0.00 (0.00, 3.00) | 0.00 (0.00, 1.75) | 3.901 | 0.048 |
| Cough | 0.00 (0.00, 3.00) | 0.50 (0.00, 3.00) | 0.204 | 0.878 |
| Rehospitalization | 1 | |||
| No | 71 (97.3) | 72 (98.6) | ||
| Yes | 2 (2.7) | 1 (1.4) | ||
| 3 months | ||||
| Physical performance | 9.00 (8.00, 9.00) | 9.00 (8.00, 9.00) | 0.581 | 0.446 |
| Pain | 0.00 (0.00, 2.00) | 0.00 (0.00, 0.00) | 4.832 | 0.028 |
| Cough | 0.00 (0.00, 2.00) | 0.00 (0.00, 1.00) | 0.456 | 0.499 |
| Follow-up compliance | 3.064 | 0.216 | ||
| Poor | 3 (4.1) | 7 (9.6) | ||
| Average | 26 (35.6) | 31 (42.5) | ||
| Good | 44 (60.3) | 35 (47.9) |
a, continuous data are shown as median (range) and categorical variables as the number (%). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery.
Table 8
| Variablea | Non-intubation group (n=73) | Intubation group (n=73) | χ2/t/Z/U | P |
|---|---|---|---|---|
| Before surgery | 1.858 | 0.173 | ||
| No | 63 (86.3) | 68 (93.2) | ||
| Yes | 10 (13.7) | 5 (6.8) | ||
| During hospitalization | 0.695 | 0.404 | ||
| No | 69 (94.5) | 71 (97.3) | ||
| Yes | 4 (5.5) | 2 (2.7) | ||
| 1 month after surgery | 0.429 | 0.512 | ||
| No | 69 (94.5) | 67 (91.8) | ||
| Yes | 4 (5.5) | 6 (8.2) | ||
| 3 months after surgery | 0 | 1 | ||
| No | 65 (89.0) | 65 (89.0) | ||
| Yes | 8 (11.0) | 8 (11.0) |
a, categorical variables are shown as the number (%). NI-SP-VALS, nonintubated anesthesia single-port video-assisted lung surgery; I-SP-VALS, intubated anesthesia single-port video-assisted lung surgery.
Discussion
In our study, NI-SP-VALS did not prolong the operation or anesthesia times, and no difference in lymph node dissection was found between the two groups. It is suggested that nonintubated anesthesia with spontaneous breathing does not increase the difficulty of our procedure. This is related to the stability of anesthesia. In nonintubated patients, we used a laryngeal mask to provide ventilation and vagal nerve block to reduce the possibility of intraoperative choking interfering with the procedure. In addition, most of our patients underwent wedge resection, which did not involve the separation of vessels or the removal of lymph nodes and was thus simpler than segmentectomy and lobectomy. Even if the amplitude of diaphragmatic and mediastinal swings is low, it does not affect the operation. A study has shown that even in relatively difficult segmentectomy and lobectomy, the operation time of NI-SP-VALS is shorter than that of I-SP-VALS, and the lymph nodes can be well dissected (12).
SpO2 maintenance in the NI-SP-VALS group was worse than that in the I-SP-VALS group (P=0.035). The lowest SpO2 was lower than 90% in 4 patients in the NI-SP-VALS group and 2 patients in the I-SP-VALS group. Intraoperative SpO2 hypoxia was transient in all patients and was sufficiently improved by simple oxygen supplementation with a laryngeal mask in the NI-SP-VALS group and through an endotracheal tube in the I-SP-VALS group. No patient was converted to intubated anesthesia because of persistent hypoxia. During general intubated anesthesia, mechanical ventilation with positive end-expiratory pressure (PEEP) can maintain adequate ventilation and avoid dependent atelectasis. In patients undergoing NI-SP-VALS, surgical pneumothorax, patient position, spontaneous breathing, and the use of sedative and analgesic drugs during anesthesia affect the respiratory physiology of patients (11). Surgical pneumothorax and patient position are unavoidable, but patients can maintain spontaneous breathing with minimal interference in diaphragmatic function and lung-dependent residual gas capacity (FRC) (13,14). Intraoperative ventilation and oxygenation can be maintained with laryngeal masks, which are more effective than are masks and nasal catheters, even when supplemental oxygen via a mask is sufficient to maintain oxygen saturation during anesthesia (11). In addition, the use of intraoperative “respiratory inhibitors,” such as sedation and analgesia, can been minimized by closely monitoring the depth of anesthesia and measuring end-tidal carbon dioxide levels (14). Furthermore, the combination of thoracic paravertebral nerve block can reduce the body movement and tachypnea caused by incision pain during the operation (15), and the block of the thoracic vagus nerve can reduce the cough reaction during the operation (16), ensuring ventilation and oxygenation of the patients during the operation.
Irreversible hypoxemia and carbon dioxide retention are the main causes of intraoperative conversion to intubation anesthesia. In addition, extensive pleural adhesion, massive intraoperative bleeding, and large central tumors are high-risk factors for intraoperative conversion to intubation anesthesia (17). In our study, no patient was converted to endotracheal intubation anesthesia due to irreversible risk factors during surgery. We used laryngeal mask anesthesia combined with paravertebral and vagus nerve blocks to effectively maintain ventilation and oxygenation during the operation. Through strict screening of patients before surgery, we excluded those with a history of pulmonary surgery and large tumors to avoid high-risk factors that may lead to intraoperative conversion to intubation anesthesia. Additionally, the standard operation of the surgeon can ensure operational safety. Lateral decubitus is often used in thoracic surgery, which increases the difficulty of intraoperative conversion to intubation anesthesia (15). Therefore, surgeons should screen patients strictly according to the indications before surgery to reduce the possibility of intraoperative conversion to intubation anesthesia. Our experience shows that the following measures should be implemented for successful NI-SP-VALS: (I) the intraoperative respiratory rate should be controlled at about 10 breaths per minute; (II) as obesity is a high risk factor for intraoperative conversion to intubation (18), nonintubation anesthesia should be avoided in obese patients (BMI >25 kg/m2); (III) the surgeon should perform the operation according to the respiratory rate and try to avoid a delicate operation during the exchange of inspiratory and expiratory breath; and (IV) close cooperation should be maintained between the anesthesia and surgical team.
In our study, the WBC on the first postoperative day was lower in the NI-SP-VALS group than in the I-SP-VALS group. This suggests that patients undergoing NI-SP-VALS have fewer postoperative inflammatory responses and less stress. The postoperative stress response is a factor that affects rapid recovery. Nonintubation with spontaneous breathing can reduce surgical stress and accelerate postoperative recovery (19). Some authors have reported that thoracic epidural anesthesia (TEA) can inhibit the sympathetic nervous system by blocking the afferent and efferent neural pathways, reducing the postoperative stress response and its effect on the postoperative lymphocyte response (20,21). In our study, no differences were observed between lymphocytes and neutrophils, and no important indicators of the inflammatory response, such as C-reactive protein (CRP) or tumor necrosis factor (TNF), were measured after the operation; only differences in WBC were observed. This may be because we only performed a thoracic paravertebral block, which did not block the phrenic nerve that conducts injury stimulation and thus could not completely reduce the stress response (19,22). In addition, stress responses in awake patients may potentially be enhanced by emotional factors (22).
In our study, there was no difference in the duration of antibiotic and analgesic use between the two groups. This may be related to the generally short duration of medication use in our patients after surgery, with the vast majority of patients discontinuing analgesics and antibiotics after 2 days due to good postoperative recovery.
Although recent studies have suggested that thoracic drainage is not necessary in selected patients undergoing wedge resection of the lungs (23,24), we believe that thoracic drainage is necessary after thoracic surgery. In our patients, we routinely indwelled thoracic drains. Indwelling of the thoracic drain is an adverse factor for postoperative chest discomfort and pain due to (I) compression and injury of the intercostal nerves and (II) stimulation of the diaphragm and pleura (25). Cattoni et al. showed that chest tube size and postoperative drainage time are risk factors for chronic chest pain and paresthesia (25). Prolonged chest tube use increases chronic pain and limits early postoperative activity. In our study, chest radiography is routinely performed on the second day after surgery to evaluate pulmonary recruitment and decide whether to remove the chest tube based on the drainage situation. The chest tube was removed in most patients on the second day after surgery, and there was no significant difference in drainage time or volume between the two groups, suggesting that nonintubated anesthesia does not prolong the indwelling time of the chest tube and increases the thoracic drainage volume.
During thoracic surgery, it is generally accepted that a catheter should be routinely inserted to accurately assess urine output and reduce the risk of postoperative urinary retention. The catheter should be removed as early as possible in the early postoperative period (days 1–2) to reduce the incidence of catheter-related adverse events and relieve catheter-induced discomfort (26). Recent studies have suggested that indwelling catheterization can be avoided after VATS (27). In our patients, catheter placement was determined by the surgeon in the operating room. In patients with temporary catheter placement during the operation, the catheter was removed immediately after evaluation. In our study, the time of postoperative catheterization in the NI-SP-VALS group was significantly shorter than that in the I-SP-VALS group, which was related to the use of anesthesia to preserve spontaneous breathing in the NI-VALS group. Avoiding the use of muscle relaxants allowed patients to quickly recover their spontaneous defecation function after surgery, and they could defecate with the bedpan on or near the bed, even when going to the bathroom with a family member or caregiver.
Previous studies have shown that nonintubation anesthesia promotes early feeding and ambulation. Simultaneously, because it avoids the use of muscle relaxants, it can reduce the incidence of postoperative vomiting, and the ventilation method of a laryngeal mask or face mask can reduce the possibility of throat injury, reduce the incidence of postoperative throat discomfort, and improve patient satisfaction (28,29). In our study, no significant differences were observed between the two groups. Most of our patients began to eat and get out of bed 1 day after surgery, and the incidence of vomiting was low. Tracheal intubation was guided by a video laryngoscope, which reduced the chance of airway injury and may account for the comparable probability of throat discomfort in the two groups. Under ERAS management, our patients’ overall satisfaction was high, and no between-group differences were observed.
NI-SP-VALS promoted patient recovery and shortened the length of hospital stay. The median length of patients’ hospital stay was 0.5 days shorter than that of patients in the I-SP-VALS group (P=0.029). In addition, in our patients, the total hospital, surgical, and anesthesia costs were lower in the observation group than in the control group, but the difference was not statistically significant. The difference in time to discharge between the two groups was small, and our patients, who both underwent ERAS, had a shorter overall hospital stay, which may explain why no difference was observed in hospital costs. Anesthesia reduces the cost of intubation and the use of muscle relaxants, but it also incurs the cost of thoracic paravertebral and vagal blocks, which may explain why no difference in anesthesia expense was observed between the two groups. Both groups of patients underwent the same surgical procedure under the same surgical instruments; therefore, there was no significant difference in surgical costs.
The patients were followed up at 1 and 3 months after the operation, and there were statistically significant differences in postoperative pain scores between the two groups (P<0.05). There were no significant differences in physical recovery, cough, readmission rate, postoperative anxiety, or follow-up compliance between the two groups. This suggests that there was no difference in the short-term postoperative quality of life between the NI-SP-VALS and I-SP-VALS groups. We observed gradual pain relief in both groups, but pain relief in the NI-SP-VALS group seemed slightly worse than that in the I-SP-VALS group. However, the mechanism of this phenomenon is not clear in the current study. More research is needed to confirm whether patients under nonintubated anesthesia experience worse chronic pain relief after surgery than do those under intubation anesthesia.
Our study has some limitations. First, we used a retrospective design with a small sample size. Although baseline data were adjusted for PSM, selection bias was still present. Second, this study only assessed the short-term outcomes of patients. Further research is needed to evaluate long-term outcomes, such as tumor outcomes and long-term prognosis. In addition, our patients had good preoperative conditions after the rigorous preoperative screening, and the applicability and benefits for some specific patient groups, such as large tumors, being overweight, and impaired lung function, need to be further studied.
Conclusions
In our data after PSM, it demonstrated that nonintubated anesthesia in single-port VALS is a safe and beneficial option that contributes to rapid patient recovery. Compared to intubation, this technique has advantages in postoperative clinical rehabilitation. However, this is a single center retrospective study, further prospective randomized studies are needed to better compare tumor and long-term outcomes of nonintubated versus intubated VALS. NI-SP-VALS promotes rapid patient recovery and may be an effective choice for clinical surgeons in the future. As the surgical team gains experience, the NI-SP-VALS strategy can be extended to more challenging procedures.
Acknowledgments
We sincerely appreciate the patients for their active participation in this study as well as the departments of anesthesiology, nursing, nutrition, and social work for their support.
Funding: This research was supported by the National Key Research and Development Program (No. 2019YFE0105600), the Clinical Science and Technology Innovation Project of Shanghai Hospital Development Center (No. SHDC22021218), and the Clinical Medical Research Project of Shanghai Science and Technology Commission (No. 21MC1930200).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1689/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1689/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1689/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This study was approved by our ethics committee of Huadong Hospital Affiliated to Fudan University (No. 2021K010). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg 2011;13:539-41. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189-98. [Crossref] [PubMed]
- Gotlib Conn L, McKenzie M, Pearsall EA, et al. Successful implementation of an enhanced recovery after surgery programme for elective colorectal surgery: a process evaluation of champions' experiences. Implement Sci 2015;10:99. [Crossref] [PubMed]
- Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19:5-10. [Crossref] [PubMed]
- Janík M, Juhos P, Lučenič M, et al. Non-intubated Thoracoscopic Surgery-Pros and Cons. Front Surg 2021;8:801718. [Crossref] [PubMed]
- Peng G, Cui F, Ang KL, et al. Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. [Crossref] [PubMed]
- Elkhouly A, Pompeo E. Nonintubated Subxiphoid Bilateral Redo Lung Volume Reduction Surgery. Ann Thorac Surg 2018;106:e277-9. [Crossref] [PubMed]
- Jiang L, Liu J, Gonzalez-Rivas D, et al. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J Thorac Cardiovasc Surg 2018;155:2746-54. [Crossref] [PubMed]
- Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [Crossref] [PubMed]
- Liu YJ, Hung MH, Hsu HH, et al. Effects on respiration of nonintubated anesthesia in thoracoscopic surgery under spontaneous ventilation. Ann Transl Med 2015;3:107. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Lohser J, Ishikawa S. Physiology of the Lateral Decubitus Position, Open Chest and One-Lung Ventilation. In: Principles and Practice of Anesthesia for Thoracic Surgery. 2011:71-82. Available online: https://link.springer.com/chapter/10.1007/978-1-4419-0184-2_5
- Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med 2005;31:1327-35. [Crossref] [PubMed]
- Yang JT, Hung MH, Chen JS, et al. Anesthetic consideration for nonintubated VATS. J Thorac Dis 2014;6:10-3. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Hung WT, Hung MH, Wang ML, et al. Nonintubated Thoracoscopic Surgery for Lung Tumor: Seven Years' Experience With 1,025 Patients. Ann Thorac Surg 2019;107:1607-12. [Crossref] [PubMed]
- Guo Z, Yin W, Pan H, et al. Video-assisted thoracoscopic surgery segmentectomy by non-intubated or intubated anesthesia: a comparative analysis of short-term outcome. J Thorac Dis 2016;8:359-68. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109-17. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Lu TY, Chen JX, Chen PR, et al. Evaluation of the necessity for chest drain placement following thoracoscopic wedge resection. Surg Today 2017;47:606-10. [Crossref] [PubMed]
- Nakashima S, Watanabe A, Mishina T, et al. Feasibility and safety of postoperative management without chest tube placement after thoracoscopic wedge resection of the lung. Surg Today 2011;41:774-9. [Crossref] [PubMed]
- Cattoni M, Rotolo N, Mastromarino MG, et al. Chronic chest pain and paresthesia after video-assisted thoracoscopy for primary pneumothorax. J Thorac Dis 2021;13:613-20. [Crossref] [PubMed]
- Darrah DM, Griebling TL, Silverstein JH. Postoperative urinary retention. Anesthesiol Clin 2009;27:465-84. table of contents. [Crossref] [PubMed]
- Lai Y, Wang X, Zhou K, et al. The Feasibility and Safety of No Placement of Urinary Catheter Following Lung Cancer Surgery: A Retrospective Cohort Study With 2,495 Cases. J Invest Surg 2021;34:568-74. [Crossref] [PubMed]
- Liu HY, Chiang XH, Hung MH, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Formos Med Assoc 2020;119:1396-404. [Crossref] [PubMed]
- Cai LS, Hou B, Jin H, et al. Clinical evaluation of the rapid recovery of patients who underwent video-assisted thoracoscopic lung surgery under non-intubated anesthesia. Ann Transl Med 2021;9:1783. [Crossref] [PubMed]


