Clinical characteristics and survival of esophageal cancer patients: annual report of the surgical treatment in Shanghai Chest Hospital, 2016
Highlight box
Key findings
• Surgical treatment conferred acceptable short- and long-term results in a high-volume center in China.
What is known and what is new?
• More than 50% of newly diagnosed global esophageal cancers cases located in China. McKeown and Ivor Lewis surgery of the right thoracic approach had gradually occupied the mainstream of esophageal cancer surgery in China. Surgical safety with less than 1% of operative death within 30 days was becoming the norm in large centers in China.
• The proportion of early-stage patients is a critical factor in determining the ultimate survival outcome.
What is the implication, and what should change now?
• Single-center and large-sample data annual reports with standardized treatment will be of great significance in determining treatment strategies for the future.
• More early-stage patients will be recruited for individualized neoadjuvant therapy with the aim of improving treatment outcomes.
Introduction
Esophageal cancer is the sixth leading cause of death resulting from cancer worldwide with esophageal squamous cell carcinoma (ESCC) accounting for over 90% of total esophageal cancer in China (1,2). Most esophageal cancers are locally advanced when diagnosed and the associated extensive lymph node metastasis, especially occult lymph node metastasis, contributes to the complexity of the treatment decisions (3,4). Multidisciplinary treatment including preoperative and postoperative chemoradiotherapy/chemotherapy has been increasingly performed worldwide since it has shown a survival benefit over surgery alone; however, surgery remains the cornerstone of curative treatment of esophageal cancer. Unfortunately, few studies to date have evaluated the real-world data of surgical treatment of esophageal cancer. Analysis of a large volume of homogeneous data may provide meaningful information that is crucial to improve treatment development. Representative database systems of this type include the Japan Esophageal Society (JES) annual report in Japan (5), the National Cancer Database (NCDB) database in the United States (6), and the European Society of Thoracic Surgeons (ESTS) registry database in Europe (7). In 2016, the first esophageal cancer annual report of JES had published, providing a comprehensive introduction on all aspects of diagnosis and treatment-related outcome of esophageal cancer in Japan, served as a reference parameter in surgical treatment of esophageal cancer. However, except for JES database, annual reports regarding esophageal cancer treatment from the NCDB and ESTS database are also warranted, and China with no exception.
More than 50% of newly diagnosed global esophageal cancers cases being in China (8), yet, the current national data registration system is lacking and incapable to involve all high-volume esophageal cancer centers. There are many single centers with huge volumes of data (more than 500 cases of esophageal cancer operations per year) in China. Although they are not representative of multiple regions, they have unique advantages in terms of therapeutic homogeneity. Notably, many reports have suggested that the future treatment of esophageal cancer should be concentrated in large centers that are able to provide better therapeutic effects (9-12). For this reason, single-center and large-sample data annual reports with standardized treatment will be of great significance in determining treatment strategies for the future treatment development.
Shanghai Chest Hospital (SCH) is a tertiary referral center with more than 800 esophageal cancer surgical operations per year [2019–2021], we have published the first annual report of surgical treatment of esophageal cancer in the Chinese journals in 2015, and plan to provide annual reports in SCH since 2016 to the worlds as reference scientific data. Here, we briefly summarized the comprehensive clinical characteristics and survival of esophageal cancer surgical treatment in SCH, 2016. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1672/rc).
Methods
Clinical data
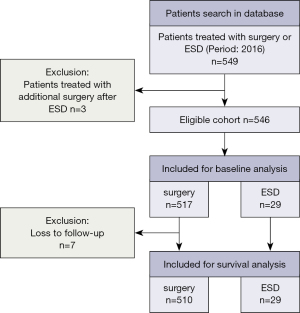
All patients who had undergone surgical treatments including esophagectomy and endoscopic resection with esophageal cancer at SCH in 2016 were enrolled in this study. Data were extracted from the esophageal cancer single disease database in our center for analysis. A total of 546 eligible patients with esophageal cancer who underwent primarily esophagectomy, as well as minor endoscopic submucosal dissection (ESD) at SCH in 2016 were included. All operations had more than 10 attending thoracic surgeons, but were mainly performed by 3 attending surgeons affiliated to the Section of Esophageal Surgery (Z Li, Y Sun, and T Mao). Although more than 87% of the pathologic types were squamous cell carcinomas, we also included the rare histology of gastroesophageal junction adenocarcinoma, small cell neuroendocrine tumors, etc. Endoscopic resections were performed by esophageal surgeons and gastrointestinal physicians (Z Li, H Zhang, and Y Su) and were therefore also included. Clinicopathological characteristics was analyzed in all 546 patients, but a total of 7 patients who were lost to follow-up in the first 2 years after the operation were excluded from the long-term survival analysis (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the Shanghai Chest Hospital (No. IS21111). Individual consent for this retrospective analysis was waived.
Evaluation
All patients underwent preoperative esophagogastroduodenoscopy (EGD) to locate the lesion and biopsy for histological diagnosis. Endoscopic ultrasonography (EUS) was only used for the diagnosis of T staging in early stage patients, but was not routinely used for T staging and EUS/fine needle aspiration (FNA) biopsy for advanced tumors. T and metastatic lymph node staging were performed for each patient using contrast-enhanced computed tomography (CT) scan of the chest and abdomen. Cervical paraesophageal and supraclavicular lymph node staging was routinely performed using neck ultrasound. For tumors or bulking metastatic lymph nodes in the upper thoracic mediastinum, a bronchoscopy was routinely performed. Endobroncheal ultrasonography (EBUS) was not commonly used. Positron emission tomography (PET)/CT was routinely used after 2020, but was not mandatory during this current study period [2016]. For suspected liver metastases, brain metastases, or bone metastases, additional enhanced liver magnetic resonance imaging (MRI), enhanced brain MRI, and bone radionuclide scans were performed. The staging was based on the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging criteria for esophageal cancer (13).
To assess the patient’s general condition, echocardiography, treadmill exercise testing, pulmonary function, and arterial blood gas assessments were conducted. Nutritional assessment was mandatory for admission.
Multidisciplinary treatment
Multidisciplinary therapy was used in patients with advanced tumors. The indication and strategy varied depending on the physician or patient preference. Neoadjuvant therapy was mainly used for patients with extensive lymph node metastasis (≥2 fields) or T3 to T4a tumor invasion. In neoadjuvant chemotherapy, fluorouracil (5-Fu), paclitaxel, or docetaxel supplemented with cisplatin or carboplatin was used commonly, mostly with two cycles. Neoadjuvant chemoradiotherapy was largely based on the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery (CROSS) regimen. Postoperative adjuvant therapy was more commonly used than preoperative adjuvant therapy. For patients with tumors in the upper thoracic esophagus or lymph node metastases at the upper mediastinum or neck, or patients with R2 resection, postoperative adjuvant radiotherapy was routinely recommended. For other patients with pN+/ypN+, an additional three cycles of platinum-containing chemotherapy were administered, otherwise only active surveillance was recommended.
Esophagectomy or endoscopic resection
In our center, the right thoracic approach of McKeown or Ivor-Lewis procedure and thoracoabdominal two-field or selective three-field lymph node dissection were routinely used to complete radical esophagectomy. Very few patients with esophageal squamous cell carcinoma used the left thoracic approach (Sweet procedure). Gastroesophageal junction adenocarcinomas were mostly operated by the transhiatal or left thoracic approach. Thoracoscopic and robotic assisted minimally invasive esophagectomy were routinely performed in 2016, but some cases of open approach were still performed by some doctors.
Endoscopic resection was used in patients with clinically intramucosal invasion and no lymph node metastasis (cT1aN0). The procedure was performed under general anesthesia and endotracheal intubation. ESD-en bloc resection was the preferred method, and circumferential resection was avoided as much as possible to prevent intractable stenosis after the operation. Standard pathological evaluations were performed postoperatively.
Data collection
A prospectively single-disease database regarding esophageal cancer was established in SCH. The following baseline patient characteristics were collated: age, gender, height and weight at admission, diagnosis, tumor related information (such as location of tumor, pathological type, differentiation status, residual tumor status, and pathological stage), treatment relevant outcomes (including surgical technique, route, substitute organ, fields of lymph node dissection, radiotherapy, chemotherapy, or chemoradiotherapy), and perioperative results (including postoperative morbidity, 30- and 90-day mortality, length of in-hospital stay, length of intensive care unit (ICU) stay, and numbers of lymph node dissection). For pathological results, resected tumor specimens were recoded with proximal and distal margins. R0 resection was defined as non-positive discovery in both resection margins; R1 was defined as microscopical residual tumor; and R2 was defined as a macroscopical tumor. Postoperative complications were classified using the Esophagectomy Complications Consensus Group (ECCG) system and Clavien-Dindo grading system (14,15). These data were extracted from patient’s medical records under permission. After smooth discharge from the SCH, the follow-up strategy was initiated by telephone or out-patient clinics until June 2022. The routine follow-up was conducted every 3 months in first year, and then every 6 months in the subsequent years. Through follow-up, linkage was established between the patients’ medical records and the data of the esophageal cancer registry database which could provide patients’ survival status. Overall survival (OS) was calculated from the day of esophageal relevant operation (day 0) to the day of death or the day of last follow-up. Similarly, cancer specific survival (CSS) was calculated from the intervals of day 0 to the day of cancer specific death or the last day of follow-up, as was the recurrence free survival (RFS), which measured the interval between day 0 and any recurrence or progression in cancer.
Statistical analysis
All analyses were performed using the SPSS software version 26.0 (IBM Corporation, Armonk, NY, USA). Categoric data was documented as numbers and percentages. Continuous data was recorded as median [interquartile range (IQR)] or mean ± standard deviation (SD). Multivariate Cox analysis was conducted to clarify prognostic factors affecting long-term outcome. To determine the effect of neoadjuvant therapy on survival, a propensity-score matched (PSM) analysis was performed to draw an unbiased marginal estimate of the exposure effect. Propensity score was calculated for each patient and matching was performed using a 1:1 matching protocol without replacement (greedy-matching algorithm) and a caliper width of 0.02. In this model, the included variable was clinical stage, which was used to compare the survival outcome between patients with neoadjuvant therapy and without neoadjuvant therapy. Survival data were analyzed and graphed using the Kaplan-Meier method and compared using Log-rank tests using the GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). A P value less than 0.05 two-sided was considered statistically significant.
Results
Patient background
A total of 546 cases were included (Figure 1). The majority of patients (52.4%) were aged 60–69 years, with 79.5% of patients being male (Table S1). There were 43 (7.9%) malnourished patients whose body mass index (BMI) was less than 18.5. The tumor was mainly located at the middle thoracic esophagus (51.3%) (Table 1). There were 125 cases (22.9%) of superficial carcinomas (Tis, T1a, T1b). Neoadjuvant therapy was administered in 60 cases (11.0%). Cure-intended esophagectomy and endoscopic resection was conducted in 517 cases (94.7%) and 29 cases (5.3%), respectively (Tables 2,3).
Table 1
| Variables | Cases (%) |
|---|---|
| Age (years) | |
| <30 | 0 (0.0) |
| 30–39 | 0 (0.0) |
| 40–49 | 16 (2.9) |
| 50–59 | 121 (22.2) |
| 60–69 | 286 (52.4) |
| 70–79 | 106 (19.4) |
| ≥80 | 17 (3.1) |
| Gender | |
| Male | 434 (79.5) |
| Female | 112 (20.5) |
| BMI (kg/m2)* | |
| <18.5 | 43 (7.9) |
| 18.5–23.0 | 247 (45.2) |
| >23.0 | 256 (46.9) |
| Location | |
| Cervical | 3 (0.5) |
| Upper thoracic | 67 (12.3) |
| Middle thoracic | 280 (51.3) |
| Lower thoracic | 146 (26.7) |
| EGJ | 50 (9.2) |
| Diagnosis | |
| Esophageal cancer | 538 (98.5) |
| Esophageal concurrent cancers | 8 (1.5) |
*, BMI in accordance with the Asia-Pacific standards. BMI, body mass index; EGJ, esophagogastric junction.
Table 2
| Approaches | Cases (%) |
|---|---|
| ESD | 29 (5.3) |
| Esophagectomy | 517 (94.7) |
| McKeown | 369 (67.6) |
| Ivor-Lewis | 77 (14.1) |
| Sweet | 52 (9.5) |
| Transhiatal | 16 (2.9) |
| TPLE | 2 (0.4) |
| Cervical esophagectomy | 1 (0.2) |
| Total | 546 |
ESD, endoscopic submucosal dissection; TPLE, total pharyngo-laryngo-esophagectomy.
Table 3
| Treatments | Cases (%) |
|---|---|
| Endoscopic resection* | 29 (5.3) |
| Esophagectomy alone** | 457 (83.7) |
| Perioperative multidisciplinary treatment | 283 (51.8) |
| Preoperative therapy + esophagectomy | 60 (11.0) |
| Chemotherapy | 28 (5.1) |
| Radiotherapy | 1 (0.2) |
| Chemoradiotherapy | 31 (5.7) |
| Endoscopic resection + adjuvant | 2 (0.4) |
| Esophagectomy + adjuvant*** | 248 (45.4) |
*, due to dissatisfied endoscopic resection, 3 cases received additional surgery; **, 1 case received esophagectomy as well as endoscopic resection at the same time; ***, 27 cases which received preoperative therapy were included simultaneously.
Resection-esophagectomy
Surgery
Of all esophagectomies performed (n=517), the McKeown approach accounted for 67.6%, Ivor-Lewis 14.1%, Sweet 9.5%, and transhiatal 2.9% (Table 2). Minimally invasive techniques were used in 58% of patients, including robotic assisted esophagectomy in 114 patients (22.1%) (Table 4). Complete resection (R0) was achieved in 90.3% of patients (Table 5). Among conduit selection, the stomach was used in 98.6% of patients and the posterior mediastinum was used in 59.4% of patients as the reconstruction route. In the remaining patients, 36.2% were retrosternal and 0.2% were subcutaneous. The method of 3-field lymph node dissection was used is 15.7% of all patients.
Table 4
| Outcomes | Cases (%) |
|---|---|
| Surgical technique (n=517) | |
| RAE | 114 (22.1) |
| MIE | 186 (36.0) |
| OPEN | 217 (42.0) |
| Organs (n=517) | |
| Gastric tube | 510 (98.6) |
| Colon | 7 (1.4) |
| Reconstruction route (n=517) | |
| None | 2 (0.3) |
| Posterior mediastinal | 327 (63.3) |
| Retrosternal | 187 (36.2) |
| Subcutaneous | 1 (0.2) |
| Fields of lymph node dissection (n=517) | |
| None | 1 (0.2) |
| One field-thoracic | 12 (2.3) |
| One field-abdominal | 24 (4.6) |
| Two fields-thoracic + abdominal | 436 (84.3) |
| Three fields-cervical + thoracic + abdominal | 44 (8.5) |
RAE, robotic assisted esophagectomy; MIE, minimally invasive esophagectomy; OPEN, open approach esophagectomy.
Table 5
| Residual tumor (R) | Cases (%) |
|---|---|
| R0 | 467 (90.3) |
| R1 | 18 (3.5) |
| R2 | 32 (6.2) |
| Total | 517 |
Multidisciplinary treatment
In the esophagectomy cohort, 281 patients received perioperative multidisciplinary treatment. The composition ratio was as follows: 28 patients (10.0%) received neoadjuvant chemotherapy, and the clinical staging ranged from cII to cIVA; 31 patients (11.0%) received neoadjuvant chemoradiotherapy, with the clinical staging ranging from cII to cIVA (the corresponding clinical stage distribution of patients is provided in Table S2); and the last 1 patient who received radiotherapy was in stage cIVA. Postoperative adjuvant therapy was performed in 248 patients (88.3%), and 11 patients were postoperative pathological stage I, 54 patients were stage II, 142 patients were stage III, and 41 patients were stage IV. A total of 27 patients (9.6%) received both preoperative and postoperative treatments.
Safety-complications
A total of 214 patients satisfied the complications definition of ECCG. Total complications occurred in 41.4% of patients and Clavien-Dindo stage III–IV morbidity was evident in 11.2%. Postoperative pneumonia was one of the most common morbidities observed in 2016, with an incidence of 15.9%, followed by recurrent laryngeal nerve injury (13.9%). Other in-hospital complications were listed in Table 6.
Table 6
| Complications | Cases (%) |
|---|---|
| Total complications (ECCG) | 214 (41.4) |
| Clavien-Dindo complications ≥III | 58 (11.2) |
| Pulmonary | |
| Pneumonia | 82 (15.9) |
| Pleural effusion | 50 (9.7) |
| Pneumothorax | 4 (0.8) |
| Respiratory failure | 7 (1.4) |
| ARDS | 30 (5.8) |
| Cardiovascular | |
| Cardiac arrhythmia | 3 (0.6) |
| Gastrointestinal | |
| Anastomotic leakage | 39 (7.5) |
| Gastrointestinal bleeding | 1 (0.2) |
| Liver dysfunction | 1 (0.2) |
| Thromboembolic | |
| Deep venous thrombosis | 2 (0.4) |
| Neurologic/psychiatric | |
| Recurrent laryngeal nerve injury | 72 (13.9) |
| Delirium | 2 (0.4) |
| Infection | |
| Wound infection | 13 (2.5) |
| Chyle leak | 10 (1.9) |
| Total | 517 |
ECCG, Esophagectomy Complications Consensus Group; ARDS, acute respiratory distress syndrome.
Resection-endoscopic therapy
Among the 29 cases that underwent ESD therapy, all were staged as cT1aN0M0. The en bloc resection rate was 96.6% and the margin-negative resection (R0) rate was 79.3% (22/29). There were 7 patients who underwent non-radical resection, including 5 patients with positive horizontal margin and 2 patients with positive vertical margin. In post-resection pathology, the tumor invaded to the mucous epithelium in 15 cases, the lamina propria in 4 cases, the muscularis mucosa in 4 cases, and the submucosa in 6 cases. Lymphovascular invasion (LVI) only occurred in 1 patient who presented with submucosal invasion. The median length of the lesions was 2 cm. No severe perioperative complications occurred, except for 4 strictures. Adjuvant therapy was conducted in 2 patients and salvage esophagectomy was performed in 3 patients to prevent recurrence (Table 3).
Pathology profile
Postoperative pathology analysis showed that squamous cell carcinoma was the most common pathology type in our center, with 453 cases out of 517 patients (87.6%). In the endoscopic resection cohort, 14 out of 29 patients (48.3%) presented with squamous cell carcinoma, and 15 cases were high grade dysplasia (HGD). The pathological depth of tumor invasion was dominated by pT3 (48.4%) in esophagectomy. Lymph node metastasis was detected in 50.7% of patients. A total of 194 cases (37.5%) were predominately pStage III in esophagectomy. There were 214 (41.4%) cases of moderately differentiated specimens of esophagectomy, 208 cases (40.2%) of poorly differentiated or undifferentiated specimens, and 65 cases (12.6%) of well differentiated specimens. LVI was detected in 69 patients (13.3%) in the esophagectomy cohort, and 1 patient (3.4%) in the ESD cohort (Tables 7-14). The total number of lymph node dissection in surgery was 17.1±9.4 (mean ± SD). The average postoperative stay of all 546 patients was 17.7±15.9 days (mean ± SD), and the ICU length of stay was 3.3±4.6 days (mean ± SD) (Table 15), it seemed that pT3 patients encountered the most cases of ICU readmission (Table 16). The postoperative 30- and 90-day mortality was 0.73% and 1.1%, respectively.
Table 7
| Histological classification | Cases (%) |
|---|---|
| Squamous cell carcinoma | 453 (87.6) |
| Adenocarcinoma | 47 (9.1) |
| Absolute adenocarcinoma | 44 (8.5) |
| Signet-ring cell carcinoma | 1 (0.2) |
| Adenocarcinoma mixed with others | 2 (0.4) |
| Neuroendocrine carcinoma | 10 (1.9) |
| Big cell carcinoma | 1 (0.2) |
| Small cell carcinoma | 8 (1.5) |
| Neuroendocrine carcinoma (undefined) | 1 (0.2) |
| Mixed neuroendocrine/non-neuroendocrine carcinoma | 1 (0.2) |
| Combined small cell-squamous cell carcinoma | 1 (0.2) |
| Adenosquamous carcinoma | 2 (0.4) |
| Multiple carcinoma | 4 (0.8) |
| Squamous cell carcinoma+ adenocarcinoma | 4 (0.8) |
| Total | 517 |
Table 8
| Histological classification | Cases (%) |
|---|---|
| High grade dysplasia | 15 (51.7) |
| Squamous cell carcinoma | 14 (48.3) |
| Total | 29 |
Table 9
| Pathological depth of tumor invasion | Cases (%) |
|---|---|
| pT0* | 18 (3.5) |
| pTis | 6 (1.2) |
| pT1a | 17 (3.3) |
| pT1b | 73 (14.1) |
| pT2 | 100 (19.3) |
| pT3 | 250 (48.4) |
| pT4a | 21 (4.1) |
| pT4b | 32 (6.2) |
| Total | 517 |
*, including 16 patients who received neoadjuvant therapy and 2 patients who had undergone ESD previously (in another medical center). AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; ESD, endoscopic submucosal dissection.
Table 10
| Pathological depth of tumor invasion | Cases (%) |
|---|---|
| pTis-M1 | 15 (51.7) |
| pT1a-M2 | 4 (13.8) |
| pT1a-M3 | 4 (13.8) |
| pT1b-SM1 | 1 (3.4) |
| pT1b-SM2 | 1 (3.4) |
| pT1b-undefined | 4 (13.8) |
| Total | 29 |
Table 11
| Lymph node metastasis | Cases (%) |
|---|---|
| pN0 | 255 (49.3) |
| pN1 | 150 (29.0) |
| pN2 | 82 (15.9) |
| pN3 | 30 (5.8) |
| Total | 517 |
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis.
Table 12
| pTNM stage | Cases (%) | |
|---|---|---|
| Esophagectomy | Endoscopy | |
| 0 | 7 (1.4) | 15 (51.7) |
| I | 93 (18.0) | 14 (48.3) |
| II | 151 (29.2) | – |
| III | 201 (38.9) | – |
| IV | 65 (12.6) | – |
| Total | 517 | 29 |
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis.
Table 13
| Differentiation | Cases (%) | |
|---|---|---|
| Esophagectomy | Endoscopy | |
| G1-well | 65 (12.6) | 3 (10.3) |
| G2-moderately | 214 (41.4) | 4 (13.8) |
| G3-poorly/undifferentiated | 208 (40.2) | 2 (6.9) |
| GX | 30 (5.8) | 20 (69.0) |
| Total | 517 | 29 |
Table 14
| Lymphovascular invasion | Cases (%) | |
|---|---|---|
| Esophagectomy | Endoscopy | |
| Negative | 448 (86.7) | 28 (96.6) |
| Positive | 69 (13.3) | 1 (3.4) |
| Total | 517 | 29 |
Table 15
| Variables | Mean ± SD |
|---|---|
| Length of postoperative stay (days) | 17.7±15.9 |
| Length of ICU stay (days) | 3.3±4.6 |
| Number of lymph node examined (n=517) | 17.1±9.4 |
ICU, intensive care unit; SD, standard deviation.
Table 16
| Pathological depth of tumor invasion | ICU cases (%) |
|---|---|
| pT0/Tis | 0 (0.0) |
| pT1a | 1 (4.3) |
| pT1b | 4 (17.4) |
| pT2 | 4 (17.4) |
| pT3 | 11 (47.8) |
| pT4a | 1 (4.3) |
| pT4b | 2 (8.7) |
| Total | 23 |
ICU, intensive care unit.
OS and subtype analysis
The routine postoperative follow-up lasted to June 2022, with a median (IQR) of 60 [19–61] months. A total of 7 cases lacked postoperative follow-up data and finally, 539 cases were included in the survival analysis. As for the endoscopic treatment cohort, the postoperative 1-, 2-, 3-, 4-, and 5-year OS were 100.0%, 96.4%, 96.4%, 96.4%, and 96.4%, respectively (Figure S1). For the surgery cohort, the relevant 1-, 2-, 3-, 4-, and 5-year OS were 86.5%, 67.8%, 59.9%, 54.5%, and 51.8%, respectively (Figure 2) and the CSS rates were 91.8%, 74.2%, 66.6%, 61.2%, and 59.1%, respectively. Regarding the stratification of pT, pN, and pTNM stage, the OS, RFS, and CSS in each subgroup were analyzed (Figures 3-8, Figure S2). The Kaplan-Meier survival curves diverged in nodal-staging (pN) for both OS and CSS. In pT analysis, the survival of pT4a was better than pT4b in OS, however, the survival curves were almost identical between pT4a and pT4b in CSS. The survival curves showed a good discriminatory ability in the pathological stage for both OS and CSS. The residual tumor (R0/R1/R2) exhibited a satisfying discrimination in OS (P<0.001). However, there was no significant difference in the CSS subtype analysis (P=0.063). To determine why radical resection could not be applied, the location of tumor invasion in R2 resection patients was analyzed (Table 17). For post pathologically verified ESCC patients after esophagectomy, the postoperative 1-, 2-, 3-, 4-, and 5-year OS were 85.9%, 67.8%, 60.7%, 55.0%, and 52.4%, respectively (Figure 9). A total of 250 deaths during follow-up was documented and 193 cases of them (77.2%) were due to recurrence (Table 18).
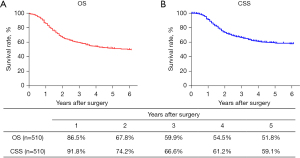
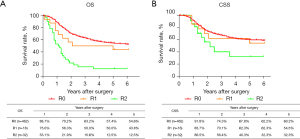
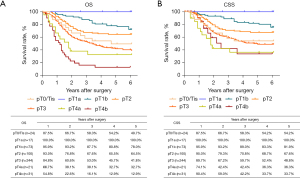
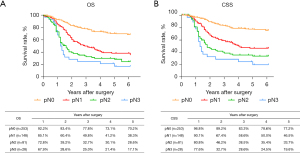
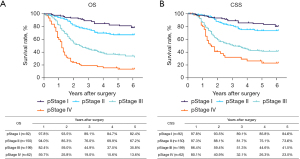
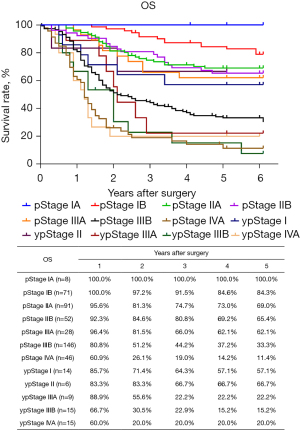
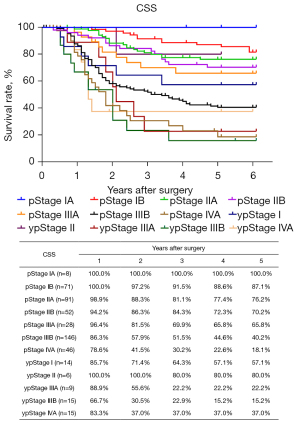
Table 17
| Invasion spot of R2 resection | Cases (%) |
|---|---|
| Trachea | 15 (42.9) |
| Aorta | 10 (28.6) |
| Recurrent laryngeal nerve | 2 (5.7) |
| Atrium | 1 (2.9) |
| Pancreas | 1 (2.9) |
| Lung | 1 (2.9) |
| Thyroid | 1 (2.9) |
| Missing data | 4 (11.4) |
| Total spots | 35 |
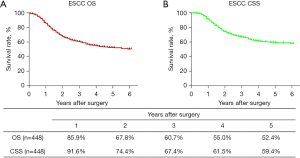
Table 18
| Cause of death | Cases (%) |
|---|---|
| Death due to recurrence | 193 (77.2) |
| Death due to other cancer | 1 (0.4) |
| Death due to other disease (recurrence+) | 2 (0.8) |
| Death due to other disease (recurrence−) | 54 (21.6) |
| Death due to other disease (recurrence?) | 0 (0.0) |
| Operative death* | 4 (1.6) |
| Postoperative hospital death** | 0 (0.0) |
| Total of death cases | 250 |
*, operative death refers to death within 30 days after operation in or out of hospital. The operative mortality rate was 0.73%; **, postoperative hospital death is defined as death during the same hospitalization, regardless of department at time of death. The hospital death rate in our center was 0.
In multivariate Cox regression analysis, male gender, deeper tumor invasion layers, positive lymph nodes metastasis, and induction of neoadjuvant therapy were prognostic factors associated with poor OS and CSS both in esophagectomy cohort and ESCC cohort (Tables S3,S4).
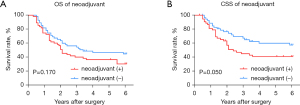
In the subtype analysis for the ESCC cohort (Figure 10), after matching according to clinical stage using the PSM method, a total of 55 pairs of patients with neoadjuvant therapy or not under a similar clinical staging background were analyzed. There was no divergence in OS between patients who accepted neoadjuvant therapy and patients who did not (P=0.170), however, as for CSS, patients who underwent esophagectomy without neoadjuvant therapy showed a better survival rate over patients who underwent preoperative inducement plus surgery (P=0.049).
Discussion
This annual report summarized the baseline characteristics, tumor pathological results, and surgery-related outcomes of esophageal cancer patients in SCH. The results demonstrated that following standard surgical quality control in a large-scale esophageal surgery center, relatively satisfactory tumor treatment results can be obtained, including good surgical risk control and long-term survival.
In recent years, the basic biological characteristics of esophageal cancer patients in China has changed, including a later age of onset and better nutritional conditions (16,17). This is closely related to the improvement of medical living conditions in China. However, other parameters have changed little, such as the increasing number of squamous cell carcinoma patients, more tumors located in the middle esophagus, and the advanced stage at primary diagnosis.
In our limited number of endoscopically treated patients, the R0 resection rate was only 79%. Although our previous study showed that positive lateral margins did not affect long-term prognosis (18), for patients with deep invasion to the submucosa, or those patients with muscularis mucosae invasion and positive LVI, adjuvant therapy is still necessary. The Japan Clinical Oncology Group Study (JCOG0508) clinical trial provided adequate salvage therapy recommendations for patients with low-risk submucosal invasion (19), but it remains unknown whether patients with more generalized submucosal invasion will necessarily achieve satisfactory long-term tumor control with nonsurgical management. Our previous study has shown that reoperation is safe for patients who have failed endoscopic therapy (20), however, a further study named Ad-ESD trial is warranted to verify these results (21).
In China, the left thoracic operation (Sweet approach) has dominated esophageal surgery for decades (22). In many town hospitals, surgeons can complete a Sweet operation for esophageal squamous cell carcinoma in 2 hours. However, with the pursuit of total mediastinal lymph node dissection, especially the rise of minimally invasive surgery (23,24), the McKeown and Ivor Lewis surgery of the right thoracic approach has gradually occupied the mainstream of esophageal cancer surgery in China. This study demonstrated that in 2016, the proportion of minimally invasive surgery in SCH exceeded 50%, and the right thoracic surgery approach exceeded 80%. The adoption of new surgical approaches, especially minimally invasive surgery, may increase surgical risks and reoperation rates (25). However, this report demonstrated that the surgical safety of the entire cohort was very stable, with less than 1% of operative death within 30 days being the norm in large centers in China.
The 5-year survival rate of patients undergoing R0 resection was 54.6% in our center. This result is similar to that of another surgical treatment retrospective report from a Chinese multicenter (17). However, compared with Japan’s 2013 annual report, in which the 5-year survival of surgically treated patients was 59.5% (26), our long-term survival results were not very satisfactory (Table 19). To explain this disparity, we conducted a comparative analysis of the two groups of people. There are two main differences in the basic composition of the populations and the intervention methods. First, the proportion of pT1 in Japanese patients reached 39%, while in our group of patients, it was only 17.6%. Furthermore, there were more early-stage patients in the Japanese group. The second difference is the proportion of patients receiving neoadjuvant therapy, which was 37.5% in the Japanese cohort compared to 11% in the SCH cohort, meaning that almost all Japanese patients above T3 received preoperative treatment. Despite the proportion of patients above pT3 in our group being close to 60%, the current use of neoadjuvant was only 11%. We suggest that earlier staging confers better survival compared to neoadjuvant therapy. A similar conclusion can be drawn among pT2 patients, where neoadjuvant therapy was rarely used in both groups, and the surgical results in our cohort was superior to that observed in the Japanese group. For pT3 and pT4a, the two most common local progression for tumors, preoperative neoadjuvant therapy was commonly used in Japan, while it was less frequently administered by the Shanghai team. Our results were comparable to those in Japan. Therefore, for the entire population, the proportion of early-stage patients is a more critical factor in determining the ultimate survival outcome.
Table 19
| pT | 2013 Japanese annual report | 2016 SCH annual report | |||
|---|---|---|---|---|---|
| Proportions | Survival rate | Proportions | Survival rate | ||
| pT0/Tis | 3.3% | 81.1% | 4.7% | 49.7% | |
| pT1a | 12% | 81.6% | 3.3% | 100% | |
| pT1b | 27% | 76% | 14.3% | 78% | |
| pT2 | 12.4% | 56.4% | 19.6% | 64.5% | |
| pT3 | 38.7% | 43.4% | 47.8% | 41.8% | |
| pT4a | 0.8% | 24.5% | 4.1% | 32.7% | |
| pT4b | 2.2% | 15.2% | 6.1% | 12.9% | |
SCH, Shanghai Chest Hospital.
Both the CROSS and NEROTEC5010 studies clearly showed that preoperative neoadjuvant improved OS in the intervention group (27,28), but neither group of patients were routinely given postoperative adjuvant therapy, establishing a highly unbalanced setting in terms of full perioperative care. Although the JCOG9907 trial clearly confirmed that preoperative neoadjuvant chemotherapy is superior to postoperative adjuvant therapy (29), the control group only received two cycles of chemotherapy after surgery. Obviously, the course and dose of chemotherapy were very weak. Moreover, in the postoperative adjuvant therapy group of patients in the JCOG9907 trial, none of the pN0 patients received adjuvant therapy, and all the neoadjuvant group received neoadjuvant chemotherapy. From this point of view, there was obvious selection bias in the study. In our patients, the postoperative adjuvant therapy was mainly radiotherapy and chemoradiotherapy, with the intensity being significantly higher than that of the postoperative adjuvant group in the JCOG9907 trial. In our study, we found that OS in the neoadjuvant treatment group was even worse than that in the primary surgery population, which may reflect a very narrow window of benefit from neoadjuvant treatment. Furthermore, the JCOG9907 trial concluded that neoadjuvant treatment was more effective in patients in clinical stage II, or cases involving the upper and the middle third of the esophagus, or cases which do not invade the deeper layers. The development of novel neoadjuvant treatment regimens with high intensity is warranted and immunotherapy may be an important future direction for preoperative treatment (30,31).
This report summarized the real-world data of esophageal cancer surgical treatment in a high-volume single center of China. The use of standardized surgical treatment and minimally invasive surgery has helped patients obtain acceptable early and long-term postoperative outcomes. However, there were some limitations in this study. First, the present study was a real-world observational-retrospective study, and bias might exist in the reporting and collection of patient information. Second, the included operations were performed by more than 10 different surgeons. Although a few surgeons accounted for the vast majority of operations, some bias in medical treatment by different surgeons may have influenced the estimate in the overall therapeutic outcomes. Third, the proportion of patients receiving neoadjuvant therapy was low at SCH in 2016, since the NEOCRTEC5010 trial comparing neoadjuvant chemoradiotherapy plus surgery versus surgery alone was not published until 2018 (32) and included in the Chinese guidelines; in other words, neoadjuvant therapy was not recommended in China during our study period [2016]; for this reason, most patients in our study with locally advanced stage esophageal cancer usually received postoperative adjuvant therapy. This situation has changed dramatically since 2020, and the current proportion of neoadjuvant patients has exceeded 40%. Our future work will recruit more early-stage patients for individualized neoadjuvant therapy, with the aim of improving treatment outcomes.
Conclusions
Through a standardized surgical procedure, surgical treatment conferred acceptable short- and long-term results in a high-volume reference center in China. The 5-year OS and CSS rate were 51.8% and 59.1%, respectively. The efficacy of neoadjuvant therapy was not tested in this cohort for limited number of patients, as the surgery plus adjuvant therapy was the preferred treatment regimen for locally advanced stage patients in 2016. This report reveals important surgical treatment effects of esophageal cancer patients and provides helpful data for their clinical management and future treatment development.
Acknowledgments
Funding: This work was supported by the Shanghai Esophageal Cancer Cohort Database of Shanghai Hospital Development Center (No. SHDC2020CR6002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1672/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1672/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1672/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the Shanghai Chest Hospital (No. IS21111). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020;159:335-349.e15. [Crossref] [PubMed]
- McGuill MJ, Byrne P, Ravi N, et al. The prognostic impact of occult lymph node metastasis in cancer of the esophagus or esophago-gastric junction: systematic review and meta-analysis. Dis Esophagus 2008;21:236-40. [Crossref] [PubMed]
- Liu YP, Ma L, Wang SJ, et al. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol 2010;36:155-9. [Crossref] [PubMed]
- Watanabe M, Toh Y, Ishihara R, et al. Comprehensive registry of esophageal cancer in Japan, 2014. Esophagus 2022;19:1-26. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Falcoz PE, Brunelli A. The European general thoracic surgery database project. J Thorac Dis 2014;6:S272-5. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- He Y, Liang D, Du L, et al. Clinical characteristics and survival of 5283 esophageal cancer patients: A multicenter study from eighteen hospitals across six regions in China. Cancer Commun (Lond) 2020;40:531-44. [Crossref] [PubMed]
- Wouters MW, Wijnhoven BP, Karim-Kos HE, et al. High-volume versus low-volume for esophageal resections for cancer: the essential role of case-mix adjustments based on clinical data. Ann Surg Oncol 2008;15:80-7. [Crossref] [PubMed]
- Voeten DM, Gisbertz SS, Ruurda JP, et al. Overall Volume Trends in Esophageal Cancer Surgery Results From the Dutch Upper Gastrointestinal Cancer Audit. Ann Surg 2021;274:449-58. [Crossref] [PubMed]
- Speicher PJ, Englum BR, Ganapathi AM, et al. Traveling to a High-volume Center is Associated With Improved Survival for Patients With Esophageal Cancer. Ann Surg 2017;265:743-9. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Wu Y, Li Y, Giovannucci E. Potential Impact of Time Trend of Lifestyle Risk Factors on Burden of Major Gastrointestinal Cancers in China. Gastroenterology 2021;161:1830-1841.e8. [Crossref] [PubMed]
- Mao YS, Gao SG, Wang Q, et al. Analysis of a registry database for esophageal cancer from high-volume centers in China. Dis Esophagus 2020;33:doz091. [Crossref] [PubMed]
- Liu Z, Li Z. Letter to the Editor: Comparison of Outcomes Between Additional Esophagectomy After Noncurative Endoscopic Resection and Upfront Esophagectomy for T1N0 Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2021;28:837-8.
- Kurokawa Y, Muto M, Minashi K, et al. A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan Clinical Oncology Group Study JCOG0508. Jpn J Clin Oncol 2009;39:686-9. [Crossref] [PubMed]
- Liu Z, Zhang J, Su Y, et al. Additional Esophagectomy Following Noncurative Endoscopic Resection for Early Esophageal Squamous Cell Carcinoma: A Multicenter Retrospective Study. Ann Surg Oncol 2021;28:7149-59. [Crossref] [PubMed]
- Yang Y, Su Y, Zhang X, et al. Esophagectomy versus definitive chemoradiotherapy for patients with clinical stage N0 and pathological stage T1b esophageal squamous cell carcinoma after endoscopic submucosal dissection: study protocol for a multicenter randomized controlled trial (Ad-ESD Trial). Trials 2020;21:603. [Crossref] [PubMed]
- Mao YS, He J, Cheng GY. Current status of surgical management of esophageal cancer in China and the future strategy. Zhonghua Zhong Liu Za Zhi 2010;32:401-4.
- Zhu C, Jin K. Minimally invasive esophagectomy for esophageal cancer in the People's Republic of China: an overview. Onco Targets Ther 2013;6:119-24. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. Updated experiences with minimally invasive McKeown esophagectomy for esophageal cancer. World J Gastroenterol 2015;21:12873-81. [Crossref] [PubMed]
- Oshikiri T, Takiguchi G, Miura S, et al. Current status of minimally invasive esophagectomy for esophageal cancer: Is it truly less invasive? Ann Gastroenterol Surg 2019;3:138-45. [Crossref] [PubMed]
- Watanabe M, Tachimori Y, Oyama T, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus 2021;18:1-24. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Yang Y, Zhu L, Cheng Y, et al. Three-arm phase II trial comparing camrelizumab plus chemotherapy versus camrelizumab plus chemoradiation versus chemoradiation as preoperative treatment for locally advanced esophageal squamous cell carcinoma (NICE-2 Study). BMC Cancer 2022;22:506. [Crossref] [PubMed]
- de Castro G Junior, Segalla JG, de Azevedo SJ, et al. A randomised phase II study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial. Eur J Cancer 2018;88:21-30. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
(English Language Editor: J. Teoh)