Prognosis of the second predominant subtype in lung adenocarcinoma: a retrospective single-center cohort study
Highlight box
Key findings
• In the low-grade group, patients had shorter DFS while the high-grade group was the second predominant subtype. In the intermediate and high-grade groups, the low-grade group, as the second major subtype, has a certain protective effect on patients' OS.
What is known and what is new?
• In previous studies, patients in the low-grade group had a better prognosis, while patients in the intermediate and high-grade groups had a worse prognosis.
• We analyzed and compared the influence of the second predominant subtype group on the prognosis and survival of patients in different levels of groups.
What is the implication, and what should change now?
• Our study shows that in different grade groups of lung adenocarcinoma, the second predominant subtype may also influence the prognosis of patients. In the future clinical treatment process, attention should also be paid to the second subtype.
Introduction
Lung cancer is a common malignant tumor with a high mortality rate (1), and lung adenocarcinoma (LUAD) is currently the most common histological subtype of non-small cell lung cancer (2). A new classification method of lung cancer was provided by the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the International Association for the Study of Lung Cancer (IASLC) in 2011 (3) and was published by the World Health Organization (WHO) in 2015 (4). The pathological features and clinical behaviors of LUAD have been described and verified by experts, and after several investigations, the main subtypes were divided into three groups: The low-grade subtype group (including the lepidic subtype), the intermediate-grade subtype group (including acinar and papillary subtypes), and the high-grade subtype group (including solid and micropapillary subtypes) (5,6). In daily clinical practice, pure adenocarcinoma of a single subtype is relatively rare, and most are comprised of at least two or more subtypes. In these LUADs, a predominant pathological subtype can be determined via a semiquantitative analysis in 5% increments. Subsequently, the second predominant pathological subtype can also be determined. The second predominant subtype was defined as the pathological subtype accounting for the second-largest percentage of lung adenocarcinoma. While the prognostic effects of the predominant pathological subtypes of LUAD have been confirmed (7-12), the possible prognostic effects of the second predominant pathological subtypes have rarely been mentioned in the literature, and there are no consistent results among relevant studies (13-15).
In our study, the main objective was to examine the prognostic influence of the second predominant subtype group on the overall survival (OS) and disease-free survival (DFS) of patients. The secondary aim was to identify whether the second predominant subtype group was the independent prognostic factor for LUAD. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1524/rc).
Methods
Patients
We collected data from patients January 2015 to December 2018. All patients underwent surgical treatment in Tianjin Chest Hospital. Postsurgical pathological diagnoses were confirmed as LUAD, and relevant information was recorded preoperatively, during the operation, and postoperatively, as shown in Table 1.
Table 1
| Variables | The entire cohort | Group 1 | Group 2 | Group 3 | P value |
|---|---|---|---|---|---|
| Male, n (%) | 138 (47.1) | 74 (43.8) | 41 (46.1) | 23 (65.7) | 0.059 |
| Mean age (years) (± SD) | 61.9 (±7.8) | 61.2 (±7.8) | 60.5 (±7.8) | 60.5 (±7.8) | 0.672 |
| Smoking status, n (%) | 0.210 | ||||
| Active | 70 (23.9) | 32 (24.2) | 24 (32.4) | 14 (45.2) | |
| Former | 26 (8.9) | 16 (12.1) | 7 (9.5) | 3 (9.7) | |
| Never | 141 (48.1) | 84 (63.6) | 43 (58.1) | 14 (45.2) | |
| Missing | 56 (19.1) | ||||
| Mean BMI (kg/m2) (± SD) | 24.8 (±9.3) | 25.3 (±11.9) | 24.0 (±3.2) | 24.4 (±3.3) | 0.439 |
| Previous tumor history, n (%) | 0.987 | ||||
| Yes | 35 (11.9) | 20 (11.8) | 11 (12.4) | 4 (11.4) | |
| Diabetes, n (%) | 0.029 | ||||
| Yes | 31 (10.6) | 20 (11.8) | 4 (4.5) | 7 (20.0) | |
| Hypertension, n (%) | 0.427 | ||||
| Yes | 105 (35.8) | 65 (38.5) | 27(30.3) | 13 (37.1) | |
| CHD, n (%) | 0.272 | ||||
| Yes | 42 (14.3) | 25 (14.8) | 15 (16.9) | 2 (5.7) | |
| Respiratory diseases, n (%) | 0.393 | ||||
| Yes | 40 (13.7) | 27 (16.0) | 9 (10.1) | 4 (11.4) | |
| location, n (%) | 0.313 | ||||
| Upper lobe | 177 (61.0) | 101 (60.5) | 55 (62.5) | 21 (60.0) | |
| Middle lobe | 14 (4.8) | 8 (4.8) | 2 (2.3) | 4 (11.4) | |
| Middle lobe | 99 (34.1) | 58 (34.3) | 31 (35.2) | 10 (28.6) | |
| Type of resection, n (%) | 0.962 | ||||
| Segmentectomy | 9 (3.1) | 5 (3.0) | 3 (3.4) | 1 (2.9) | |
| Pulmonary lobectomy | 278 (94.9) | 160 (94.7) | 85 (95.5) | 33 (94.3) | |
| Pneumonectomy | 6 (2.0) | 4 (2.3) | 1 (1.1) | 1 (2.9) | |
| Surgical technique, n (%) | 0.886 | ||||
| Open | 13 (4.4) | 8 (4.7) | 4 (4.5) | 1 (2.9) | |
| Minimally invasive (VATS) | 280 (95.6) | 161 (95.3) | 85 (95.5) | 34 (97.1) | |
| The second predominant subtype, n (%) | NA | ||||
| Low-grade | 71 (24.2) | 59 (66.3) | 12 (34.3) | ||
| Intermediate-grade | 123 (42.0) | 100 (59.2) | 23 (65.7) | ||
| High-grade | 99 (33.8) | 69 (40.8) | 30 (33.7) | ||
| Mean tumor diameter, cm (± SD) | 2.6 (±1.3) | 2.4 (±1.4) | 2.7 (±1.0) | 3.1 (±1.3) | 0.001 |
| UICC stage, n (%) | 0.026 | ||||
| I | 174 (59.4) | 112 (66.3) | 49 (55.1) | 13 (37.1) | |
| II | 44 (15.0) | 21 (12.4) | 16 (18.0) | 7 (20.0) | |
| III | 67 (22.9) | 33 (19.5) | 22 (24.7) | 12 (34.3) | |
| IV | 8 (2.7) | 3 (1.8) | 2 (2.2) | 3 (8.6) | |
| Nodal status, n (%) | 0.008 | ||||
| N0 | 208 (71.0) | 132 (78.1) | 58 (65.2) | 18 (51.4) | |
| N1 | 29 (9.9) | 14 (8.3) | 11 (12.4) | 4 (11.4) | |
| N2 | 56 (19.1) | 23 (13.6) | 20 (22.5) | 13 (37.1) | |
| Visceral pleural invasion, n (%) | 0.709 | ||||
| Yes | 191 (65.2) | 109 (64.5) | 57 (64.0) | 25 (71.4) | |
| Tumor communicates with bronchus, n (%) | 0.004 | ||||
| Yes | 85 (29.0) | 38 (22.5) | 30 (33.7) | 17 (48.6) | |
| Tumor thrombus in the vascular lumen, n (%) | 0.363 | ||||
| Yes | 233 (79.5) | 132 (78.1) | 75 (84.3) | 26 (74.3) |
Group 1 is the low-grade predominant group, which includes lepidic subtypes. Group 2 is the intermediate-grade predominant group, which includes papillary and acinar nodule subtypes. Group 3 is the high-grade predominant group, which includes micropapillary and solid subtypes. CHD, coronary heart disease; BMI, body mass index; VATS, video-assisted thoracic surgery; UICC, International Union Against Cancer; SD, standard deviation.
For the homogenization of patients, all in our study underwent maximum anatomic lobectomy (i.e., segmentectomy, pulmonary lobectomy, and pneumonectomy). In addition, we excluded cases of mural pleural metastases, pure adenocarcinoma, invasive mucinous adenocarcinoma, and patients in whom the predominant pathological subtype group and second predominant pathological subtype group could not be distinguished based on their proportions.
According to the individual, surgical techniques were selected between traditional thoracotomy and minimally invasive techniques [such as video-assisted thoracic surgery (VATS)].
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. The study was approved by Tianjin Chest Hospital ethics board (No. 2021KY-012-02).
Histological classification
Pathological results confirmed the samples according to IASLC/ATS/ERS classification criteria. The proportions of pathological subtypes in each section were determined based on the principle of a semiquantitative analysis in 5% increments, and the second predominant pathological subtype was then determined. Patients were excluded if the percentages of the predominant pathological subtype group and the second predominant pathological subtype group were identical. All pathologic results were graded according to the eighth edition of the International Union against Cancer (UICC)/American Joint Committee on Cancer TNM classification.
We then divided patients into three groups as follows: Group 1, in which the second predominant subtype groups were the intermediate-grade group and the high-grade group; group 2, in which the second predominant subtype groups were the low-grade group and high-grade group; and group 3, in which the second predominant subtype groups were the low-grade group and the intermediate-grade group.
Follow-up
We stipulated a time period from the end of surgery to the date of death for any reason or to the last follow-up as the follow-up period. The follow-up time was recorded in months and was determined until September 2021. The methods of follow-up included telephone follow-up, letter follow-up, or outpatient follow-up.
Statistical methods
Data analysis was performed using IBM SPSS 26.0 software (IBM SPSS INC.). The means with standard deviations or medians with ranges were used for representing the continuous variables, whereas categorical variables are represented by frequencies. The comparison of the categorical variables was performed by utilizing the two-tailed Pearson χ2 test, whereas continuous variables were analyzed using the Student’s t test. The time from the day of surgical treatment to the first recurrence event that occurred or to the last follow-up was regarded as DFS. In the same manner, the time from the day of surgical treatment to the time of death for any reason or to the last follow-up was regarded as OS. Kaplan-Meier method was used to perform a survival analysis and survival curve plot, and survival and recurrence times were evaluated. Univariate analysis was performed for survival differences via the log-rank test. Moreover, a Cox proportional risk regression model was used for the multivariate analysis of possible preoperative and postoperative prognostic factors. As only variables with significant influences in the univariate analysis were used in the multivariate analysis, the “second predominant subtype” group was included in the multivariate analysis. P value ≤0.05 was considered as a statistically significant variable and covariates were recorded using their risk ratios and 95% confidence intervals (see Tables 2,3 for details).
Table 2
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| The entire cohort | |||||
| Male gender | 0.134 | 1.385 (0.904–2.120) | |||
| Age (years) | 0.707 | 0.995 (0.968–1.022) | |||
| Smoking status | <0.001 | 1.442 (1.184–1.757) | 0.018 | 1.269 (1.042–1.547) | |
| BMI | 0.295 | 1.008 (0.993–1.022) | |||
| Previous tumor history | 0.583 | 1.194 (0.634–2.251) | |||
| Diabetes | 0.135 | 0.502 (0.203–1.239) | |||
| Hypertension | 0.107 | 0.681 (0.427–1.087) | |||
| CHD | 0.111 | 0.552 (0.267–1.145) | |||
| Respiratory diseases | 0.001 | 0.438 (0.265–0.724) | 0.004 | 0.458 (0.268–0.782) | |
| Location | 0.7 | 0.956 (0.759–1.203) | |||
| Type of resection | 0.131 | 2.433 (0.767–7.720) | |||
| Surgical technique | 0.001 | 0.293 (0.147–0.586) | 0.002 | 0.331 (0.162–0.677) | |
| Second predominant subtype group | 0.985 | 0.997 (0.751–1.324) | 0.262 | 0.855 (0.651–1.124) | |
| Tumor size | <0.001 | 1.474 (1.283–1.694) | 0.008 | 1.281 (1.067–1.537) | |
| UICC stage | <0.001 | 1.938 (1.573–2.388) | 0.053 | 1.476 (0.994–2.192) | |
| Nodal status | <0.001 | 1.904 (1.515–2.393) | 0.722 | 1.074 (0.723–1.596) | |
| Visceral pleural invasion | 0.002 | 2.295 (1.363–3.865) | 0.171 | 1.478 (0.845–2.584) | |
| Tumor communicates with bronchus | 0.001 | 2.073 (1.348–3.186) | 0.127 | 1.455 (0.899–2.354) | |
| Tumor thrombus in the vascular lumen | 0.007 | 2.703 (1.304–5.600) | 0.349 | 1.442 (0.671–3.101) | |
| Group 1 | |||||
| Male gender | 0.495 | 1.242 (0.667–2.311) | |||
| Age | 0.812 | 0.995 (0.957–1.035) | |||
| Smoking status | 0.002 | 1.592 (1.192–2.216) | 0.02 | 1.415 (1.056–1.895) | |
| BMI | 0.191 | 1.010 (0.995–1.024) | |||
| Previous tumor history | 0.454 | 1.394 (0.584–3.324) | |||
| Diabetes | 0.082 | 0.172 (0.024–1.251) | |||
| Hypertension | 0.032 | 0.456 (0.223–0.935) | 0.189 | 0.610 (0.292–1.275) | |
| CHD | 0.222 | 0.524 (0.186–1.477) | |||
| Respiratory diseases | 0.001 | 0.320 (0.164–0.632) | 0.028 | 0.249 (0.202–0.912) | |
| Location | 0.88 | 1.026 (0.734–1.434) | |||
| Type of resection | 0.124 | 3.026 (0.736–12.737) | |||
| Surgical technique | 0.067 | 0.381 (0.135–1.070) | |||
| Second predominant subtype group | 0.041 | 1.959 (1.028–3.732) | 0.944 | 0.972 (0.446–2.119) | |
| Tumor size | 0.001 | 1.413 (1.157–1.726) | 0.254 | 1.174 (0.891–1.546) | |
| UICC stage | <0.001 | 2.067 (1.532–2.805) | 0.004 | 2.022 (1.249–3.274) | |
| Nodal status | <0.001 | 1.872 (1.333–2.629) | 0.701 | 0.901 (0.530–1.532) | |
| Visceral pleural invasion | 0.125 | 1.751 (0.856–3.584) | |||
| Tumor communicates with bronchus | 0.097 | 1.753 (0.903–3.402) | |||
| Tumor thrombus in the vascular lumen | 0.058 | 2.722 (0.968–7.654) | |||
| Group 2 | |||||
| Male gender | 0.18 | 1.682 (0.787–3.596) | |||
| Age | 0.769 | 0.993 (0.947–1.041) | |||
| Smoking status | 0.154 | 1.291 (0.909–1.835) | |||
| BMI | 0.283 | 1.065 (0.949–1.196) | |||
| Previous tumor history | 0.468 | 0.586 (0.139–2.478) | |||
| Diabetes | 0.38 | 1.907 (0.451–8.057) | |||
| Hypertension | 0.599 | 1.246 (0.557–2.760) | |||
| CHD | 0.876 | 0.919 (0.317–2.659) | |||
| Respiratory diseases | 0.389 | 0.627 (0.217–1.814) | |||
| Location | 0.517 | 0.889 (0.592–1.336) | |||
| Type of resection | 0.741 | – | |||
| Surgical technique | 0.001 | 0.164 (0.056–0.483) | 0.004 | 0.181 (0.056–0.587) | |
| Second predominant subtype group | 0.434 | 1.169 (0.791–1.728) | 0.473 | 1.168 (0.764–1.787) | |
| Tumor size | 0.042 | 1.408 (1.012–1.960) | 0.16 | 1.334 (0.892–1.994) | |
| UICC stage | 0.001 | 1.844 (1.273–2.669) | 0.885 | 1.068 (0.434–2.632) | |
| Nodal status | 0.001 | 2.012 (1.339–3.024) | 0.319 | 1.587 (0.640–3.931) | |
| Visceral pleural invasion | 0.007 | 4.315 (1.480–12.580) | 0.013 | 4.071 (1.344–12.382) | |
| Tumor communicates with bronchus | 0.094 | 1.908 (0.896–4.061) | |||
| Tumor thrombus in the vascular lumen | 0.1 | 5.350 (0.726–39.443) | |||
| Group 3 | |||||
| Male gender | 0.444 | 0.690 (0.267–1.785) | |||
| Age | 0.836 | 1.006 (0.949–1.067) | |||
| Smoking status | 0.709 | 1.085 (0.707–1.665) | |||
| BMI | 0.166 | 0.892 (0.760–1.048) | |||
| Previous tumor history | 0.095 | 2.954 (0.829–10.524) | |||
| Diabetes | 0.303 | 0.462 (0.106–2.010) | |||
| Hypertension | 0.521 | 0.724 (0.270–1.942) | |||
| CHD | 0.43 | – | |||
| Respiratory diseases | 0.245 | 0.474 (0.135–1.667) | |||
| Location | 0.827 | 0.944 (0.561–1.588) | |||
| Type of resection | 0.369 | 2.556 (0.330–19.819) | |||
| Surgical technique | 0.013 | 0.030 (0.002–0.447) | 0.066 | 0.068 (0.004–1.192) | |
| Second predominant subtype group | 0.317 | 0.615 (0.238–1.592) | 0.29 | 0.568 (0.199–1.619) | |
| tumor size | 0.008 | 1.498 (1.110–2.022) | 0.011 | 1.542 (1.105–2.152) | |
| UICC stage | 0.168 | 1.404 (0.867–2.272) | |||
| Nodal status | 0.244 | 1.353 (0.813–2.249) | |||
| Visceral pleural invasion | 0.324 | 1.756 (0.573–5.378) | |||
| Tumor communicates with bronchus | 0.222 | 1.814 (0.698–4.715) | |||
| Tumor thrombus in the vascular lumen | 0.262 | 2.038 (0.588–7.067) | |||
Group 1 is the low-grade predominant group, which includes lepidic subtypes. Group 2 is the intermediate-grade predominant group, which includes papillary and acinar nodule subtypes. Group 3 is the high-grade predominant group, which includes micropapillary and solid subtypes. CHD, coronary heart disease; BMI, body mass index; VATS, video-assisted thoracic surgery; UICC, International Union Against Cancer; HR, hazard ratio; CI, confidence interval.
Table 3
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| The entire cohort | |||||
| Male gender | 0.134 | 1.385 (0.904–2.120) | |||
| Age (years) | 0.707 | 0.995 (0.968–1.022) | |||
| Smoking status | <0.001 | 1.442 (1.184–1.757) | 0.018 | 1.269 (1.042–1.547) | |
| BMI | 0.295 | 1.008 (0.993–1.022) | |||
| Previous tumor history | 0.583 | 1.194 (0.634–2.251) | |||
| Diabetes | 0.135 | 0.502 (0.203–1.239) | |||
| Hypertension | 0.107 | 0.681 (0.427–1.087) | |||
| CHD | 0.111 | 0.552 (0.267–1.145) | |||
| Respiratory diseases | 0.001 | 0.438 (0.265–0.724) | 0.004 | 0.458 (0.268–0.782) | |
| Location | 0.7 | 0.956 (0.759–1.203) | |||
| Type of resection | 0.131 | 2.433 (0.767–7.720) | |||
| Surgical technique | 0.001 | 0.293 (0.147–0.586) | 0.002 | 0.331 (0.162–0.677) | |
| Second predominant subtype group | 0.985 | 0.997 (0.751–1.324) | 0.262 | 0.855 (0.651–1.124) | |
| Tumor size | <0.001 | 1.474 (1.283–1.694) | 0.008 | 1.281 (1.067–1.537) | |
| UICC stage | <0.001 | 1.938 (1.573–2.388) | 0.053 | 1.476 (0.994–2.192) | |
| Nodal status | <0.001 | 1.904 (1.515–2.393) | 0.722 | 1.074 (0.723–1.596) | |
| Visceral pleural invasion | 0.002 | 2.295 (1.363–3.865) | 0.171 | 1.478 (0.845–2.584) | |
| Tumor communicates with bronchus | 0.001 | 2.073 (1.348–3.186) | 0.127 | 1.455 (0.899–2.354) | |
| Tumor thrombus in the vascular lumen | 0.007 | 2.703 (1.304–5.600) | 0.349 | 1.442 (0.671–3.101) | |
| Group 1 | |||||
| Male gender | 0.495 | 1.242 (0.667–2.311) | |||
| Age | 0.812 | 0.995 (0.957–1.035) | |||
| Smoking status | 0.002 | 1.592 (1.192–2.216) | 0.02 | 1.415 (1.056–1.895) | |
| BMI | 0.191 | 1.010 (0.995–1.024) | |||
| Previous tumor history | 0.454 | 1.394 (0.584–3.324) | |||
| Diabetes | 0.082 | 0.172 (0.024–1.251) | |||
| Hypertension | 0.032 | 0.456 (0.223–0.935) | 0.189 | 0.610 (0.292–1.275) | |
| CHD | 0.222 | 0.524 (0.186–1.477) | |||
| Respiratory diseases | 0.001 | 0.320 (0.164–0.632) | 0.028 | 0.249 (0.202–0.912) | |
| Location | 0.88 | 1.026 (0.734–1.434) | |||
| Type of resection | 0.124 | 3.026 (0.736–12.737) | |||
| Surgical technique | 0.067 | 0.381 (0.135–1.070) | |||
| Second predominant subtype group | 0.041 | 1.959 (1.028–3.732) | 0.944 | 0.972 (0.446–2.119) | |
| Tumor size | 0.001 | 1.413 (1.157–1.726) | 0.254 | 1.174 (0.891–1.546) | |
| UICC stage | <0.001 | 2.067 (1.532–2.805) | 0.004 | 2.022 (1.249–3.274) | |
| Nodal status | <0.001 | 1.872 (1.333–2.629) | 0.701 | 0.901 (0.530–1.532) | |
| Visceral pleural invasion | 0.125 | 1.751 (0.856–3.584) | |||
| Tumor communicates with bronchus | 0.097 | 1.753 (0.903–3.402) | |||
| Tumor thrombus in the vascular lumen | 0.058 | 2.722 (0.968–7.654) | |||
| Group 2 | |||||
| Male gender | 0.18 | 1.682 (0.787–3.596) | |||
| Age | 0.769 | 0.993 (0.947–1.041) | |||
| Smoking status | 0.154 | 1.291 (0.909–1.835) | |||
| BMI | 0.283 | 1.065 (0.949–1.196) | |||
| Previous tumor history | 0.468 | 0.586 (0.139–2.478) | |||
| Diabetes | 0.38 | 1.907 (0.451–8.057) | |||
| Hypertension | 0.599 | 1.246 (0.557–2.760) | |||
| CHD | 0.876 | 0.919 (0.317–2.659) | |||
| Respiratory diseases | 0.389 | 0.627 (0.217–1.814) | |||
| Location | 0.517 | 0.889 (0.592–1.336) | |||
| Type of resection | 0.741 | – | |||
| Surgical technique | 0.001 | 0.164 (0.056–0.483) | 0.004 | 0.181 (0.056–0.587) | |
| Second predominant subtype group | 0.434 | 1.169 (0.791–1.728) | 0.473 | 1.168 (0.764–1.787) | |
| Tumor size | 0.042 | 1.408 (1.012–1.960) | 0.16 | 1.334 (0.892–1.994) | |
| UICC stage | 0.001 | 1.844 (1.273–2.669) | 0.885 | 1.068 (0.434–2.632) | |
| Nodal status | 0.001 | 2.012 (1.339–3.024) | 0.319 | 1.587 (0.640–3.931) | |
| Visceral pleural invasion | 0.007 | 4.315 (1.480–12.580) | 0.013 | 4.071 (1.344–12.382) | |
| Tumor communicates with bronchus | 0.094 | 1.908 (0.896–4.061) | |||
| Tumor thrombus in the vascular lumen | 0.1 | 5.350 (0.726–39.443) | |||
| Group 3 | |||||
| Male gender | 0.444 | 0.690 (0.267–1.785) | |||
| Age | 0.836 | 1.006 (0.949–1.067) | |||
| Smoking status | 0.709 | 1.085 (0.707–1.665) | |||
| BMI | 0.166 | 0.892 (0.760–1.048) | |||
| Previous tumor history | 0.095 | 2.954 (0.829–10.524) | |||
| Diabetes | 0.303 | 0.462 (0.106–2.010) | |||
| Hypertension | 0.521 | 0.724 (0.270–1.942) | |||
| CHD | 0.43 | – | |||
| Respiratory diseases | 0.245 | 0.474 (0.135–1.667) | |||
| Location | 0.827 | 0.944 (0.561–1.588) | |||
| Type of resection | 0.369 | 2.556 (0.330–19.819) | |||
| Surgical technique | 0.013 | 0.030 (0.002–0.447) | 0.066 | 0.068 (0.004–1.192) | |
| Second predominant subtype group | 0.317 | 0.615 (0.238–1.592) | 0.29 | 0.568 (0.199–1.619) | |
| tumor size | 0.008 | 1.498 (1.110–2.022) | 0.011 | 1.542 (1.105–2.152) | |
| UICC stage | 0.168 | 1.404 (0.867–2.272) | |||
| Nodal status | 0.244 | 1.353 (0.813–2.249) | |||
| Visceral pleural invasion | 0.324 | 1.756 (0.573–5.378) | |||
| Tumor communicates with bronchus | 0.222 | 1.814 (0.698–4.715) | |||
| Tumor thrombus in the vascular lumen | 0.262 | 2.038 (0.588–7.067) | |||
Group 1 is the low-grade predominant group, which includes lepidic subtypes. Group 2 is the intermediate-grade predominant group, which includes papillary and acinar nodule subtypes. Group 3 is the high-grade predominant group, which includes micropapillary and solid subtypes. CHD, coronary heart disease; BMI, body mass index; VATS, video-assisted thoracic surgery; UICC, International Union Against Cancer; HR, hazard ratio; CI, confidence interval.
Results
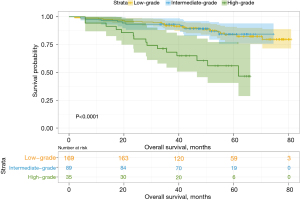
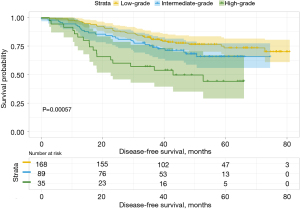
Among a total of 293 patients, 169 (57.7%) were included in the low-grade predominant subtype group, 89 (30.4%) were included in the intermediate-grade predominant subtype group, and 35 (11.9%) were included in the high-grade predominant subtype group. In the predominant pathological subtypes and their subtype groups, OS and DFS were significantly different (Figures 1,2), while for the entire cohort, the 3- and 5-year DFS rates were 76.4% and 67.3%, respectively, and the 3- and 5-year OS rates were 89.7% and 80.5%, respectively. These results were consistent with those of other studies. The 3- and 5-year OS and DFS rates for the second predominant group are recorded in Table 4.
Table 4
| Group | The second predominant group | Rate of OS (%) | Rate of DFS (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 3-year | 5-year | P value | 3-year | 5-year | P value | |||
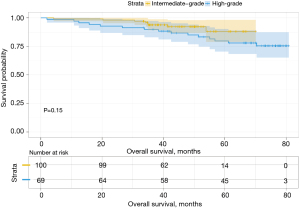
| 1 | Intermediate-grade | 93.8 | 88.0 | 86.5 | 86.5 | |||
| High-grade | 89.8 | 79.8 | 0.150 | 72.4 | 65.2 | 0.037 | ||
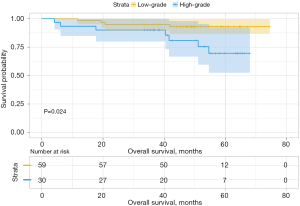
| 2 | Low-grade | 94.9 | 93.0 | 79.4 | 69.4 | |||
| High-grade | 90.0 | 69.6 | 0.024 | 69.0 | 62.1 | 0.300 | ||
| 3 | Low-grade | 50.0 | 31.3 | 41.7 | 41.7 | |||
| Intermediate-grade | 77.4 | 70.4 | 0.033 | 65.2 | 45.5 | 0.312 | ||
Group 1 is the low-grade predominant group, which includes lepidic subtypes. Group 2 is the intermediate-grade predominant group, which includes papillary and acinar nodule subtypes. Group 3 is the high-grade predominant group, which includes micropapillary and solid subtypes. OS, overall survival; DFS, disease-free survival.
Information on the characteristics of all patients is recorded in Table 1. Of the entire cohort, male patients were the minority group (138, 47.1%), and the mean age was 62 years (±7.8). In addition, there were 237 smokers (at the time of intervention, 70 patients were still smokers, accounting for 23.9% and 26 were former smokers, accounting for 8.9%), and a history of previous tumors was seen in 35 patients (24 benign and 11 malignant). Pulmonary lobectomy was performed in 278 patients (94.9%), anatomic segmentectomy in nine (3.1%), and left or right pneumonectomy in six patients (2.0%). All patients underwent systematic lymph node dissection.
Histologically, the distribution of the second predominant subtype group was demonstrated and showed that in the low-grade predominant subtype group, the second predominant subtype group included 100 cases (59.2%) of the intermediate-grade group and 69 cases (40.8%) of the high-grade group. Among the intermediate-grade predominant subtypes, the second predominant subtype group included 59 cases (66.3%) of the low-grade groups and 30 cases (33.7%) of the high-grade groups. In the high-grade predominant subtype, the second predominant subtype group included 12 cases (34.3%) in the intermediate-grade subtype group and 23 cases (65.7%) in the intermediate-grade subtype group. As shown in Table 1, the perioperative period and pathological characteristics were similar in the three groups, but the distribution of diabetes (P=0.029), tumor diameter (P=0.001), UICC stage (P=0.026), N stage (P=0.008), and tumor communication with the bronchus (P=0.004) were significantly different. These factors were more common in patients in the low-grade predominant subtype group. In contrast, male sex (P=0.059), age (P=0.672), smoking status (P=0.210), mean BMI (P=0.439), history of cancer (P=0.987), history of hypertension (P=0.427), history of coronary heart disease (P=0.272), history of respiratory disease (P=0.393), tumor location (P=0.313), resection method (P=0.962), surgical method (P=0.886), right visceral pleural invasion (P=0.709), or tumor embolism (P=0.363) were not significantly different among all of the groups.
Results of survival recurrence analysis for the entire cohort
In the entire cohort, the median follow-up time was 49.3 months (range: 2–80.9 months). In different predominant subtype groups, the 3- and 5-year OS rates for the low-grade predominant subtype group were 92.2% and 83.8%, respectively; the 3- and 5-year OS rates for the intermediate-grade predominant subtype group were 93.3% and 84.4%, respectively; and the 3- and 5-year OS rates for the high-grade predominant subtype group were 68.2% and 55.9%, respectively. The 3- and 5-year DFS rates were 80.6% and 73.2% in the low-grade predominant subtype group, 76.0% and 66.5% in the intermediate-grade predominant subtype group, and 57.1% and 44.1% in the high-grade predominant subtype group, respectively.
Analysis of the influence of different second predominant subtype groups on OS, produced various results. For example, when we considered the low-grade group as the predominant subtype group, the second predominant groups were the intermediate-grade group and the high-grade group. The 3- and 5-year OS rates were 93.8% and 88.0% in the intermediate-grade group and 89.8% and 79.8% in the high-grade group (Table 4), respectively. Moreover, their difference in OS was not statistically significant (P=0.15, Figure 3). Subsequently, we regarded the intermediate-grade group as the predominant subtype group, wherein the second predominant subtype groups were the low-grade group and high-grade group. Their 3- and 5-year OS rates were 94.9% and 93.0% in the low-grade group and 90.0% and 69.6% in the high-grade group (Table 4), respectively, and their difference in OS was statistically significant (P=0.024, Figure 4). In the same manner, we considered the high-grade group as the predominant subtype group, in which the second predominant subtype groups were the low-grade group and intermediate-grade group. The influence of the second dominant subtype on OS was also statistically significant (P=0.033, Figure 5). Moreover, the 3- and 5-year OS rates were 50.0% and 31.3% in the low-grade group and 77.4% and 70.4% in the intermediate-grade group (Table 4), respectively.
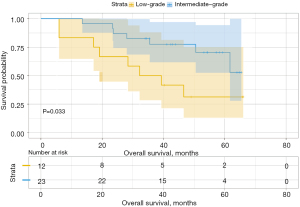
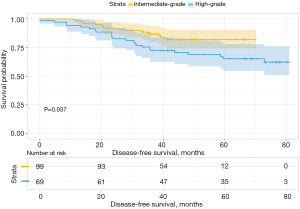
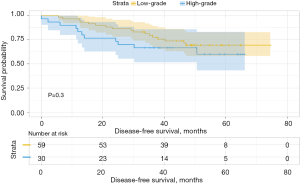
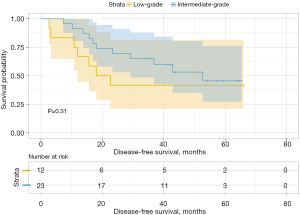
We then analyzed the influence of the second group on DFS and found that when the second predominant groups were the intermediate-grade group and high-grade group, they had statistical significance for DFS in this group (P=0.037; Figure 6). Additionally, the 3- and 5-year DFS rates were 86.5% and 86.5% in the intermediate-grade group and 72.4% and 65.2% in the high-grade group (Table 4), respectively. However, when the low-grade group and the high-grade group were the second predominant subtype, there was no significant influence on DFS in the subgroup (P=0.3, Figure 7), and their 3- and 5-year DFS rates were 79.4% and 69.4% in the low-grade group and 69.0% and 62.1% in the high-grade group (Table 4), respectively. When the low-grade group and the intermediate-grade group were the second predominant subtype, no significant influence on the restructured DFS was seen (P=0.31, Figure 8). In this scenario, their 3- and 5-year DFS rates were 41.7% and 41.7% in the low-grade group and 65.2% and 45.5% in the intermediate-grade group (Table 4), respectively.
Univariate analysis showed smoking status (P=0.003), BMI (P=0.037), previous respiratory disease (P=0.007), minimally invasive surgical method (P=0.041), tumor size (P<0.001), UICC stage (P<0.001), N stage (P<0.001), visceral pleural invasion (P=0.038), and communication between the tumor and bronchus (P=0.001) obviously affected OS. In the multivariate analysis, only smoking status (P=0.045), prior respiratory disease (P=0.021), and tumor size (P=0.001) confirmed the prognostic effects (Table 2). For DFS, smoking history (P<0.001), previous respiratory disease (P=0.001), surgical method (P=0.001), tumor diameter (P<0.001), UICC stage (P<0.001), N stage (P<0.001), visceral pleural invasion (P=0.002), tumor communication with the bronchus (P=0.001), and tumor thrombus in the vascular lumen (P=0.007) were significant factors. In the multivariate analysis, only smoking status (P=0.018), previous respiratory disease (P=0.004), surgical method (P=0.002), and tumor diameter (P=0.008) had a significant influence on DSF.
Group 1 results
Group 1 comprised 169 patients.
As summarized in Table 2, the univariate analysis showed no significant differences in prognostic factors that might affect OS, which included the male sex (P=0.574), age (P=0.560), lymphatic vascular invasion (P=0.228), resection method (P=0.616), minimally invasive surgery vs. traditional open surgery (P=0.448), previous tumor history (P=0.781), diabetes (P=0.650), coronary heart disease (P=0.288), location (P=0.657), visceral pleural invasion (P=0.072), tumor communication with the bronchus (P=0.192), and tumor thrombus found in the vascular lumen (P=0.141). In contrast, smoking history, BMI, hypertension, previous respiratory disease, tumor size, UICC stage, and N stage had significant adverse effects on DFS (P=0.002, P=0.015, P=0.012, P=0.004, P=0.001, P<0.001, and P=0.003, respectively). However, in the multivariate analysis, only smoking history (P=0.020; HR: 1.613, 95% CI: 1.078–2.415) was confirmed to have adverse prognostic effects on OS.
The univariate analysis of DSF prognosis showed male sex (P=0.495), age (P=0.812), BMI (P=0.191), previous tumor history (P=0.454), history of diabetes (P=0.082), history of coronary heart disease (P=0.222), location (P=0.880), resection method (P=0.124), surgical technique (P=0.067), visceral pleural invasion (P=0.125), tumor communication with the bronchus (P=0.097), and tumor thrombus in the vascular lumen (P=0.058) had no significant influence on the prognosis of DFS. The prognosis of DFS was significantly affected by smoking status (P=0.002), history of hypertension (P=0.032), history of previous respiratory diseases (P=0.001), second predominant pathological subtype group (P=0.041), tumor size (P=0.001), UICC stage (P<0.001), and N stage (P<0.001). In the multivariate analysis, smoking status (P=0.020; HR: 1.415, 95% CI: 1.056–1.895), respiratory diseases (P=0.028; HR: 0.249, 95% CI: 0.202–0.912), and UICC stage (P=0.004; HR: 2.022, 95% CI: 1.249–3.274) were confirmed to have an adverse prognostic effect on DFS.
Group 2 results
This subgroup included 89 patients.
The univariate analysis showed male sex (P=0.027), surgical technique (P=0.025), second predominant pathological subtype group (P=0.036), tumor size (P=0.004), UICC stage (P=0.005), and N stage (P=0.005) had a significant influence on OS, and the influence of other factors was not statistically significant. Only the surgical technique (P=0.031; HR: 0.145, 95% CI: 0.025–0.837) demonstrated an effect on the prognosis in the multivariate analysis.
The univariate analysis of the influence of DFS showed other factors had statistically significant effects on it, except for surgical technique (P=0.001), tumor size (P=0.042), UICC stage (P=0.001), N stage (P=0.001), and visceral pleural invasion (P=0.007). Tumor size (P=0.004, HR: 0.181, 95% CI: 0.056–0.587) and visceral pleural invasion (P=0.013; HR: 4.071, 95% CI: 1.344–12.382) were confirmed to be important prognostic factors affecting DFS in the multivariate analysis.
Group 3 results
The cohort of the high-grade group consisted of 35 patients.
The prognostic factors that might affect OS showed no statistical significance, except for the surgical technique (P=0.013), the second predominant pathological subtype group (P=0.042), and the tumor size (P=0.046). In the multivariate analysis, the second predominant pathological subtype group (P=0.037; HR: 0.297, 95% CI: 0.095–0.927), and tumor size (P=0.035; HR: 1.548, 95% CI: 1.033–2.429) confirmed their prognostic effect on OS in this group of patients.
The univariate analysis of prognostic factors that may affect DFS showed male sex (P=0.444), age (P=0.836), smoking status (P=0.709), BMI (P=0.166), previous tumor history (P=0.095), diabetes (P=0.303), hypertension (P=0.521), coronary heart disease (P=0.430), history of respiratory diseases (P=0.245), location (P=0.872), resection method (P=0.369), second predominant pathological subtype group (P=0.371), UICC stage (P=0.168), N stage (P=0.244), visceral pleural invasion (P=0.324), tumor communication with the bronchus (P=0.222), and tumor thrombus in the vascular lumen (P=0.262) showed no significant effect on the prognosis. In contrast, surgical technique (P=0.013) and tumor size (P=0.024) were significantly correlated with DFS. The multivariate analysis showed that only tumor size (P=0.011; HR: 1.542, 95% CI: 1.105–2.152) confirmed its prognostic effect on DFS.
Discussion
The classification of lung adenocarcinoma published by the WHO in 2015 was revised (4). After this revision, the diagnosis and treatment of the disease have become increasingly standardized (16). In this new classification, each of the different histological subtypes has been shown to have different growth characteristics and aggressiveness, which are associated with long-term OS and DFS (8,17-20).
In previous studies, most researchers have focused on the prognostic effects of the predominant subtypes of LUAD. Warth et al. (21) found these had significant effects on OS and DFS, and the lepidic predominant subtype ADC had the best prognosis, followed by the acinar type, solid type, papillary type, and micropapillary type. Moreover, Da Cruz et al. (22) found that patients with solid pathological subtypes were related to poor OS and short progression-free survival, especially in stage IV lung LUAD. Additionally, in the study of Motono et al. (23), in patients with Stage I LUAD, the high-grade predominant subtype group was significantly associated with a poor prognosis. In our study, the analysis of these factors found different predominant subtypes did have different long-term OS and DSF effects. Specifically, the low-grade group had a better prognosis (2), and the high-grade group, which includes solid and micropapillary tumors, was frequently associated with a poor prognosis (16,24-27). This is consistent with results from previous studies.
Regarding the investigation of the second predominant pathological subtype, Bertoglio et al. (13) found the high-grade second predominant subtype was often associated with a poor prognosis as its proportion increased, while in an earlier study by Ito et al. (14) evaluting the intermediate-grade dominant subtype, the lepidic subtype and nonlepidic subtype components could be used as factors for further grading and exhibited significance for prognoses. Furthermore, Bertoglio et al. (15) found that as the second predominant subtype, the lepidic subtype had a better level of DFS, whereas the micropapillary type often predicted a poorer prognosis. Recently, Tsai et al. (28) found no significant difference in DFS between the two groups, regardless of whether the second pathological subtype was invasive (acinar, papillary, micropapillary, and solid) or noninvasive (lepidic).
Our results showed that when a predominant subtype group was determined, the influence of the second predominant pathological subtype on the OS and DFS of different groups could be summarized as follows. For the low-grade predominant subtypes (i.e., the lepidic subtype), the intermediate-grade group, as the second predominant subtype, had significantly better DFS (P=0.037) than the high-grade group, and in terms of OS, the intermediate-grade group (acinar and/or papillary subtypes) showed a better prognosis than the high-grade group (solid and/or micropapillary subtypes). However, our study did not observe statistically significant results (P=0.15). When we considered the intermediate-grade predominant subtype group, the analysis showed the low-grade group had a better prognostic effect on OS than the high-grade group as the second predominant subtype (P=0.024), while in terms of DFS, although the low-grade group exhibited some advantages, the results were not statistically significant (P=0.3). For the high-grade predominant subtype group analysis, we observed a strange phenomenon. When the low-grade group was the second predominant pathological subtype, its long-term OS for this group of patients was worse than the intermediate-grade second predominant subtype (P=0.033). For DFS, although the low-grade second predominant subtype group also had a poorer prognosis than the intermediate-grade second predominant subtype, the results were still not statistically significant (P=0.31).
Tumor size was observed to be an independent prognostic factor in several studies exploring the OS and DFS of patients with LUAD (3,29). In our study cohort, tumor size was solely identified as having its prognostic effect for OS and DFS in all cohorts but was distributed differently in the subgroups. In previous study, the distribution of tumor size in different predominant subtypes has been described, which was confirmed in our cohort study because the low-grade predominant subtype group < intermediate-grade predominant subtype group < high-grade predominant subtype group (P=0.001) (13).
Nevertheless, our study has several limitations. First, this was a single-center study, and the results require verification by multicenter studies. Second, the independent prognostic effect of the second predominant subtype was not confirmed in our study, and the analysis results were not statistically significant. There are several possible explanations for this effect. The first may be related to patient loss to follow-up, which is also one of the inevitable problems in retrospective analyses. The second reason may be attributed to the small number of patients analyzed, which can be further followed up and supplemented via multicenter studies. Finally, for the abnormal phenomenon in the high-grade dominant group, we suspect anomalies can be explained because of the small sample size.
Conclusions
In summary, our analysis indicates the influence of the second predominant pathological subtype on the OS and DFS of LUAD patients is inconsistent among the different predominant subtype groups. The intermediate-grade and high-grade groups showed aggressiveness, but their sensitivity to chemoradiotherapy was confirmed to some extent. Low-grade groups also showed protection against different predominant subtypes. Therefore, in follow-up diagnosis and treatment, the prediction of malignant outcomes and postoperative treatment should not solely consider the predominant subtypes. Similarly, the second predominant pathological subtype should also be of clinical and pathological concern. Large-scale prospective studies concerning the classification of treatment and monitoring of modalities for patients with different grades should be conducted in the future.
Acknowledgments
Funding: This work was supported by the Natural Funding Project of Tianjin Science and Technology Bureau (No. 20JCYBJC01350).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1524/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1524/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1524/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. The study was approved by Tianjin Chest Hospital ethics board (No. 2021KY-012-02).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsao MS, Marguet S, Le Teuff G, et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J Clin Oncol 2015;33:3439-46. [Crossref] [PubMed]
- Jeon HW, Kim YD, Sim SB, et al. Prognostic impact according to the proportion of the lepidic subtype in stage IA acinar-predominant lung adenocarcinoma. Thorac Cancer 2021;12:2072-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Fujikawa R, Muraoka Y, Kashima J, et al. Clinicopathologic and Genotypic Features of Lung Adenocarcinoma Characterized by the International Association for the Study of Lung Cancer Grading System. J Thorac Oncol 2022;17:700-7. [Crossref] [PubMed]
- Boland JM, Wampfler JA, Yang P, et al. Growth pattern-based grading of pulmonary adenocarcinoma—analysis of 534 cases with comparison between observers and survival analysis. Lung Cancer 2017;109:14-20. [Crossref] [PubMed]
- Murakami S, Ito H, Tsubokawa N, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015;90:199-204. [Crossref] [PubMed]
- Campos-Parra AD, Avilés A, Contreras-Reyes S, et al. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur Respir J 2014;43:1439-47. [Crossref] [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol 2015;33:2877-84. [Crossref] [PubMed]
- Bertoglio P, Querzoli G, Ventura L, et al. Prognostic impact of lung adenocarcinoma second predominant pattern from a large European database. J Surg Oncol 2021;123:560-9. [Crossref] [PubMed]
- Ito M, Miyata Y, Yoshiya T, et al. Second predominant subtype predicts outcomes of intermediate-malignant invasive lung adenocarcinoma. Eur J Cardiothorac Surg 2017;51:218-22. [Crossref] [PubMed]
- Bertoglio P, Cattoni M, Nachira D, et al. P2.17-29 impact of second predominant pattern on recurrence in early stage resected lung adenocarcinoma: a multicentric study. J Thorac Oncol 2019;14:S895-6.
- Rokutan-Kurata M, Yoshizawa A, Ueno K, et al. Validation Study of the International Association for the Study of Lung Cancer Histologic Grading System of Invasive Lung Adenocarcinoma. J Thorac Oncol 2021;16:1753-8. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [Crossref] [PubMed]
- Hung JJ, Yeh YC, Jeng WJ, et al. Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol 2014;32:2357-64. [Crossref] [PubMed]
- Casteillo F, Guy JB, Dal-Col P, et al. Pathologic Subtypes of Lung Adenocarcinoma Brain Metastasis Is a Strong Predictor of Survival After Resection. Am J Surg Pathol 2018;42:1701-7. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Da Cruz V, Yvorel V, Casteillo F, et al. Histopathological subtyping is a prognostic factor in stage IV lung adenocarcinoma. Lung Cancer 2020;147:77-82. [Crossref] [PubMed]
- Motono N, Matsui T, Machida Y, et al. Prognostic significance of histologic subtype in pStage I lung adenocarcinoma. Med Oncol 2017;34:100. [Crossref] [PubMed]
- Sung YE, Lee KY, Moon Y. The prognostic utility of the histologic subtype of stage I lung adenocarcinoma may be diminished when using only the invasive component to determine tumor size for tumor node metastasis (TNM) staging. J Thorac Dis 2021;13:2910-22. [Crossref] [PubMed]
- Miyahara N, Nii K, Benazzo A, et al. Solid predominant subtype in lung adenocarcinoma is related to poor prognosis after surgical resection: A systematic review and meta-analysis. Eur J Surg Oncol 2019;45:1156-62. [Crossref] [PubMed]
- Park S, Lee SM, Kim S, et al. Volume Doubling Times of Lung Adenocarcinomas: Correlation with Predominant Histologic Subtypes and Prognosis. Radiology 2020;295:703-12. [Crossref] [PubMed]
- Yeh YC, Kadota K, Nitadori J, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification predicts occult lymph node metastasis in clinically mediastinal node-negative lung adenocarcinoma. Eur J Cardiothorac Surg 2016;49:e9-e15. [Crossref] [PubMed]
- Tsai PC, Liu C, Yeh YC, et al. Prognostic histologic subtyping of dominant tumor in resected synchronous multiple adenocarcinomas of lung. Sci Rep 2021;11:9539. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [Crossref] [PubMed]
(English Language Editor: B. Draper)