
The efficacy and safety of wedge resection for peripheral stage IA lung adenocarcinoma: a real-world study based on a single center
Highlight box
Key findings
• Wedge resection can be considered a safe and effective treatment for a subset of peripheral lung adenocarcinoma (LUAD) patients with stage IA.
What is known and what is new?
• Anatomical lobectomy or segmentectomy are standard treatment for stage IA non-small cell lung cancer (NSCLC). However, the efficacy of wedge resection for peripheral stage IA NSCLC remains controversial.
What is the implication, and what should change now?
• Wedge resection may provide similar efficacy versus anatomical lobectomy or segmentectomy for stage IA LUAD patients whose maximum dimension of consolidation component (MCD), the consolidation-to-tumor ratio of the maximum dimension of the tumor (CTR), and CT value of the tumor (CTVt) less than 10 mm, 60%, and −220 HU simultaneously.
Introduction
With the widespread use of high-resolution computed tomography (HRCT) scans and increased screening awareness, a growing number of lung cancer patients whose imaging manifestation were ground glass nodules (GGN), have been diagnosed at early stages (1,2). According to the results of the randomized phase III trial from the Lung Cancer Study Group, lobectomy plus systematic lymph node dissection has been the standard treatment for clinical T1N0M0 non-small cell lung cancer (NSCLC) for over 20 years (3). However, the results of more recent studies have demonstrated that lobectomy may not be the optimal surgical approach for all patients with early T1-stage NSCLC (4,5), suggesting that surgical alternatives to anatomical lobectomy may include a less invasive wedge resection (6-12). Wedge resection offers many potential benefits by preserving lung parenchyma and pulmonary function and lowering the risk of perioperative morbidity. It offers the possibility of curative therapy for patients who may not tolerate a lobectomy as well as those who present with postoperative recurrence or a secondary primary lung cancer (11,13). Although the data suggest that recurrence in patients with stage IA NSCLC who undergo wedge resection is higher than that of patients undergoing lobectomy, it is not significant (11,12). For these reasons, the indications for wedge resection in early-stage NSCLC has undergone exploration in recent years to improve the prognosis and quality of life of patients with early-stage NSCLC (14-16). However, the efficacy and safety of wedge resection for peripheral stage IA NSCLC remain controversial.
The purpose of this study was to assess the efficacy and safety of wedge resection among patients presenting with peripheral stage IA lung adenocarcinoma (IA-LUAD). A secondary aim was to identify predictors of recurrence after wedge resection for IA-LUAD and calculate cutoffs for these predictors. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1010/rc).
Methods
Between January 2014 and December 2017, 196 consecutive patients who received wedge resection by video-assisted thoracoscopic surgery (VATS) in Shanghai Pulmonary Hospital were considered for inclusion in this retrospective study. Patients were diagnosed with pathological stage IA-LUAD by immunohistochemistry (IHC) in accordance to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system (8th edition) (17), and the World Health Organization (WHO) classification of tumors of the lung (4th edition) (18) postoperatively. At the first postoperative follow-up, no behavior of lung cancer was observed on computed tomography (CT) scan. The exclusion criteria were: (I) patient had an oncologic history; (II) the minimum distance between the tumor and resection margin was less than the maximum dimension of the tumor or 20 mm, whichever was less; (III) patient had received chemotherapy and/or radiotherapy postoperatively before recurrence; (IV) patient with LUAD nodules more than 1. Ten patients were excluded to yield a final study cohort of 186 patients. Patients underwent intentional wedge resection only when: (I) a multi-disciplinary team comprised thoracic surgeons, oncologists, pathologists, and radiologists, diagnosed the tumor as stage I LUAD, and (II) the tumor was located in the lung periphery and a wedge resection could achieve a sufficient surgical margin (≥ the maximum tumor dimension or 20 mm). In addition, patients who could not tolerate anatomical lobectomy or segmentectomy for comorbidities or other self-limited reasons underwent a compromised wedge resection. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Shanghai Pulmonary Hospital (No. K20-433) and informed consent was taken from all the patients.
Clinical and pathological parameters of patients were sorted by gender, diagnostic age, tumor laterality, concomitant disease, postoperative complications, the IHC diagnosis upgraded from intraoperative frozen section (IFS), pathological subtypes of LUAD, and the status of any driver genomic mutations. Radiological parameters were reviewed from each patient’s presenting inspiratory HRCT and abstracted as the maximum dimension of the entire tumor (MTD) and that of its consolidation component (MCD), the mean computed tomography value of the tumor (CTVt) and that of its consolidation component (CTVc), and the consolidation-to-tumor ratio of the maximum dimension of the tumor (CTR). Lung windows were used to measure the MTD and CTVt, and mediastinal windows were used to measure the MCD and CTVc.
Statistical analysis
Categorical and continuous variables were compared between recurrence and non-recurrence patients by the chi-square test and unpaired t-test, respectively. Predictors for recurrence were analyzed by Cox proportional hazards modeling. The efficacy of MTD, MCD, CTVt, and CTR in predicting recurrence was calculated by receiver operating characteristic (ROC) curve analysis. Recurrence-free survival (RFS) was calculated from the time of surgery to disease relapse (LUAD relapse was confirmed by pathological and/or radiological analysis). All patients were followed to death, last contact, or an end date of December 31, 2021. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed by SPSS Statistics 26 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of patients
Baseline characteristics are listed in Table 1. A total of 186 patients were included. The mean age was 59.9 years, and the number of female patients outnumbered male patients (115 vs. 71). On preoperative HRCT, the mean MTD was 14.4 mm (range, 5.9 to 29.5 mm), the mean MCD was 5.6 mm (range, 0 to 28.3 mm), the mean CTVt was −285.4 HU (range, −851.1 to 55.7 HU), and the mean CTR was 0.37. The numbers of patients with pure ground glass nodules (pGGN), part-solid ground glass nodules (mGGN), and solid nodules (SN) were 88, 73 and 25, respectively. After preoperative evaluation by the surgical team, 21 patients (11.3%) underwent wedge resection because they could not tolerate an anatomic resection, including 6, 10 and 5 patients with pGGN, mGGN, and SN, respectively. On pathological analysis, there were 24 patients (12.9%) whose subtypes included micropapillary (MPA) and/or solid (SPA) features; 18 (9.7%) patients were diagnosed as LUAD by IHC, which was upgraded from adenocarcinoma in situ (AIS) and microinvasive adenocarcinoma (MIA) by IFS.
Table 1
| Variables | Values |
|---|---|
| Age (years), mean (range) | 59.9 (26, 82) |
| Tumor size (mm), mean (range) | |
| MTD | 14.4 (5.9, 29.5) |
| MCD | 5.6 (0, 28.3) |
| CTVt (HU), range | −285.4 (−851.1, 55.7) |
| Gender, n (%) | |
| Male | 71 (38.2) |
| Female | 115 (61.8) |
| Number of nodules, n (%) | |
| Single | 136 (73.1) |
| Multiple | 50 (26.9) |
| Feature of nodule, n (%) | |
| Pure GGN | 88 (47.31) |
| Mixed GGN | 73 (39.25) |
| Solid nodule | 25 (13.44) |
| Laterality, n (%) | |
| Left | 85 (45.7) |
| Right | 101 (54.3) |
| Pathological upgrading, n (%) | |
| Positive | 18 (9.7) |
| Negative | 168 (90.3) |
| Pathological subtype, n (%) | |
| With MPA/SPA component | 24 (12.9) |
| Without MPA/SPA component | 162 (87.1) |
| Drive gene mutation, n (%) | |
| Positive | 82 (44.1) |
| Negative | 49 (26.3) |
| Unknown | 55 (29.6) |
| Selection of surgery, n (%) | |
| Intentional surgery | 165 (88.7) |
| Compromised surgery | 21 (11.3) |
| Recurrence, n (%) | |
| Positive | 10 (5.4) |
| Negative | 176 (94.6) |
| Recurrence rate (%), [n] | |
| 1-year | 0.54 [1] |
| 3-year | 3.76 [7] |
| 5-year | 4.84 [9] |
LUAD, lung adenocarcinoma; MTD, maximum dimension of the entire tumor; MCD, maximum consolidation component of the tumor; CTVt, mean computed tomography value of the tumor; GGN, ground glass nodules; MPA/SPA, micropapillary or solid component.
All patients survived their operation and no major surgical-related complications were observed within 90 days postoperatively. Postoperative recurrence was observed in 10 patients. The numbers of patients with distant and local recurrence were 6 and 4, respectively. With a median follow-up of 67 months (interquartile range, 52–72 months), the 1-, 3-, and 5-year recurrence rates were 0.54%, 3.76%, and 4.84%, respectively. Among the 165 patients receiving planned wedge resections, 7 developed a recurrence, of which 6 recurred within 5 years (5-year recurrence rate: 3.64%). Among the other 21 patients who underwent a compromised wedge resection due to their inability to tolerate an anatomic resection, 3 developed recurrences within 5 years postoperatively (5-year recurrence rate: 14.29%).
Predictors of recurrence
The mean MTD (14.1 vs. 19.4 mm, P=0.002), CTVt (−300 vs. −28.4 HU, P=0.001), and CTR (34.1% vs. 89.8%, P<0.001) were significantly lower in non-recurrence patients as compared to in recurrence patients (Table 2). Additionally, there were significant differences in the proportion of the following imaging features as seen on HRCT between recurrence and non-recurrence patients: spicules margin (recurrence vs. non-recurrence: 90.0% vs. 55.7%, P=0.046), lobulated shape (recurrence vs. non-recurrence: 100.0% vs. 58.0%, P=0.007), and vascular passage (recurrence vs. non-recurrence: 20.0% vs. 67.6%, P=0.004). However, neither pathological subtypes (MPA or SPA) nor pathologic diagnosis of IHC upgraded from IFS was observed to be significantly associated with postoperative recurrence.
Table 2
| Variables | Non-recurrence (n=176) | Recurrence (n=10) | P value |
|---|---|---|---|
| Age (years), mean | 59.5 | 66.4 | 0.052 |
| Gender, n (%) | 0.903 | ||
| Male | 67 (38.1) | 4 (40.0) | |
| Female | 109 (61.9) | 6 (60.0) | |
| Nodule, n (%) | 0.730 | ||
| Single | 129 (73.3) | 7 (70.0) | |
| Multiple | 47 (26.7) | 3 (30.0) | |
| Tumor size (mm), mean | |||
| MTD | 14.1 | 19.4 | 0.002 |
| MCD | 5.0 | 17.5 | 0.647 |
| CTVt (HU) | −300.0 | −28.4 | 0.001 |
| CTR (%) | 34.1 | 89.8 | <0.001 |
| Laterality, n (%) | 0.779 | ||
| Left | 80 (45.5) | 5 (50.0) | |
| Right | 96 (54.5) | 5 (50.0) | |
| Pathologic upgrading, n (%) | 0.602 | ||
| Positive | 158 (89.8) | 10 (100.0) | |
| Negative | 18 (10.2) | 0 | |
| Pathologic subtype, n (%) | 0.620 | ||
| With MPA/SPA | 22 (12.5) | 2 (20.0) | |
| Without MPA/SPA | 154 (87.5) | 8 (80.0) | |
| Drive gene mutation, n (%) | 0.090 | ||
| Positive | 75 (42.6) | 7 (70.0) | |
| Negative/unknown | 101 (57.4) | 3 (30.0) | |
| Pleural indentation, n (%) | 0.620 | ||
| Positive | 66 (37.5) | 2 (20.0) | |
| Negative | 110 (62.5) | 8 (80.0) | |
| Spicules of margin, n (%) | 0.046 | ||
| Positive | 98 (55.7) | 9 (90.0) | |
| Negative | 78 (44.3) | 1 (10.0) | |
| Lobulated shape, n (%) | 0.007 | ||
| Positive | 102 (58.0) | 10 (100.0) | |
| Negative | 74 (42.0) | 0 | |
| Vacuole sign, n (%) | 0.226 | ||
| Positive | 36 (20.5) | 4 (40.0) | |
| Negative | 140 (79.5) | 6 (60.0) | |
| Vascular passage, n (%) | 0.004 | ||
| Positive | 119 (67.6) | 2 (20.0) | |
| Negative | 57 (33.4) | 8 (80.0) | |
LUAD, lung adenocarcinoma; MTD, maximum dimension of the entire tumor; MCD, maximum consolidation component of the tumor; CTVt, mean computed tomography value of the tumor; CTR, the consolidation-to-tumor ratio of the maximum dimension of the tumor; MPA/SPA, micropapillary or solid component.
Table 3 depicts the results of the univariate analysis by Cox proportional hazards modeling. MTD [hazard ratio (HR) =1.194; 95% confidence interval (CI): 1.078–1.324; P=0.001], MCD (HR =1.212; 95% CI: 1.120–1.311; P<0.001), CTVt (HR =1.012; 95% CI: 1.004–1.019; P=0.004), CTR (HR =1.054; 95% CI: 1.018–1.092; P=0.003), and the imaging features concerning vascular passage on HRCT (HR =0.141; 95% CI: 0.030–0.666; P=0.013) were identified as predictors for recurrence.
Table 3
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age (years) | 1.059 | 0.996–1.126 | 0.065 |
| Gender | |||
| Female | 0.829 | 0.233–2.947 | 0.772 |
| Male | – | – | – |
| Number of primary nodule | |||
| Single | 1.289 | 0.331–5.017 | 0.715 |
| Multiple | – | – | – |
| MTD (mm) | 1.194 | 1.078–1.324 | 0.001 |
| MCD (mm) | 1.212 | 1.120–1.311 | <0.001 |
| CTVt (HU) | 1.012 | 1.004–1.019 | 0.004 |
| CTR (%) | 1.054 | 1.018–1.092 | 0.003 |
| Laterality | |||
| Right | 0.827 | 0.239–2.859 | 0.764 |
| Left | – | – | – |
| Pathologic upgrading | |||
| Positive | 24.143 | 0.004–138,710.739 | 0.471 |
| Negative | – | – | – |
| Pathologic subtype | |||
| Without MPA/SPA | 0.549 | 0.116–2.589 | 0.448 |
| With MPA/SPA | – | – | – |
| Drive gene mutation | |||
| Negative/unknown | 0.322 | 0.083–1.247 | 0.101 |
| Positive | – | – | – |
| Pleural indentation | |||
| Positive | 2.487 | 0.527–11.728 | 0.250 |
| Negative | – | – | – |
| Spicules of margin | |||
| Positive | 7.533 | 0.954–59.467 | 0.055 |
| Negative | – | – | – |
| Lobulated shape | |||
| Positive | 46.210 | 0.238–8,960.132 | 0.154 |
| Negative | – | – | – |
| Vacuole sign | |||
| Positive | 2.428 | 0.684–8.622 | 0.170 |
| Negative | – | – | – |
| Vascular passage | |||
| Positive | 0.141 | 0.030–0.666 | 0.013 |
| Negative | – | – | – |
LUAD, lung adenocarcinoma; HR, hazard ratio; CI, confidence interval; MTD, maximum dimension of the entire tumor; MCD, maximum consolidation component of the tumor; CTVt, mean computed tomography value of the tumor; CTR, the consolidation-to-tumor ratio of the maximum dimension of the tumor; MPA/SPA, micropapillary or solid component.
ROC curve analysis showed that the cutoffs for MCD, CTVt, and CTR were 10.5 mm, −217 HU, and 64%, and the area under the curve (AUC) for these three predictors was 0.908, 0.882, and 0.879 respectively. MCD was the most valuable predictor for recurrence (Figure 1). When the MCD was less than 10 mm, the postoperative recurrence rate of patients with IA-LUAD was 0.69% whereas it was 21.4% in patients whose MCD was greater than 10 mm. Furthermore, for patients with tumors that met all the cutoffs for MCD, CTR, and CTVt simultaneously, no recurrence was observed.
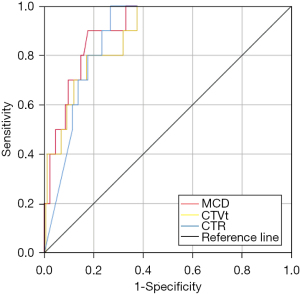
Non-recurrence and recurrence patients after wedge resection
A total of 106 patients whose predictors of recurrence were within the identified safety thresholds were defined as a “selected group” of which none developed a postoperative recurrence (Figure 2).
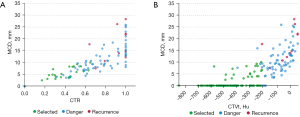
Due to the non-recurrence performance of pGGN in IA-LUAD, patients who had mGGN as their predominant imaging manifestation in selected group and patients occurred recurrence are listed in Table 4. Significant differences were observed between this selected group of patients who had no recurrence (n=21) compared to those who did (n=10). Compared with the mGGN patients in the selected group, the diagnostic age (66.4 vs. 60.2 years, P=0.049), MTD (19.5 vs. 14.7 mm, P=0.006), MCD (17.5 vs. 5.8 mm, P<0.001), CTR (90% vs. 41%, P<0.001), CTVt (−28.4 vs. −335.5 HU, P<0.001), and CTVc (17.3 vs. −138.7 HU, P<0.001) were significantly higher among the patients who developed recurrence. Details about these 10 patients who developed recurrence are presented in Table 5.
Table 4
| Variables | Selected group (n=21) | Recurrence (n=10) | P value |
|---|---|---|---|
| Age (years), mean | 60.2 | 66.4 | 0.049 |
| Gender, n (%) | 0.458 | ||
| Male | 12 (57.1) | 4 (40.0) | |
| Female | 9 (42.9) | 6 (60.0) | |
| Nodule, n (%) | 1.000 | ||
| Single | 15 (71.4) | 7 (70.0) | |
| Multiple | 6 (28.6) | 3 (30.0) | |
| Tumor size (mm), mean | |||
| MTD | 14.7 | 19.5 | 0.006 |
| MCD | 5.8 | 17.5 | <0.001 |
| CTR (%) | 41 | 90 | <0.001 |
| CTV (HU) | |||
| CTVt | −335.5 | −28.4 | <0.001 |
| CTVc | −138.7 | 17.3 | <0.001 |
| Laterality, n (%) | 0.709 | ||
| Left | 9 (42.9) | 5 (50.0) | |
| Right | 12 (57.1) | 5 (50.0) | |
| Pathologic upgrading, n (%) | 0.533 | ||
| Positive | 3 (14.3) | 0 | |
| Negative | 18 (85.7) | 10 (100.0) | |
| Pathologic subtype, n (%) | 0.237 | ||
| With MPA/SPA | 1 (4.8) | 2 (20.0) | |
| Without MPA/SPA | 20 (95.2) | 8 (80.0) | |
| Drive gene mutation, n (%) | 1.000 | ||
| Positive | 13 (61.9) | 7 (70.0) | |
| Negative/unknown | 8 (38.1) | 3 (30.0) | |
| Resected size (mm) | |||
| Maxima | 80.7 | 81.0 | 0.983 |
| Minimal | 23.2 | 21.5 | 0.641 |
| Pleural indentation, n (%) | 0.607 | ||
| Positive | 12 (57.1) | 8 (80.0) | |
| Negative | 9 (42.9) | 2 (20.0) | |
| Spicules of margin, n (%) | 0.106 | ||
| Positive | 12 (57.1) | 9 (90.0) | |
| Negative | 9 (42.9) | 1 (10.0) | |
| Lobulated shape, n (%) | 0.012 | ||
| Positive | 11 (52.4) | 10 (100.0) | |
| Negative | 10 (47.6) | 0 | |
| Vacuole sign, n (%) | 0.417 | ||
| Positive | 5 (23.8) | 4 (40.0) | |
| Negative | 16 (76.2) | 6 (60.0) | |
| Vascular passage, n (%) | 0.003 | ||
| Positive | 16 (76.2) | 2 (20.0) | |
| Negative | 5 (23.8) | 8 (80.0) | |
mGGN, part-solid ground glass nodules; MTD, maximum dimension of the entire tumor; MCD, maximum consolidation component of the tumor; CTR, the consolidation-to-tumor ratio of the maximum dimension of the tumor; CTVt, mean computed tomography value of the tumor; CTVc, the mean computed tomography value of the consolidation component of the tumor; MPA/SPA, micropapillary or solid component.
Table 5
| No. | Gender | Age (years) | Location | MTD (mm) | MCD (mm) | CTVt (HU) | CTVc (HU) | Pathology subtype | Gene mutation | Status [RFS, months] | Recurrent site |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 72 | LUL | 27.6 | 17.7 | −217.1 | 18.1 | APA | L858R | Alive [40] | Pleural |
| 2 | M | 69 | LUL | 10.7 | 7.5 | −156.4 | −44.8 | PPA + MPA | Unknown | Dead [28] | Liver |
| 3 | M | 71 | RLL | 13.5 | 10.7 | −62.8 | 5.9 | LPA | Wild type | Censored [21] | Brain, bone |
| 4 | M | 63 | RLL | 12.2 | 12.2 | −27.7 | −27.7 | SPA + MPA | Wild type | Dead [19] | Brain |
| 5 | F | 75 | LUL | 14.9 | 13.9 | −1.5 | 40.4 | APA | L858R | Alive [27] | Lung |
| 6 | F | 62 | RLL | 14.8 | 14.8 | 9.8 | 9.8 | PPA + SPA | 19-Del | Alive [68] | Bone |
| 7 | F | 66 | LLL | 21.8 | 21.8 | 54.2 | 54.2 | APA | 19-Del | Alive [51] | Lung |
| 8 | F | 59 | LLL | 22.1 | 22.1 | 54.6 | 54.6 | SPA | ROS1 | Alive [14] | Bone |
| 9 | F | 64 | RUL | 28.6 | 26.2 | 34.0 | 34.0 | APA | L858R | Alive [11] | Brain, bone |
| 10 | M | 63 | RUL | 28.3 | 28.3 | 28.5 | 28.5 | APA | L858R | Alive [31] | Lung |
MTD, maximum dimension of the entire tumor; MCD, maximum consolidation component of the tumor; CTVt, mean computed tomography value of the tumor; CTVc, the mean computed tomography value of the consolidation component of the tumor; RFS, recurrence-free survival; F, female; LUL, left upper lobe; APA, acinar predominant LUAD; LUAD, lung adenocarcinoma; M, male; PPA, papillary predominant LUAD; MPA, micropapillary predominant LUAD; RLL, right lower lobe; LPA, lepidic predominant LUAD; SPA, solid predominant LUAD; LLL, left lower lobe; RUL, right upper lobe.
Discussion
In recent years, there has been ongoing debate regarding the optimal surgical approach for patients presenting with IA-LUAD. A few studies have demonstrated equivalent outcomes between lobectomy and sub-lobar resection in this population (6,11). There are several benefits of a wedge resection over an anatomic resection. The former is more likely to preserve postoperative pulmonary function and lung parenchyma (11), reduce the duration of the operation and intraoperative complications, and may be better suited for elderly patients or those with significant comorbidities or other contraindications to an anatomic lobectomy (7). In this retrospective study, we assessed the safety and efficacy of wedge resection for patients with peripheral IA-LUAD and explored the indications for a wedge resection in this population, which may help guide the selection of an optimal surgical strategy.
To our knowledge, previously published prospective randomized clinical trials (RCT) and retrospective studies have mainly focused on the feasibility of sub-lobar resections for patients with IA-LUAD. Prior studies have shown that MTD plays an important role in determining the pathological features of NSCLC (2). Clinical trial JCOG0201 defined non-invasive NSCLC to be a tumor with radiological criteria of CTR 0.25 or less in stage cT1a-b patients (≤20 mm size) (14), and demonstrated the feasibility of a limited surgical resection such as segmentectomy and wedge resection for IA-LUAD with a CTR 0.5 or less in cT1 patients (≤30 mm size) (9,14,19-21). A phase II prospective clinical trial (JCOG0804) is currently evaluating the safety and efficacy of wedge resection for early-stage peripheral NSCLC with a CTR of 0.25 or less (7). Finally, CALGB-140503 has been launched to compare wedge resection and lobectomy for peripheral early-stage NSCLC (22). However, these studies did not assess the predictive factors for recurrence in IA-LUAD patients clearly, nor did they contribute a predictive model to guide the surgical procedure.
The results of our study demonstrated that the most valuable predictive factors for recurrence were MCD, CTR, and CTVt, and no recurrence was observed among peripheral IA-LUAD patients with pGGN after wedge resection. Our ROC curve analysis confirmed that when the imaging features of a peripheral IA-LUAD tumor met the requirements of MCD less than 10 mm, CTR less than 0.6, and CTVt less than −220 HU simultaneously, a wedge resection was not only feasible, but no recurrence was also observed postoperatively. With these radiographic criteria met, no recurrence patient was observed after wedge resection, suggesting that these radiographic criteria were appropriate indicators for a limited resection in patients with IA-LUAD. In addition, for patients with tumor CTR greater than 0.6, recurrence rate after sub-lobar resection was improved over lobectomy, and the former may increase the risk of recurrence in this subset of patients.
Consistent with previous research results, the most important factor for the successful treatment of IA-LUAD is the surgical margin (7,12,23). Based on the experience at our center, in patients with peripheral IA-LUAD, a wedge resection, compared to a segmentectomy or lobectomy, could ensure sufficient distance between nodules and resected margins equally. Moreover, wedge resection is a time-tested and safe surgical technique with many benefits over an anatomical resection such as decreasing the duration of chest tube placement and the inpatient length of stay.
Furthermore, one of the disadvantages of a sub-lobar resection is inadequate lymph node dissection, especially for cT1-LUAD with a solid component. Wedge resection without lymph node sampling is considered to be inadequate even for small and peripheral nodules due to the possibility for locoregional recurrence (6,24-26). Therefore, lymph node sampling plays a crucial role in wedge resection to stage cT1-stage LUAD pathologically and guide adjuvant treatment and follow-up strategies. In this retrospective analysis, all patients received wedge resection combined with regional lymph node sampling or dissection, and the results of their IHC postoperatively confirmed that none of them had lymph node involvement.
It is worth point out that, from a previous study, pathological subtypes (27) and the status of drive-gene mutations (28) may influence the recurrence of early-stage LUAD postoperatively. However, a significant difference in the distribution of these 2 factors between recurrence and non-recurrence patients was not observed in our study, which demonstrated that the status of drive gene mutations and the component of MPA/SPA did not affect recurrence in wedge-treated patients with IA-LUAD postoperatively. This seemingly contradictory phenomenon may be attributed to the fact that there was a relatively small number of patients developed recurrence in our study.
Limitations
There are several limitations to our findings. First, this research is a retrospective analysis based on a single center, which is inherently prone to selection bias. Second, the sample size of this study is small. Third, while a limited resection for early-LUAD has similar 5-year survival as an anatomical resection, it may decrease long-term survival (6). Due to our limited follow-up, this study does not explore outcomes beyond ten years. As mentioned, a larger sample size and a longer follow-up period is warranted and will be the focus of future study. Fourth, this study included 21 patients who should undergo lobectomy according to guideline, but only compromising wedge resection was performed for various reasons, including cardiovascular diseases, limitation of pulmonary function, and others comorbidities. Fifth, the detail information of lymph nodes dissection or sampling was not included. However, patients without lymph nodes sampling were excluded in this study to ensure accurate staging and prognostic information.
Conclusions
Among patients with peripheral IA-LUAD, wedge resection provides a similar recurrence rate and decreased morbidity compared to an anatomic resection. MCD, CTR, and CTVt are predictors for recurrence. The cutoffs for these 3 predictors are 10 mm, 60%, and −220 HU. When these cutoffs were met simultaneously, patients developed no postoperative recurrence over the course of follow-up. A wedge resection for IA-LUAD patients can be performed safely and efficaciously, but larger scale and long-term follow-up are necessary for further study.
Acknowledgments
Funding: This study was supported by the National Key R&D Program of China (No. 2019YFC1315803), and the National Natural Science Foundation of China (No. 82070022).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1010/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1010/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1010/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Shanghai Pulmonary Hospital (No. K20-433), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Hayashi T, et al. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:954-62. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. Identification of early t1b lung adenocarcinoma based on thin-section computed tomography findings. J Thorac Oncol 2013;8:1289-94. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Tanaka K, Tsutani Y, Wakabayashi M, et al. Sublobar resection versus lobectomy for patients with resectable stage I non-small cell lung cancer with idiopathic pulmonary fibrosis: a phase III study evaluating survival (JCOG1708, SURPRISE). Jpn J Clin Oncol 2020;50:1076-9. [Crossref] [PubMed]
- Yerokun BA, Yang CJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:675-86.e4. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Fan X, Liang Y, Bai Y, et al. Conditional survival rate estimates of lobectomy, segmentectomy and wedge resection for stage IA1 non-small cell lung cancer: A population-based study. Oncol Lett 2020;20:1607-18. [Crossref] [PubMed]
- Matsumura Y, Yano M, Yoshida J, et al. Early and late recurrence after intentional limited resection for cT1aN0M0, non-small cell lung cancer: from a multi-institutional, retrospective analysis in Japan. Interact Cardiovasc Thorac Surg 2016;23:444-9. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Xi J, Yin J, Liang J, et al. Prognostic Impact of Radiological Consolidation Tumor Ratio in Clinical Stage IA Pulmonary Ground Glass Opacities. Front Oncol 2021;11:616149. [Crossref] [PubMed]
- Mimae T, Saji H, Nakamura H, et al. Survival of Octogenarians with Early-Stage Non-small Cell Lung Cancer is Comparable Between Wedge Resection and Lobectomy/Segmentectomy: JACS1303. Ann Surg Oncol 2021;28:7219-27. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Speicher PJ, Gu L, Gulack BC, et al. Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States. Clin Lung Cancer 2016;17:47-55. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Qiu ZB, Zhang C, Chu XP, et al. Quantifying invasiveness of clinical stage IA lung adenocarcinoma with computed tomography texture features. J Thorac Cardiovasc Surg 2022;163:805-15.e3. [Crossref] [PubMed]
- Sun K, Li M, Shang M, et al. Impact of genetic status on the survival outcomes of patients with clinical stage I non-small cell lung cancer with a radiological pure-solid appearance. Lung Cancer 2022;166:63-9. [Crossref] [PubMed]


