
Allergen-specific immunotherapy with Alutard SQ improves allergic inflammation in house-dust mites-induced allergic asthma rats through inactivation of the HMGB1/TLR4/NF-κB pathway
Highlight box
Key findings
• AIT with Alutard SQ improves allergic inflammation in HDM -induced allergic asthma rats through inactivation of the HMGB1/TLR4/NF-κB pathway.
What is known and what is new?
• House dust mites induced the allergic asthma.
• AIT with Alutard SQ ameliorates allergic inflammation in HDM-induced allergic asthma rats by suppressing the HMGB1/TLR4/NF-κB pathway.
What is the implication, and what should change now?
• The results of this study can clarify the role and molecular mechanism of AIT with Alutard SQ in ameliorating airway inflammation on HDM-induced allergic asthma.
Introduction
Asthma is a chronic respiratory disease estimated to affect nearly 339 million individuals globally (1). Emerging evidence shows that asthma is associated with airway inflammation and remodeling, mucus production, eosinophilia and lymphocytosis, and bronchospasm, resulting in premature death and lower quality of life of individuals across all ages (2,3). Nearly 30 million people in China have asthma, the majority of whom were diagnosed before the age of 5 years (4). Inhaled corticosteroids are the common drugs for asthma treatment because of their efficacy in controlling asthma exacerbation and progression. However, they are prone to relapse after drug discontinuation or reduction yet, patients require lifelong medication (5). Therefore, there is an urgent need for a novel safe, and effective treatment.
House dust mites (HDMs) are one of the most potent and frequent sources of allergens worldwide, causing allergic diseases, such as allergic rhinoconjunctivitis, allergic asthma, and other allergic skin diseases (6). More than 85% of asthma patients in Europe, Southeast Asia, Australia, and North and South America are typically HDM-sensitized (7).
The preferred treatment options for allergic asthma include symptom control medications, such as inhaled corticosteroids and risk reduction strategies such as allergen prevention (8). Pharmacotherapy approaches including corticosteroids, short- or long-acting β-agonists and leukotriene receptor antagonists improve the symptoms in most allergic asthma patients (9). However, these drug treatments hardly change the course of asthma, and patients may need lifelong medication, since drug withdrawal or reduction may aggravate asthma attack. Although environmental control measures may decrease allergen levels, they are not always effective in improving allergic asthma symptoms (9).
Allergen-specific immunotherapy (AIT) is the only available disease-modifying treatment for patients with allergies, presenting long-lasting effects which counteract the progression from rhinoconjunctivitis to asthma (10). AIT alleviates allergic asthma by decreasing the symptoms and medication use; it also improves the quality of life, with a long-lasting benefit after cessation of treatment (11). Since 2017, the annual Global Initiative for Asthma (GINA) guidelines recommend the addition of immunotherapy for HDM allergic asthma (8). However, the role and molecular mechanism of AIT in asthma management are not fully established.
In view of the above information, we hypothesized that AIT with Alutard SQ improves allergic asthma. HDM-induced asthma rat model was used to investigate the role and potential regulatory mechanisms of AIT with Alutard SQ in allergic asthma. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-715/rc).
Methods
Experimental animals and allergic asthma model
Six weeks old healthy male Sprague-Dawley rats (weighing 200±20 g) were obtained from the Laboratory Animal Center of Southern Medical University (Guangzhou, China), and housed in a standard climate-controlled animal facility (25 ℃, 50% humidity, 12-h light-dark cycle) with free access to water and food. Experiments were performed under a project license (No. 2021-156) granted by the Animal Committee of First Affiliated Hospital of Guangzhou Medical University, in compliance with First Affiliated Hospital of Guangzhou Medical University institutional guidelines for the care and use of animals. The HDM-sensitized rats were intra-peritoneally injected with 50 µg HDM Dermatophagoides pteronyssinus (GREER, Lenoir, USA) and 50 µg alum adjuvant on 0, 7th, and 14th days. Thereafter, 42 rats were randomly divided into seven groups (N=6 rats/group): normal group (rats sensitized with saline), asthma group (HDM), asthma + Alutard SQ (ALK-Abello A/S, Hörsholm, Denmark) low dose group (HDM + ALD; asthma rats subcutaneously injected with 10,000 SQ-U/mL Alutard SQ on days 21, 23, 25), asthma + Alutard SQ middle dose group (HDM + AMD; asthma rats subcutaneously injected with 40,000 SQ-U/mL Alutard SQ on days 21, 23, 25), asthma + Alutard SQ high dose group (HDM + AHD; asthma rats subcutaneously injected with 100,000 SQ-U/mL Alutard SQ on days 21, 23, 25), HDM + AHD + high mobility group box 1 (HMGB1) lentivirus group (HDM + AHD + HMGB1; asthma rats subcutaneously injected with 100,000 SQ-U/mL Alutard SQ and intravenously injected with HMGB1 lentivirus in the tail on days 21, 23, 25, HDM + AHD + HMGB1 inhibitor ammonium glycyrrhizinate (AMGZ) group (HDM + AHD+ AMGZ; asthma rats subcutaneously injected with 100,000 SQ-U/mL Alutard SQ, and intravenously injected with AMGZ in the tail on days 21, 23, 25). Subsequently, rats were challenged for 30 min by intranasal instillation of HDM (50 µg/per rat) alone on days 32, 33, 34, 35, 36, 37, and 38. Anaphylactic responses including motility and body temperature were assessed by changes in rat behavior. Rats were sacrificed 24 h after the final challenge. Subsequently, we obtained blood, bronchoalveolar lavage fluid (BALF), and lung tissues for subsequent analysis.
Collection and analysis of BALF
Rats were lavaged with 1 mL of Hank’s balanced salt solution via injection into the lungs and drawing to collect cells three times. BALF was centrifuged for 10 min (400 g at 4 ℃), and the supernatant was collected for further analysis. The cell pellet was resuspended in 500 µL of saline. For the total cell counts, 100 µL BALF was added to 390 µL of Turk solution (0.5% methylene blue in 30% acetic acid) in a Neubauer chamber. The remaining BALF was centrifuged onto glass slides, and stained with Wright-Giemsa stain. Differential counts were quantified by counting to 200 cells/slide.
Hematoxylin & eosin (H&E) staining
The lung tissues were fixed, embedded in paraffin, and cut into 5-µm sections. The resultant sections were stained with H&E reagent and examined under an optical microscope (Olympus, Tokyo, Japan).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from lung tissues with TRIzol reagent according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA with a PrimeScript RT reagent Kit (Takara, China). qRT-PCR detection was performed on an ABI PRISM 7300 Sequence Detection system (Applied Biosystems, Foster City, CA, USA) with SYBR green qPCR Master Mix kit (Takara). β-actin served as an internal parameter. The fold change of gene expression was quantified using the 2−ΔΔCt method. All primers used are as follows: interleukin (IL)-4 primers: forward 5'- TACGGCAACAAGGAACACCA-3', reverse 5'- CAGAGTTTCCTCAGTTCACCG -3'; IL-5 primers: forward 5'-TGTTGACGAGCAATGAGACGAT-3', reverse 5'- AAGTTTTGGAATGGTATTTCCA-3'; IL-6 primers: forward 5'- GACCAAGACCATCCAACTCATC-3', reverse 5'-CCACAGTGAGGAATGTCCACA-3'; IL-13 primers: forward 5'-AGCAACATCACACAAGACCAGA-3', reverse 5'- GAGGCCATTCAATATCCTCTGG-3'; immunoglobulin (Ig)E primers: forward 5'- GGCTCCAACCAACGCTTCT-3', reverse 5'- GGTTCCCGAAGTGCCTCA-3'; Interferon-gamma (IFN-γ) primers: forward 5'- GAACAACCCACAGATCCAGC-3', reverse 5'-CAGAATCAGCACCGACTCCT-3'; transforming growth factor beta 1 (TGF-β1) primers: forward 5'-GCCCTGGATACCAACTACTGC-3', reverse 5'- CAGACAGAAGTTGGCATGGTA-3'; β-actin primers: forward 5'- GGAGATTACTGCCCTGGCTCCTA-3', reverse 5'-GACTCATCGTACTCCTGCTTGCTG-3'.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were centrifuged (3,000 g at 4 ℃) for 20 min. Supernatants were collected and stored at −80 ℃. The expression levels of IFN-γ, IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE in the serum, BALF and lung tissues and HMGB1 expression in the BALF were detected using ELISA kit (eBioscience) according to manufacturers' instructions.
Western blotting
Lung tissues were lysed in RIPA buffer (Beyotime, Shanghai, China) supplemented with protease inhibitors. The total protein was examined using the Pierce BCA protein assay kit (Pierce Chemical Co., Rockford, IL, USA). Subsequently, 30 µg of the protein was loaded and separated on 10% SDS-PAGE gel, then transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were placed in a blocking buffer, and probed with primary antibodies HMGB1 (1:1,000; Cell Signaling Technology, Beverly, MA, USA), Τoll-like receptor 4 (TLR4) (1:1,000, Proteintech, Wuhan, China), Phospho-p65 (1:1,000; Cell Signaling Technology), and p65 (1:1,000; Cell Signaling Technology) overnight at 4 ℃. The membranes were then incubated with a secondary antibody for 1 h. The band was visualized by chemiluminescence.
Statistical analysis
GraphPad Prism 9.0 Software (GraphPad Inc., San Diego, CA, USA) was employed for all statistical analyses. Data were expressed as mean ± standard deviation (SD). The data distribution was analyzed with the Shapiro-Wilk test. Statistical significance was analyzed using a one-way analysis of variance followed by Tukey’s multiple comparison test. P value <0.05 was considered statistically significant.
Results
Alutard SQ ameliorates HDM-induced airway inflammation
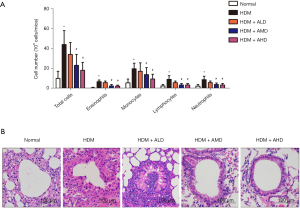
Airway inflammation is a typical pathological characteristic of asthma. We investigated whether Alutard SQ improves airway inflammation in rats with allergic asthma by analyzing the total and differential cell counts in the bronchoalveolar lavage fluid (BALF) from normal, HDM, HDM + ALD, HDM + AMD, and HDM + AHD, and H&E stained lung tissues. Consequently, total cell counts in the HDM-induced rat were significantly higher than those in the normal group, whereas Alutard SQ treatment significantly decreased the total cell counts compared with that in the HDM-induced group (Figure 1A). Furthermore, the number of differential cell counts (eosinophils, monocytes, and lymphocytes) in the HDM-induced group was significantly higher than that in the normal group, the effects of which were reversed by Alutard SQ treatment (Figure 1A). In support of the above data, histopathological examination inflammation in the airway region was substantially higher in the HDM-induced asthma model group than that in the normal group (Figure 1B). Rats in the Alutard SQ-treated group displayed fewer inflammatory cells in the airway region compared with the normal group (Figure 1B). Collectively, Alutard SQ significantly ameliorated the airway inflammation of the HDM-induced asthma model group.
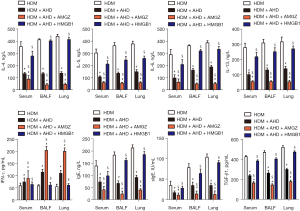
Alutard SQ attenuates IL-4, IL-5, IL-6, IL-13, TGF-β, total and allergen-specific Ig E expression, while it induces the expression of IFN-γ in allergic asthma rat models
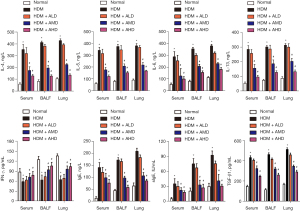
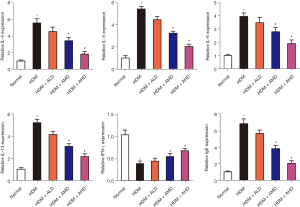
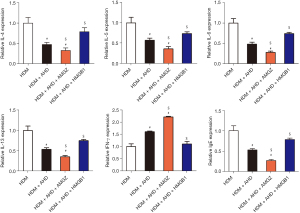
Expression levels of total and allergen-specific IgE, TGF-β1, Th (T helper)2 cytokines (IL-4, IL-5, IL-6, IL-13) and Th1 cytokines (IFN-γ) in the BALF, serum and lung samples derived from rats were detected to explore whether Alutard SQ treatment regulated the expression of inflammatory cytokines. The HDM-challenged group exhibited significantly higher levels of IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE than that in the normal group, whereas IFN-γ were significantly lower in the BALF, serum, and lung tissues from the HDM-challenged group (Figures 2,3). Nevertheless, the Alutard SQ treatment reverses the promotive effects of HDM on the expression of IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE, and the inhibitory effect of HDM on the IFN-γ expression (Figure 2). The mRNA expression level also showed a similar trend. IL-4, IL-5, IL-6, IL-13, TGF-β1, and total IgE mRNA expression were upregulated and IFN-γ was downregulated in HDM induced asthma model. However, Alutard SQ treatment restored these effects (Figure 3 and Figure S1A). Taken together, Alutard SQ impede the expression of HDM-induced IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE, and inhibit IFN-γ expression in the BALF, serum, and lung tissues.
Alutard SQ prevents HDM-induced airway inflammation by suppressing the HMGB1/TLR4/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signal pathway
A study recently reported HMGB1 upregulation in HDM-induced asthmatic rats, HMGB1 overexpression aggravated HDM-induced inflammatory responses and airway damage (12). Herein, we investigated whether Alutard SQ could regulate HMGB1 expression in HDM-challenged asthma. The results revealed a remarkably higher expression of HMGB1 in the HDM-induced group than that in the normal group (Figure 4A). Alutard SQ treatment exerted opposite effects by significantly decreasing HMGB1 protein expression in the HDM-induced group (Figure 4A and Figure S1B). Evidence from previous work demonstrated that HMGB1 regulates the expression of TLR4, which consequently induces a downstream inflammatory response by activating NF-κB. With these data, we presumed that Alutard SQ potentially activates the TLR4/NF-κB pathway. To validate this hypothesis, we examined TLR4 and p-p65 protein levels in the lung tissue of allergic asthma rats through Western blot analysis. Notably, the levels of TLR4 and p-p65 proteins significantly increased in the HDM-induced group, however, this increase was attenuated after Alutard SQ treatment (Figure 4A). These data confirm that Alutard SQ treatment restrains the upregulation of HMGB1, TLR4, and p-p65 in the HDM-induced asthma rat model.
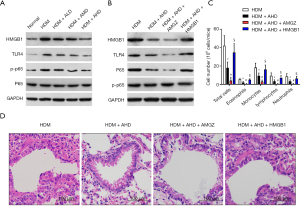
Furthermore, we established whether Alutard SQ could alleviate airway inflammation via the HMGB1/TLR4/NF-κB signal pathway in the HDM-sensitized rat model. Rats were intratracheally administered AMGZ (an HMGB1 antagonist) or HMGB1 lentivirus 30 min before each HDM challenge on days 21–23. The protein levels of HMGB1, TLR4, and p-p65 in the lung tissues were noticeably reduced in the Alutard SQ-treated asthma group, whereas the reduction was pronounced following AMGZ administration. However, protein levels of HMGB1, TLR4, and p-p65 in the lung tissues or BALF markedly increased by recombinant rat HMGB1 antibody treatment (Figure 4B and Figure S1C). The number of total cells, eosinophils, monocytes, and lymphocytes was lower in the BALF of Alutard SQ-treated asthma rats than that in the PBS-treated asthma group. AMGZ administration further lowered the total cells, eosinophils, monocytes, and lymphocytes in BALF. However, the HMGB1 lentivirus-treated group had an elevated number of total cells, eosinophils, monocytes, and lymphocytes (Figure 4C). In addition, the administration of Alutard SQ significantly weakened the asthmatic typical histological change compared to that in the PBS-treated asthma group. AMGZ treatment further weakened the asthmatic typical histological change. Nevertheless, administration of HMGB1 lentivirus increased the infiltration of inflammatory cells into the lung airways (Figure 4D). Taken together, these results provide convincing evidence that Alutard SQ improves allergic airway inflammation in the HDM-sensitized rat by inhibiting the HMGB1/TLR4/NF-κB signal pathway.
Alutard SQ improves HDM-provoked asthmatic inflammation by inactivating the HMGB1/TLR4/NF-κB signal pathway
We investigated whether Alutard SQ could regulate the HDM-mediated asthmatic inflammation by modulating the HMGB1/TLR4/NF-κB signal pathway. Intriguingly, Alutard SQ administration significantly overturned the HDM-induced upregulation of IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE, and the HDM-induced downregulation of IFN-γ protein expression in the BALF, serum, and lung tissues (Figure 5). These changes were pronounced after intervention with AMGZ, whereas HMGB1 lentivirus treatment showed opposite effects (Figure 5). Meanwhile, Alutard SQ treatment markedly downregulated mRNA expression levels of IL-4, IL-5, IL-6, IL-13, TGF-β, and total IgE, and upregulated IFN-γ mRNA expression levels in the asthma group (Figure 6 and Figure S1D). Stimulation with AMGZ further improved these effects, whereas the administration of HMGB1 lentivirus lowered these effects (Figure 6). These results suggest that Alutard SQ ameliorates Th2 and the expression of total and allergen-specific IgE as well as improves Th1 responses in the HDM-induced allergic asthma rat by blocking the HMGB1/TLR4/NF-κB signal pathway.
Discussion
Asthma is one of the most common chronic respiratory diseases, characterized by chronic airway inflammation and associated with high mortality and morbidity worldwide (13-16). In this study, using an HDM-induced asthma model of rats, we found that AIT with Alutard SQ can improve HDM-induced airway inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway.
The migration of inflammatory cells, including eosinophils and lymphocytes into the lung majorly contributes to the development of allergic airway inflammation (17). Increased eosinophil counts in blood, sputum and BALF are characteristic of eosinophilic asthma (18). Mepoluzimab, an anti-interleukin 5 (IL-5) monoclonal antibody, has been approved for the treatment of severe eosinophilic asthma resistant to glucocorticoids. Moreover, clinical and real-world studies have shown favorable efficacy and safety (19,20). In HDM-induced rats, Alutard SQ distinctly lowers the number of total cell counts, eosinophils, monocytes, and lymphocytes in the BALF. Furthermore, Th2 cytokines were previously reported to play an important role in airway inflammation and airway hyperresponsiveness (AHR), which are the hallmarks of allergic asthma (21). The expression of Th2 cytokines (IL-4, IL-5, IL-6, and IL-13) also increases the infiltration of inflammatory cells, eosinophils, and the production of airway mucus and allergen-specific IgE (22,23). Specifically, the Th2 response is a characteristic feature of allergic eosinophilic asthma (24). Previous evidence shows that the administration of Th1 cytokines such as IFN-γ can restrain the induction of AHR and Th2-driven inflammation (17). Antibodies targeting type 2 inflammatory factors, including IL-4R antibodies, have been used to treat severe asthma (25). Also, asthma is closely related to the augmented secretion of Th2 cytokines and reduced secretion of Th1 cytokines (15). AIT is a well-recognized treatment for allergic asthma (26). AIT reduces the production of allergen-specific IgE and induces a major change in allergen-specific T-cell subsets (i.e., stimulation of Th1 lymphocytes with increased IFN-γ and IL-2 production, decreased production of Th2, Th17, Tr17 lymphocyte counts, and Th2 cytokines, as well as induction of regulatory T-lymphocytes), producing IL-10 and TGF-β cytokines (27-29). Consistent with the above reports, Alutard SQ remarkedly downregulated the expression levels of IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE. On the other hand, the expression levels of IFN-γ were noticeably upregulated in the HDM-challenged allergic asthma rats. The interaction of HDM inhalation and airway epithelial cells directly caused airway epithelium dysfunction. HDM stimulates airway epithelial cells to release epithelial-derived cytokines, and recruit neighboring innate and adaptive immune cells including DCs, T-cells (such as Th2, Th1, and Th17), B-cells, eosinophils, and neutrophils, which are essential for asthma pathogenesis (12,30,31). Amongst these cytokines, IL-13, IL-33, TSLP, CCL5, CCL7, CCL17, CCL22, IFN-γ, IL-4, and several eotaxins strongly direct or support the development of a Th2 polarized inflammation (30,32). Additionally, GM-CSF secreted from airway epithelial cells leads to maturation and survival of eosinophils (30). Both effects promote the inflammation of allergic asthma. Huang et al. showed that AIT attenuates both upper and lower airway immune response to nasal allergen exposure in patients with asthma to improve airway inflammatory response (33). AIT increases secretoglobin 1A1 expression in cells of the lower airways from patients with allergic asthma to improve airway inflammatory response (34). It also suppresses TGF-β1 in asthma airway epithelial cells, which regulate airway inflammation and remodeling in asthma due to its anti-inflammatory and profibrotic effect (35,36). Similarly, our study showed that Alutard SQ inhibited the HDM-induced TGF-β1 protein secretion, and airway inflammation. This is similar to the findings of Park et al., who demonstrated favorable efficacy and safety in transdermal immunotherapy using biodegradable microneedle patches in a mouse asthma model (37). Several analyses have demonstrated the efficacy and safety of allergen-specific immunotherapy in the treatment of allergic asthma (38), and the GINA guidelines have endorsed dust mite desensitization therapy to their asthma treatment ladder since 2017 (8).
HMGB1 is a ubiquitous and highly conserved DNA-binding protein secreted by dendritic cells, mononuclear cells, and macrophages following stimulation by lipopolysaccharide (LPS), tumor necrosis factor (TNF)-α or IL-1. HMGB1 is also released from injured and dead cells to induce the production and secretion of a number of inflammatory mediators (39,40). HMGB1 has been implicated in many inflammatory conditions, including sepsis, rheumatoid arthritis, and an intestinal inflammatory disorder, and lung inflammatory diseases (41). For instance, HMGB1-mediated eosinophil recruitment to inflammatory sites aggravates the inflammatory response. Related studies have reported that HMGB1 is overexpressed in lung tissue, serum, and sputum of patients with severe asthma, and blockade of HMGB1 alleviates airway inflammation and Th2 cytokine release and promotes Th1 cytokine in OVA-primed allergic asthma rats (4,39,42,43). Herein, HMGB1 was mainly expressed in airway epithelium from the HDM-induced allergic asthma model; its overexpression abolished the protective effect of CC16 against HDM-induced airway damage, and airway epithelial cell apoptosis (12). TLR4, a major receptor of HMGB1, has been implicated in the pathophysiology of asthma, mainly in the initiation and exacerbation of asthma (44). TLR4 activates NF-κB via MyD88- or non-MyD88-dependent signaling, and these events promote the secretion of pro-inflammatory factors (45). NF-κB is a potent inflammatory mediator and has potent effects on asthma-related airway inflammation (46). HMGB1 activates TLRs, including TLR2 and TLR4, to induce the release of pro-inflammatory cytokines through the mitogen-activated protein kinase (MAPK), and NF-κB pathways; these events exacerbate airway inflammation (47,48). In line with previous studies, Alutard SQ inhibited HMGB1 expression in the lungs and BALF from the HDM-sensitized rat model and TLR4 and p-p65 expression in the lungs from the HDM-sensitized rat model; these inhibitory effects were reversed following HMGB1 overexpression and further suppressed by AMGZ treatment. Moreover, Alutard SQ suppressed the HDM-induced airway inflammation, downregulated the expression of IL-4, IL-5, IL-6, IL-13, TGF-β1, total and allergen-specific IgE, as well as increased IFN-γ expression levels. These changes were further enhanced by AMGZ treatment but abrogated by enforced HMGB1 expression. These findings have important implications for the development of drugs, or allergen-specific immunotherapy.
The present study has some worth-mentioning limitations. First, we only used Western blotting to detect the effects of Alutard SQ on HMGB1, TLR4, and NF-κB expression. Thus, additional experimental methods should be adopted in future investigations. One study reported that Constitutive immune activity promotes JNK- and FoxO-dependent remodeling of Drosophila airways (49). Further studies are necessary to assess whether the Alutard SQ-related effects on NF-κB-pathway and HMGB1 would affect constitutive immune activity, which promotes JNK- and FoxO-dependent remodeling. Therefore, future studies should focus on the relationship between the the HMGB1/TLR4/NF-κB axis and Alutard SQ.
Conclusions
In conclusion, AIT with Alutard SQ alleviates HDM-induced airway inflammation by suppressing inflammatory cytokine secretion via the HMGB1/TLR4/NF-κB pathway. These findings provide convincing evidence supporting the role of AIT with Alutard SQ as a therapeutic strategy for patients with allergic asthma.
Acknowledgments
Funding: This study was funded by the Municipal schools (colleges) jointly funded project basic and applied basic research project (No. 202201020504).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-715/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-715/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-715/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-715/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2021-156) granted by the Animal Committee of First Affiliated Hospital of Guangzhou Medical University, in compliance with First Affiliated Hospital of Guangzhou Medical University institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Husseini ZW, Gosens R, Dekker F, et al. The genetics of asthma and the promise of genomics-guided drug target discovery. Lancet Respir Med 2020;8:1045-56. [Crossref] [PubMed]
- Ellwood P, Asher MI, Billo NE, et al. The Global Asthma Network rationale and methods for Phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J 2017;49:1601605. [Crossref] [PubMed]
- Abu Khweek A, Kim E, Joldrichsen MR, et al. Insights Into Mucosal Innate Immune Responses in House Dust Mite-Mediated Allergic Asthma. Front Immunol 2020;11:534501. [Crossref] [PubMed]
- Lin J, Fu X, Jiang P, et al. INITIAL - An observational study of disease severity in newly diagnosed asthma patients and initial response following 12 weeks' treatment. Sci Rep 2019;9:1254. [Crossref] [PubMed]
- Corrao G, Arfè A, Nicotra F, et al. Persistence with inhaled corticosteroids reduces the risk of exacerbation among adults with asthma: A real-world investigation. Respirology 2016;21:1034-40. [Crossref] [PubMed]
- Rodríguez-Domínguez A, Berings M, Rohrbach A, et al. Molecular profiling of allergen-specific antibody responses may enhance success of specific immunotherapy. J Allergy Clin Immunol 2020;146:1097-108. [Crossref] [PubMed]
- Dust mite allergens and asthma--a worldwide problem. J Allergy Clin Immunol 1989;83:416-27. [Crossref] [PubMed]
- Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021. Executive Summary and Rationale for Key Changes. Arch Bronconeumol 2022;58:35-51. [Crossref] [PubMed]
- Richards JR, Stumpf JL. House Dust Mite Sublingual Immunotherapy for Pediatric Patients With Allergic Asthma. Ann Pharmacother 2018;52:1019-30. [Crossref] [PubMed]
- Hagen A, Gorenoi V, Schönermark MP. Specific immunotherapy (SIT) in the treatment of allergic rhinitis. GMS Health Technol Assess 2010;6:Doc01. [Crossref] [PubMed]
- Tosca MA, Olcese R, Licari A, et al. Allergen immunotherapy and asthma. Pediatr Allergy Immunol 2020;31:46-8. [Crossref] [PubMed]
- Liu M, Lu J, Zhang Q, et al. Clara cell 16 KDa protein mitigates house dust mite-induced airway inflammation and damage via regulating airway epithelial cell apoptosis in a manner dependent on HMGB1-mediated signaling inhibition. Mol Med 2021;27:11. [Crossref] [PubMed]
- Padem N, Saltoun C. Classification of asthma. Allergy Asthma Proc 2019;40:385-8. [Crossref] [PubMed]
- Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet 2018;391:783-800. [Crossref] [PubMed]
- Asayama K, Kobayashi T, D'Alessandro-Gabazza CN, et al. Protein S protects against allergic bronchial asthma by modulating Th1/Th2 balance. Allergy 2020;75:2267-78. [Crossref] [PubMed]
- Casaro M, Souza VR, Oliveira FA, et al. OVA-Induced Allergic Airway Inflammation Mouse Model. Methods Mol Biol 2019;1916:297-301. [Crossref] [PubMed]
- Liu J, Cheng Y, Zhang X, et al. Astragalin Attenuates Allergic Inflammation in a Murine Asthma Model. Inflammation 2015;38:2007-16. [Crossref] [PubMed]
- Van Hulst G, Batugedara HM, Jorssen J, et al. Eosinophil diversity in asthma. Biochem Pharmacol 2020;179:113963. [Crossref] [PubMed]
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198-207. [Crossref] [PubMed]
- Harrison T, Canonica GW, Chupp G, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020;56:2000151. [Crossref] [PubMed]
- Choy DF, Hart KM, Borthwick LA, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 2015;7:301ra129. [Crossref] [PubMed]
- Li R, Wang J, Zhu F, et al. HMGB1 regulates T helper 2 and T helper17 cell differentiation both directly and indirectly in asthmatic mice. Mol Immunol 2018;97:45-55. [Crossref] [PubMed]
- Robinson D, Humbert M, Buhl R, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy 2017;47:161-75. [Crossref] [PubMed]
- Mahmutovic Persson I, Menzel M, Ramu S, et al. IL-1β mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir Res 2018;19:16. [Crossref] [PubMed]
- Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy 2020;50:5-14. [Crossref] [PubMed]
- Zhang W, Lin C, Sampath V, et al. Impact of allergen immunotherapy in allergic asthma. Immunotherapy 2018;10:579-93. [Crossref] [PubMed]
- Zissler UM, Jakwerth CA, Guerth FM, et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. EBioMedicine 2018;36:475-88. [Crossref] [PubMed]
- Yang L, Zhu R. Immunotherapy of house dust mite allergy. Hum Vaccin Immunother 2017;13:2390-6. [Crossref] [PubMed]
- Nakagome K, Nagata M. Allergen Immunotherapy in Asthma. Pathogens 2021;10:1406. [Crossref] [PubMed]
- Frey A, Lunding LP, Ehlers JC, et al. More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front Immunol 2020;11:761. [Crossref] [PubMed]
- Alessandrini F, de Jong R, Wimmer M, et al. Lung Epithelial CYP1 Activity Regulates Aryl Hydrocarbon Receptor Dependent Allergic Airway Inflammation. Front Immunol 2022;13:901194. [Crossref] [PubMed]
- Zissler UM, Chaker AM, Effner R, et al. Interleukin-4 and interferon-γ orchestrate an epithelial polarization in the airways. Mucosal Immunol 2016;9:917-26. [Crossref] [PubMed]
- Huang R, Qin R, Hu Q, et al. Effect of Dermatophagoides pteronyssinus Immunotherapy on Upper and Lower Airway Eosinophilic Inflammatory Response to Nasal Allergen Challenge. Allergy Asthma Immunol Res 2020;12:844-58. [Crossref] [PubMed]
- Zissler UM, Jakwerth CA, Guerth F, et al. Allergen-specific immunotherapy induces the suppressive secretoglobin 1A1 in cells of the lower airways. Allergy 2021;76:2461-74. [Crossref] [PubMed]
- Musiol S, Alessandrini F, Jakwerth CA, et al. TGF-β1 Drives Inflammatory Th Cell But Not Treg Cell Compartment Upon Allergen Exposure. Front Immunol 2022;12:763243. [Crossref] [PubMed]
- Huo R, Tian X, Chang Q, et al. Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1. Ther Adv Respir Dis 2021;15:1753466620981858. [Crossref] [PubMed]
- Park KH, Oh EY, Han H, et al. Efficacy of transdermal immunotherapy with biodegradable microneedle patches in a murine asthma model. Clin Exp Allergy 2020;50:1084-92. [Crossref] [PubMed]
- Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017;72:1825-48. [Crossref] [PubMed]
- Lv Y, Li Y, Zhang D, et al. HMGB1-induced asthmatic airway inflammation through GRP75-mediated enhancement of ER-mitochondrial Ca(2+) transfer and ROS increased. J Cell Biochem 2018;119:4205-15. [Crossref] [PubMed]
- Jiang H, Duan J, Xu K, et al. Resveratrol protects against asthma-induced airway inflammation and remodeling by inhibiting the HMGB1/TLR4/NF-κB pathway. Exp Ther Med 2019;18:459-66. [Crossref] [PubMed]
- Lee CC, Lai YT, Chang HT, et al. Inhibition of high-mobility group box 1 in lung reduced airway inflammation and remodeling in a mouse model of chronic asthma. Biochem Pharmacol 2013;86:940-9. [Crossref] [PubMed]
- Di Candia L, Gomez E, Venereau E, et al. HMGB1 is upregulated in the airways in asthma and potentiates airway smooth muscle contraction via TLR4. J Allergy Clin Immunol 2017;140:584-587.e8. [Crossref] [PubMed]
- Shang L, Wang L, Shi X, et al. HMGB1 was negatively regulated by HSF1 and mediated the TLR4/MyD88/NF-κB signal pathway in asthma. Life Sci 2020;241:117120. [Crossref] [PubMed]
- Shalaby KH, Al Heialy S, Tsuchiya K, et al. The TLR4-TRIF pathway can protect against the development of experimental allergic asthma. Immunology 2017;152:138-49. [Crossref] [PubMed]
- Wang Q, Cui Y, Wu X, Wang J. Evodiamine protects against airway remodelling and inflammation in asthmatic rats by modulating the HMGB1/NF-κB/TLR-4 signalling pathway. Pharm Biol 2021;59:192-9. [Crossref] [PubMed]
- Tian C, Gao F, Li X, et al. Icariside II attenuates eosinophils-induced airway inflammation and remodeling via inactivation of NF-κB and STAT3 in an asthma mouse model. Exp Mol Pathol 2020;113:104373. [Crossref] [PubMed]
- Imbalzano E, Quartuccio S, Di Salvo E, et al. Association between HMGB1 and asthma: a literature review. Clin Mol Allergy 2017;15:12. [Crossref] [PubMed]
- Hwang YH, Lee Y, Paik MJ, et al. Inhibitions of HMGB1 and TLR4 alleviate DINP-induced asthma in mice. Toxicol Res (Camb) 2019;8:621-9. [Crossref] [PubMed]
- Wagner C, Uliczka K, Bossen J, et al. Constitutive immune activity promotes JNK- and FoxO-dependent remodeling of Drosophila airways. Cell Rep 2021;35:108956. [Crossref] [PubMed]


