
Active immunization with Pseudomonas aeruginosa vaccine protects mice from secondary Pseudomonas aeruginosa challenge post-influenza virus infection
Highlight box
Key findings
• The prior influenza infection greatly enhanced the susceptibility to secondary infection of Pseudomonas aeruginosa and increased morbidity and mortality in mice.
What is known and what is new?
• Active immunization with inactivated Pseudomonas aeruginosa cells could protect mice from secondary Pseudomonas aeruginosa challenge in influenza virus infected mice.
What is the implication, and what should change now?
• To develop an effective Pseudomonas aeruginosa vaccine might be a promising strategy to decrease the threat of secondary Pseudomonas aeruginosa infection in influenza patients.
Introduction
Pneumonia is the leading cause of infectious death worldwide (1), and acute lower respiratory infection is the leading cause of death in children under 5 years old in developing countries (2). Influenza virus is one of the predominant causative agents of seasonal flu and infectious mortality worldwide. Most influenza-related mortality is not due to the viral infection alone (3,4). Recent studies suggested that bacterial pneumonia secondary to influenza virus infection was identified as the major cause of increased hospitalizations and deaths during influenza virus epidemics (5,6).
Earlier studies about influenza-related secondary infections were concentrated on the gram-positive bacterium. Several gram-positive bacterial pathogens have been isolated from patients who suffered from secondary infections during influenza pandemics, such as Streptococcus pneumoniae (S. pneumoniae), Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes) and methicillin resistant S. aureus (MRSA) (7-9). Recently, a gram-negative bacterium such as Pseudomonas aeruginosa (P. aeruginosa), Haemophilus influenzae (H. influenzae), Klebsiella pneumoniae (K. pneumoniae) and anaerobes were also isolated from institutionalized elderly influenza patients (10-12). During avian influenza A virus H7N9 epidemic, H7N9 patients older than 60 were more likely to suffer from secondary infections of P. aeruginosa, K. pneumoniae or Acinetobacter baumannii (A. baumannii) (13,14). Secondary infection with gram-negative bacteria caused more serious disease and higher mortality than gram-positive bacteria (15,16).
Active immunization with a vaccine is a common strategy to protect humans from microbial infections. Vaccination with attenuated bacterial or inactivated bacterial cells were often used to induce a protective immunity response. In the 1990’s, a candidate inactivated vaccine was tested in acute P. aeruginosa pneumonia model (17). It showed that vaccination not only decreased the P. aeruginosa titers in the lungs but also improved the survival rate.
In this study, we prepared candidate vaccines of inactivated whole P. aeruginosa cells and recombinant type III secretion system PcrV protein, and established a secondary infection mouse model with a prior infection of influenza virus and secondary infection of P. aeruginosa. Furthermore, the protection of candidate vaccines were evaluated against P. aeruginosa with the secondary infectious model. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1012/rc).
Methods
Mice
Six to eight weeks old female BALB/c mice were housed with 24-hour access to food and water. The study protocol was approved by the Guangzhou Institutes of Biomedicine and Health (GIBH) Institutional Animal Care and Use Committee (ID: 2013028). All animal experiments were performed under the guidelines established by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Influenza virus and bacterial strain
The mouse-adapted influenza A virus (PR8) was diluted in sterile phosphate-buffered saline (PBS) and with a mouse LD50 (MLD50) of 100 PFU. The P. aeruginosa strain (ATCC27853) was purchased from China General Microbiological Culture Collection Center (CGMCC). The bacterial strain had a MLD50 of 3.2×107 colony-forming units (CFU).
Influenza virus—P. aeruginosa secondary infection model (Figure 1)
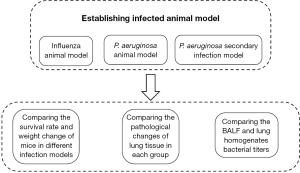
For infection, mice were anesthetized with isoflurane and held upright. Then, the mice were intranasally (i.n.) inoculated with PR8 virus and/or P. aeruginosa strain respectively. PR8 virus was administered at a dose of 0.4 MLD50 in 40 µL PBS per mouse, and the P. aeruginosa was administered at a dose of 0.0625 MLD50 (2×106 CFU). Secondary infection of P. aeruginosa was administered at day 2, 7 or 14 post PR8 virus infection (18). A group of mice inoculated with PBS served as controls. Once PR8 virus was inoculated, body weights and survival rate of mice were monitored daily for 20 days. Mice were euthanized for further experiments when their body weights fell below 70% of the initial body weights.
Bronchoalveolar lavage fluid (BALF) and lung homogenates bacterial titers
The mice were euthanized 3, 6, 8, or 24 hours post P. aeruginosa infection. The bronchoalveolar lavage was performed with three aliquots of 0.6 mL sterile PBS, and about 1.6 mL in a total of lavage fluid was retrieved for each mouse. Lungs were collected and homogenized in PBS. Ten-fold serial dilutions of BALFs and lung homogenates were inoculated onto Luria-Bertani (LB) agar plates, and bacterial CFU were counted after incubation at 37 ℃ overnight.
Histological analysis of lungs
Lungs were removed from euthanized mice and fixed immediately with 10% buffered formalin phosphate for 24 hours. After paraffin embedding, representative lateral and medial lung areas were cut into 7 µm thick sections. After deparaffinization, the sections were stained with hematoxylin and eosin, and then observed under microscopy (19).
Preparation of inactivated P. aeruginosa
The P. aeruginosa was cultured in broth to an OD600 of 1.0. The bacterial cells were washed 3 times with PBS and then inactivated with 10% formalin for 1 hour. The complete inactivation efficiency of the bacteria was confirmed by the index of no viable bacteria growing on blood agar. The complete inactivated P. aeruginosa was purified by centrifugation after being shaken in cold PBS for 1 hour, and the purification was repeated 3 times. The vaccine was prepared in PBS with a concentration of 3×1010 cells/mL and immunized after being mixed with an equal volume of aluminum phosphate adjuvant (18).
Preparation of recombinant type III secretion system PcrV protein
PcrV expression plasmid (pET22b-PcrV) was made by inserting PcrV gene into the pET-22b vector with C-terminal His-Tag. The E. coli BL21 were transformed with pET22b-PcrV, then cultured in LB containing ampicillin at 37 ℃ and shaken at 250 rpm/min. When OD600 of culture solution arrived at 0.5, the culture was induced with 1 mmol/L IPTG and then shaken for another four hours. The bacterial cells were harvested by centrifugation, and the protein expression was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). PcrV protein was purified by Nickel-Nitrilotriacetic Acid (Ni-NTA) affinity chromatography and identified by Western-blotting using rat anti-His-Tag antibody and horseradish peroxidase (HRP)-labeled goat anti-rat antibody. 1 mg/mL Purified PcrV protein in PBS was mixed with an equal volume of aluminum phosphate adjuvant for immunization.
Active immunization studies (Figure 2)
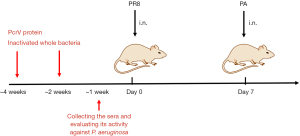
The 6-week-old female BALB/c mice were immunized by intramuscular quadriceps injection of 100 µL of the inactivated P. aeruginosa (1×108 inactivated bacterial cells), PcrV protein (0.5 mg/mL) or PBS, respectively. The booster were performed on the 14th day after the first immunization. On day 21 after immunization, the sera were collected, and its activity against P. aeruginosa was evaluated by detecting the growth of P. aeruginosa in broth containing diluted serum (1:4, 1:8, 1:16, and 1:32). The immunized mice were infected by PR8 virus on day 28 and followed by a secondary infection of P. aeruginosa on day 35 post-immunization.
Statistical analysis
The data was analyzed by the GraphPad Prism 6.01 software package (GraphPad Software) and statistical significance was determined by unpaired Student’s t-test with two-tailed analysis. Logrank trend test was used to compare the survival rate of the experimental group and the mock control. A P value less than 0.05 was statistically significant.
Results
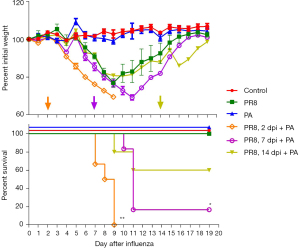
Secondary P. aeruginosa challenge increased lethality after influenza virus infection in BALB/c mice
To evaluate the effect of prior influenza virus infection on secondary P. aeruginosa infection, we monitored the body weight loss and mortality after the challenge of P. aeruginosa (0.0625 MLD50, 2×106 CFU) in PR8 influenza virus-infected mice (0.4 MLD50). The body weight showed a loss of less than 20% within 9 days post PR8 influenza virus sole infection and then gradually recovered (Figure 3). P. aeruginosa sole infection caused no apparent loss of body weight. No mouse died in the groups with sole infection of PR8 virus or P. aeruginosa. However, significant body weight loss and mortality were observed in the mice with secondary infection with P. aeruginosa. More importantly, secondary P. aeruginosa on day 2 or day 7 post-PR8 infection caused more significant body weight loss and higher mortality. Furthermore, secondary infection with an even lower dose of P. aeruginosa (2×105 CFU) on day 2 post PR8-infection (0.00625 MLD50) could also induce significant weight loss and death (Figure S1). The results indicated that mice become more susceptible to P. aeruginosa after PR8 virus infection, and secondary P. aeruginosa infection might cause severe outcomes.
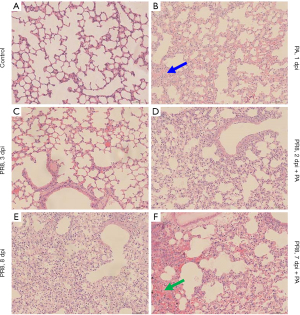
Secondary infection recruited inflammatory cells and induced severe lung injury
We next examined the histopathology of lungs in mice infected with PR8 virus and P. aeruginosa (Figure 4). The mice were sacrificed at the indicated time after PR8 virus infection or 24 hours after P. aeruginosa infection. In this study, many more inflammatory cells could be observed in the alveolar space on day 8 than on day 3 after PR8 infection, indicating that PR8 virus infection induced progressive inflammatory cell infiltration (Figure 4C,4E). However, P. aeruginosa could induce severe inflammatory cell infiltration within 24 hours (Figure 4B) compared to control mice without PR8 or P. aeruginosa instillation (Figure 4A). In comparison, more severe inflammatory cell infiltration and more severe lung injury were observed in the lung tissue with secondary infection (Figure 4D,4F) than that with sole infection of PR8 virus or P. aeruginosa (Figure 4B,4C,4E). Severe lung injury in mice with the secondary infection of P. aeruginosa was consistent with body weight loss and mortality. These findings indicated that it tended to develop severe bacterial pneumonia when a secondary P. aeruginosa happened after influenza virus infection.
Prior infection of influenza hinders the clearance of P. aeruginosa
The secondary infection mice were prepared by inoculating with P. aeruginosa on day 2 post influenza infection. BALFs were collected from the mice at 3, 6, 8, and 24 hours after P. aeruginosa inoculation (Figure 5A). The viable P. aeruginosa in BALF was detected by clone formation in an agarose medium. The number of viable P. aeruginosa in BALF gradually decreased after infection. There were almost no viable bacteria detected in the BALF from mice with sole disease of P. aeruginosa for 24 hours. In the BALF collected at 3 hours and 6 hours after P. aeruginosa infection, there was no significant difference in the number of viable bacteria between the P. aeruginosa sole infection group and the secondary infection group. However, in the BALF collected at 8 and 24 hours after P. aeruginosa infection, the number of viable bacterial from the secondary infection group was higher than that from sole P. aeruginosa infection group. Likewise, viable bacterial titers in the whole lung homogenates (collected 24 hours after P. aeruginosa infection) from the secondary infection group were about 100-folds higher than that from the sole P. aeruginosa infection group (Figure 5B). The consistent results of viable bacteria titer in BALF and lung homogenate suggest that prior infection of influenza virus attenuates the clearance of bacteria, leading to severe secondary bacterial pneumonia.

Whole bacteria vaccine protects mice from P. aeruginosa secondary challenge after influenza infection
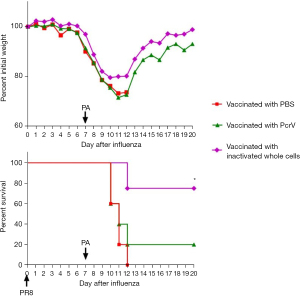
Currently, there are several vaccine candidates against P. aeruginosa are under development, including protein vaccines, inactivated bacterial and attenuated bacterial (17). Since IC43, OprI, OprF, and PcrV are expressed in most of the pathogenic strains of PA and play an essential role for P. aeruginosa, these proteins have been selected as vaccine candidates (20). Based on the results that prior influenza infection hinders the clearance of P. aeruginosa, we investigated whether a vaccine could protect against P. aeruginosa secondary infection. PcrV protein is an essential component of the type III secretion system (T3SS), which is a trans-membrane syringe-like structure and related to the secretion of Virulence factors. PcrV protein was proven to be with immunogenicity (20,21). Antibodies induced by PcrV protein have been reported to block the cytotoxicity mediated by T3SS and relieve the acute lung injury caused by P. aeruginosa (22). We expressed recombinant PcrV protein (Figure S2) and prepared inactivated P. aeruginosa cells as two candidate vaccines. Mice received intramuscular injection of either inactivated P. aeruginosa (1×108 inactivated bacterial cells) or recombinant PcrV protein (50 µg) with Alum adjuvant. The booster was vaccinated on the 14th day after the first immunization. On day 7 after booster vaccination, the sera were collected and evaluated for bacteriostatic activity against P. aeruginosa in vitro. On days 21 and 28 after booster vaccination, the mice were challenged with sublethal dose of PR8 virus and P. aeruginosa (0.0625 MLD50, 2×106 CFU), respectively. The mice vaccinated with inactivated P. aeruginosa cells had 80% survival and less body weight loss, while mice vaccinated with recombinant PcrV protein only had 20% survival (Figure 6). However, all unvaccinated mice died on day 5 after P. aeruginosa challenge.
We next detected the bacteriostatic activity of immune sera against P. aeruginosa in vitro. The growth of P. aeruginosa was inhibited in the medium with immune sera. The sera from mice vaccinated with inactivated vaccine completely inhibited P. aeruginosa at 4-fold dilution and showed significant bacteriostatic activity at 32-fold dilution. However, the growth of P. aeruginosa was only partially inhibited by sera from mice vaccinated with recombinant PcrV protein at 4-fold dilution and was not inhibited by sera from unvaccinated mice (Figure 7).
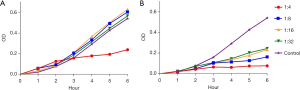
These results suggested that the vaccination with inactivated P. aeruginosa cells could provide adequate protection against secondary P. aeruginosa infection post influenza virus infection, whereas recombinant PcrV protein could only provide weak protection.
Discussion
P. aeruginosa is one of the six pathogens that are the major causes of nosocomial infections. Secondary infection of P. aeruginosa always results in severe pneumonia and even death in influenza patients. Treatment of P. aeruginosa infections is extremely challenging due to multi-drug-resistant (MDR) P. aeruginosa strains in the hospital environment, which is becoming increasingly resistant to all available antibiotics. Vaccination against P. aeruginosa would provide protection and overcome difficulties associated with antibiotic resistance. It is important to develop a P. aeruginosa secondary infection model and to explore the possibility of creating a candidate vaccine.
In this study, we successfully established an influenza-P. aeruginosa secondary infection model by sequential intranasal inoculation with influenza virus and P. aeruginosa strain. Gram-positive bacterial streptococcus pneumonia could be completely cleared within three hours after sole infection (23). However, we found that viable P. aeruginosa could still be detected in BALF until 24 hours after P. aeruginosa sole infection. Further study showed that the influenza virus prior to infection decreases the clearance of P. aeruginosa and increases the susceptibility to secondary P. aeruginosa infection. The viable bacterial titer in the lung tissues from secondary infection at 24 hours after P. aeruginosa infection was almost 100 folds higher than that from P. aeruginosa sole infection. Our study demonstrated that prior influenza virus infection damaged the ability of the host to remove bacteria, which made it possible for bacteria to invade into the lung tissue and replicate. Therefore, the P. aeruginosa secondary infection caused much more severe morbidity and mortality than sole infection with influenza or P. aeruginosa.
What’s more, challenges with P. aeruginosa at different times after influenza viral infection could all aggravate the disease, even at 14 days after influenza viral infection. This result indicates that P. aeruginosa is a severe threat to patients during the progression or recovery of influenza. Therefore, to minimize P. aeruginosa associated morbidity and mortality after influenza infection, the development of an effective P. aeruginosa vaccine is highly desirable.
Vaccines against P. aeruginosa have been explored in the past, so far there are no licensed vaccines (17). Currently, several vaccine candidates against P. aeruginosa are under clinical trials, including protein vaccines, inactivated bacterial and attenuated bacterial. Since the protein IC43, OprI, OprF, and PcrV are expressed in most pathogenic P. aeruginosa strains and are associated with P. aeruginosa reproduction or pathogenicity (20), these proteins are selected as promising vaccine candidates. It was reported that infection of P. aeruginosa with type-3 secretion system (T3SS) proteins caused 6-fold higher mortality in patients than without T3SS proteins (21). T3SS proteins expression was correlated with increasing incidence of bacteremia and organ failure induced by P. aeruginosa (19,24). PcrV protein is one of the important components of T3SS and has strong immunogenicity (25-28), which functions as an essential virulence factor to cause acute necrotic cell death and significant lung injury (29). Antibodies against PcrV protein can block the cytotoxicity mediated by T3SS and relieve the acute lung injury caused by P. aeruginosa. Mab166, a monoclonal antibody against PcrV protein from mice, was reported to protect mice from P. aeruginosa infection. In this study, serum against PcrV could inhibit P. aeruginosa growth in vitro. Still, PcrV protein vaccine showed less protection in P. aeruginosa secondary infection mice, indicating that one recombinant protein alone might not be enough to induce sufficient protection. Combination of multiple P. aeruginosa candidate proteins might be a better strategy for developing a candidate vaccine.
Attenuated bacterial and inactivated bacterial cells have been used as a common strategy to develop a vaccine, which may induce a broader immune response to multiple antigenic targets on P. aeruginosa. It was reported that immunization with inactivated P. aeruginosa significantly enhanced bacterial clearance and improved the survival rate in an acute P. aeruginosa pneumonia rat model (17). Our study demonstrated that vaccination with inactivated P. aeruginosa could provide protection and reduce mortality caused by P. aeruginosa secondary infection in mice. Further analysis showed that serum induced by the inactivated vaccine could inhibit P. aeruginosa growth in vitro. In addition to PcrV protein, delineating and evaluating the protective effects of other components from the inactivated P. aeruginosa cells might be helpful for the development of an effective vaccine.
Conclusions
In summary, prior infection with non-lethal influenza virus decreases the capacity of clearing P. aeruginosa and increases the susceptibility to P. aeruginosa secondary infection. P. aeruginosa secondary infection is a severe threat to influenza patients. Vaccination against P. aeruginosa could provide protection and should be a strategy to overcome high morbidity caused by P. aeruginosa secondary infection in influenza patients.
Acknowledgments
Funding: This work was supported by grant from National Natural Science Foundation of China (No. 91442102), National Science and Technology Major Project of China (No. 2018YFC1200100) and Guangzhou Science and Technology Program key project (No. 201904020037).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1012/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1012/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1012/coif). NZ serves as Editor-in-Chief of Journal of Thoracic Disease. All authors report funding from National Natural Science Foundation of China (No. 91442102) and National Science and Technology Major Project of China (No. 2018YFC1200100). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Guangzhou Institutes of Biomedicine and Health Institutional Animal Care and Use Committee (ID: 2013028). All animal experiments were performed under the guidelines established by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The top 10 causes of death. 2014.
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151-61. [Crossref] [PubMed]
- Lipscomb MF, Hutt J, Lovchik J, et al. The pathogenesis of acute pulmonary viral and bacterial infections: investigations in animal models. Annu Rev Pathol 2010;5:223-52. [Crossref] [PubMed]
- Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol 2009;27:61-82. [Crossref] [PubMed]
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008;198:962-70. [Crossref] [PubMed]
- Reichert TA, Simonsen L, Sharma A, et al. Influenza and the winter increase in mortality in the United States, 1959-1999. Am J Epidemiol 2004;160:492-502. [Crossref] [PubMed]
- Wang XY, Kilgore PE, Lim KA, et al. Influenza and bacterial pathogen coinfections in the 20th century. Interdiscip Perspect Infect Dis 2011;2011:146376. [Crossref] [PubMed]
- Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 2008;14:558-64. [Crossref] [PubMed]
- Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J 2004;23:S87-97. [Crossref] [PubMed]
- Chidiac C, Maulin L. Using antibiotics in case of influenza. Med Mal Infect 2006;36:181-9. [Crossref] [PubMed]
- Cillóniz C, Ewig S, Menéndez R, et al. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J Infect 2012;65:223-30. [Crossref] [PubMed]
- Martín-Loeches I, Sanchez-Corral A, Diaz E, et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest 2011;139:555-62. [Crossref] [PubMed]
- Lu H, Zhang C, Qian G, et al. An analysis of microbiota-targeted therapies in patients with avian influenza virus subtype H7N9 infection. BMC Infect Dis 2014;14:359. [Crossref] [PubMed]
- Tang X, He H, Sun B, et al. ARDS associated with pneumonia caused by avian influenza A H7N9 virus treated with extracorporeal membrane oxygenation. Clin Respir J 2015;9:380-4. [Crossref] [PubMed]
- Robinson KM, Kolls JK, Alcorn JF. The immunology of influenza virus-associated bacterial pneumonia. Curr Opin Immunol 2015;34:59-67. [Crossref] [PubMed]
- Bianchini S, Argentiero A, Camilloni B, et al. Vaccination against Paediatric Respiratory Pathogens. Vaccines (Basel) 2019;7:168. [Crossref] [PubMed]
- Buret A, Dunkley ML, Pang G, et al. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1 alpha. Infect Immun 1994;62:5335-43. [Crossref] [PubMed]
- Chaussee MS, Sandbulte HR, Schuneman MJ, et al. Inactivated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super-infections. Vaccine 2011;29:3773-81. [Crossref] [PubMed]
- Shime N, Sawa T, Fujimoto J, et al. Therapeutic administration of anti-PcrV F(ab')(2) in sepsis associated with Pseudomonas aeruginosa. J Immunol 2001;167:5880-6. [Crossref] [PubMed]
- Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 2006;444:567-73. [Crossref] [PubMed]
- Hauser AR, Cobb E, Bodi M, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 2002;30:521-8. [Crossref] [PubMed]
- Frank DW, Vallis A, Wiener-Kronish JP, et al. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J Infect Dis 2002;186:64-73. [Crossref] [PubMed]
- Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 2013;191:1250-9. [Crossref] [PubMed]
- Roy-Burman A, Savel RH, Racine S, et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 2001;183:1767-74. [Crossref] [PubMed]
- Yahr TL, Vallis AJ, Hancock MK, et al. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A 1998;95:13899-904. [Crossref] [PubMed]
- Yahr TL, Mende-Mueller LM, Friese MB, et al. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol 1997;179:7165-8. [Crossref] [PubMed]
- Finck-Barbançon V, Frank DW. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J Bacteriol 2001;183:4330-44. [Crossref] [PubMed]
- Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 2009;7:654-65. [Crossref] [PubMed]
- Ader F, Le Berre R, Faure K, et al. Alveolar response to Pseudomonas aeruginosa: role of the type III secretion system. Infect Immun 2005;73:4263-71. [Crossref] [PubMed]


