
Inflammatory myositis-associated interstitial lung disease can be distinguished from that associated with other connective tissue diseases
Highlight box
Key findings
• Idiopathic inflammatory myositis-associated interstitial lung disease (IIM-ILD) can be distinguished from other connective tissue disease-associated ILDs (CTD-ILDs) by clinical and radiological presentation.
What is known and what is new?
• Uncontrolled studies have suggested that a high proportion of IIM-ILD patients present an acute onset of the disease.
• We compared IIM-ILD with other CTD-ILDs and observed that an acute disease onset was particularly associated with IIM-ILD.
• Radiological nonspecific interstitial lung pneumonia/organizing pneumonia (NSIP/OP) overlap pattern, the simultaneous onset of both ILD and CTD symptoms, and younger age were also associated with IIM-ILD.
What is the implication, and what should change now?
• In acute respiratory failure with lung infiltrates a clinician should evaluate the possibility of IIM-ILD.
Introduction
Interstitial lung disease (ILD) increases the morbidity and mortality of the patients with connective tissue diseases (CTD), such as rheumatoid arthritis (RA), systemic sclerosis (SSc), systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD), Sjögren’s syndrome (SjS) and idiopathic inflammatory myopathies (IIM) (1).
IIM are a heterogeneous group of disorders characterized by muscle inflammation and weakness. The most common IIM in adults are dermatomyositis (DM) including clinically amyopathic dermatomyositis (CADM), polymyositis (PM) and inclusion body myositis (2). The antisynthetase syndrome (ASyS) is a distinct condition, which nowadays is also included in the IIM group (3).
The clinical presentation of any CTD-ILD can vary from an indolent disease course to an acute respiratory failure (4). Several cohorts have revealed that a high proportion of patients with IIM-associated ILD (IIM-ILD) presented an acute onset of disease (3,5-7), particularly in those patients with CADM and/or anti-melanoma differentiation-associated gene-5 (MDA5) positivity (7-9) and ASyS (10). However, a rapid disease onset has been described in other CTD-ILDs as well (11-13). More importantly, the IIM-ILD studies reporting high frequency of acute disease onset have lacked a control group consisting of other CTD-ILDs. Thus, it is unclear whether IIM-ILD has a distinct clinical presentation among CTD-ILDs.
Controlled comparative studies regarding to radiological findings and demographic data of IIM-ILD are also limited. One study has compared high-resolution computed tomography (HRCT) patterns of IIM-ILD and other ILDs, but the control group mostly consisted of idiopathic ILDs and less of CTD-ILDs (14). Thus, it is also unknown whether the radiological findings of IIM-ILD differ from other CTD-ILDs.
The aim of this study was to compare the clinical presentation and radiological findings between IIM-ILD and other CTD-ILDs. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1219/rc).
Methods
Data sources and search
The study cohort consists of patients treated in the Kuopio University Hospital (KUH) pulmonology clinic between 1.1.2000–20.11.2019. The subjects for the study were searched from the KUH database using following International Classification of Diseases (ICD-10) codes: M05.X/M06.X/J99.0*M05.1 for RA, M32.X/J99.1*M32.1 for SLE, M33.X/J99.1*M33.9/J99.1*M33.2 for IIM, M34.X/J99.1*M34.8 for SSc, M35.0/J99.1*M35.0 for SjS, M35.1 for MCTD and M35.8/M35.9 for undifferentiated connective tissue disease (UCTD). We have previously published three studies of RA-ILD, in which the patient search reached to December 2014 (15-17). These patients are included in the present study cohort and the RA searches were further extended to 20.11.2019.
Altogether 2202 new patients were identified. First the patients were screened (HMN, VAEK, HMIJ) based on medical records and HRCT or other comparable computed tomography (CT) reports. If those did not suggest a presence of ILD, the patient was excluded, as were the cases for whom a proper CT scan allowing reliable analysis of the lung parenchyma was not available. The remaining 121 patients with suspected CTD-ILD, as well as our previous RA-ILD cohort of 60 patients, were further analyzed by an experienced rheumatologist (PKE) and two radiologists (HPK, SKS).
Re-analysis of the CTD diagnoses
A rheumatologist independently evaluated laboratory findings, clinico-radiological data and other necessary examinations and re-assessed the CTD diagnoses. Patients were included in the IIM subgroup if they fulfilled either (I) the probable or definite European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and their Major Subgroups (2), or (II) the Connor’s criteria for ASyS (18). Other CTD diagnoses were confirmed by using the current International classification criteria of each disease (19-27). Eight patients with dry eyes, dry mouth, and Sjögren’s-syndrome-related antigen A/B (SSA/SSB) antibodies were included to SjS subgroup as highly probable SjS, without objective tests for dryness of mouth or eyes, but with multiple signs and symptoms supporting the diagnosis. Twenty-three patients with autoantibodies or clinical features of CTD without meeting the criteria for any specific autoimmune disease were included as UCTD (28).
Radiological re-analysis
Two radiologists independently re-assessed the baseline HRCT scans according to the 2018 international statement as a definite usual interstitial pneumonia (UIP), probable UIP, indeterminate with UIP, or alternative diagnosis (29). In cases with alternative diagnosis, the 2013 idiopathic interstitial pneumonias (IIP) classification was applied to diagnose patients as having nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), desquamative interstitial pneumonia (DIP), diffuse alveolar damage (DAD) or unspecific radiological patterns (30). Some patients displayed NSIP/OP overlap pattern, as described in the previous literature (31). In 106 patients with available follow-up HRCTs, the final radiological diagnosis was determined based on the analysis of both CTs. In case of a disagreement, the final radiological categorization was reached via discussion between the investigators (HMN, HPK, SKS).
Gathering of demographic information
After the re-analysis of rheumatological and radiological diagnoses, 27 patients were excluded for not having confirmed CTD-ILD. Clinical information and laboratory test results (Table S1) were gathered from the patient records of KUH. The results of pulmonary function tests were gathered at baseline ± three months.
The order of CTD/ILD presentation was determined from the medical records. If the patient revealed both CTD- and ILD- related symptoms, signs, or findings on the first contact to either rheumatologist or pulmonologist, the disease onset was defined as simultaneous.
The disease onset was defined as acute if the patient needed hospitalization at the onset of ILD. These patients were further divided into those who were admitted to intensive care and those admitted to pulmonology ward. If the patient was managed on an outpatient setting, the disease onset was defined as subacute/chronic. In all cases with a possibility of an acute respiratory infection, appropriate microbiological samples were collected according to the clinician’s consideration. Bronchoalveolar lavage was performed in 63.4% of the patients with an acute onset disease to exclude infections.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). According to Finnish legislation, no consents for inclusion are needed in retrospective register-based study. The study protocol was approved by the Ethics Committee, Hospital District of Northern Savo [statement No. 151/2015 (17/2013)]. In addition, organizational permission of KUH was obtained, which enabled data collection from the hospital registries.
Statistical analysis
The continuous data is expressed as medians (range) and categorical variables as absolute numbers (valid percentages). Different variables were compared using Mann-Whitney U-test, Kruskal-Wallis test or chi-squared test, as appropriate. Missing data was excluded from these comparisons. Logistic regression of features associated with IIM-ILD was used for multivariate analysis. Results are presented as odds ratio (OR) ± 95% confidence interval (CI). A receiver operating curve (ROC) was constructed to evaluate the diagnostic performance of the final multivariate model. Of the variables utilized in the multivariate analyses, there were no missing data. A sensitivity analysis omitting the patients with RA was also performed. Survival time was calculated from the baseline to the date of death or December 10, 2021, when the vital status was ascertained. The Kaplan-Meier method and log-rank test were used to calculate and compare the survival curves. P values <0.05 were considered statistically significant. All data was analyzed using IBM Statistics SPSS software, version 26.0.
Results
Altogether 154 patients with confirmed CTD-ILD were identified. The most common CTDs were RA (78/50.6%), UCTD (23/14.9%), IIM (22/14.3%) and SSc (18/11.7%). Some patients fulfilled the diagnostic criteria of two different CTDs (Figure 1). The IIM-ILD subgroup consisted of 14 patients with ASyS, 5 with CADM, 2 with DM and 5 with PM of whom four fulfilled also criteria for ASyS.
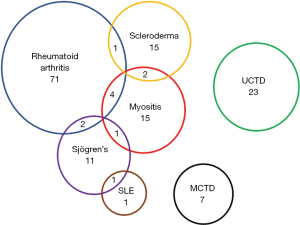
The demographics, lung functions and radiologic findings of different CTD-ILD subgroups are presented in Table 1. The IIM overlap patients are displayed in a separate column and thus the statistical comparisons in Table 1 involve only a subset of patients with IIM-ILD. The patients were mostly female and almost half of them had never smoked (Table 1). All but seven (95.5%) patients suffered from either cough or dyspnea. The median survival was 8.4 years. Comparing all the different CTD subgroups, patients with IIM-ILD had worse baseline FVC than patients with RA-ILD or MCTD-ILD (P values 0.004, 0.036, respectively, Table 1). Fever was more frequent in patients with IIM-ILD compared to RA-ILD (91.7% vs. 22.6%, P<0.001) or SSc-ILD (91.7% vs. 40.0%, P=0.022). In IIM-ILD patients the radiological NSIP/OP overlap pattern (Figure 2) was observed more commonly than in other subgroups.
Table 1
| Variables | All CTD-ILD (n=154) | RA-ILD (n=71, 46.1%) | SjS-ILD (n=11, 7.1%) | SSc-ILD (n=15, 9.7%) | IIM-ILD (n=15, 9.7%) | MCTD-ILD (n=7, 4.5%) | UCTD-ILD (n=23, 14.9%) | Two CTDs (n=11, 7.1%) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 66.8 (26.3–87.0) | 67.6 (31.8–87.0) | 70.9 (47.4–83.1) | 60.7 (43.3–82.2) | 58.8 (48.2–72.9) | 61.0 (37.4–81.7) | 67.1 (26.3–84.0) | 70.0 (35.8–78.3) | 0.178 |
| Male sex | 66 (42.9) | 39 (54.9) | 3 (27.3) | 3 (20.0) | 6 (40.0) | 3 (42.9) | 10 (43.5) | 2 (18.2) | 0.080 |
| Number of deaths | 93 (60.4) | 49 (69.0) | 5 (45.5) | 9 (60.0) | 6 (40.0) | 4 (57.1) | 12 (52.2) | 8 (72.7) | 0.300 |
| Non-smokers* | 74/150 (49.3) | 28/70 (40.0) | 7 (63.6) | 8/14 (57.1) | 10/14 (66.7) | 4/7 (57.1) | 10/23 (43.5) | 7/10 (70.0) | 0.197 |
| Lung functions | |||||||||
| FVC % pred* | 78.0 (31–123) | 82.0 (40–122) | 70.0 (52–93) | 79.0 (31–103) | 69.5 (40–102)#,ǂ | 89.0 (63–117) | 72.0 (51–106) | 83.0 (74–116) | 0.001 |
| DLco % pred* | 60.5 (16–109) | 70.0 (28–109) | 53.0 (42–77) | 56.0 (33–80) | 60.5 (25–77) | 61.5 (55–82) | 52.0 (16–106) | 68.7 (42–87) | 0.039 |
| Symptoms* | |||||||||
| Cough | 86/122 (70.5) | 39/59 (66.1) | 6/7 (85.7) | 7/10 (70.0) | 13/14 (92.9) | 2/4 (50.0) | 13/18 (72.2) | 6/10 (60.0) | 0.417 |
| Dyspnoea | 106/141 (75.2) | 41/64 (64.1) | 9 (81.8) | 13 (86.7) | 9/13 (69.2) | 6/7 (85.7) | 20/21 (95.2) | 7/9 (77.8) | 0.097 |
| Inspiratory crackles | 122/152 (80.3) | 50 (70.4) | 10 (90.9) | 10/14 (71.4) | 15 (100.0)#,¤,ǂ | 5 (71.4) | 21 (91.3) | 10/10 (100.0) | 0.046 |
| Fever | 48/100 (48.0) | 12/53 (22.6) | 4/6 (66.7) | 2/5 (40.0) | 11/12 (91.7)#,¤ | 2/2 (100.0) | 10/13 (76.9) | 6/8 (75.0) | <0.001 |
| Radiological pattern | |||||||||
| UIP | 54 (35.1) | 37 (52.1) | 1 (9.1) | 3 (20.0) | 1 (6.7)# | 1 (14.3) | 6 (26.1) | 5 (45.5) | 0.001 |
| UIP or probable UIP | 68 (44.2) | 42 (59.2) | 2 (18.2) | 4 (26.7) | 2 (13.3)# | 2 (28.6) | 10 (43.5) | 6 (54.5) | 0.006 |
| NSIP | 19 (12.3) | 7 (9.9) | 2 (18.2) | 2 (13.3) | 2 (13.3) | 1 (14.3) | 2 (8.7) | 3 (27.3) | 0.766 |
| OP | 22 (14.3) | 9 (12.7) | 3 (27.3) | 1 (6.7) | 5 (33.3) | 0 (0.0) | 4 (17.4) | 0 (0.0) | 0.127 |
| NSIP/OP overlap | 8 (5.2) | 2 (2.8) | 0 (0.0) | 0 (0.0) | 5 (33.3)#,¥,¤,§ | 0 (0.0) | 0 (0.0) | 1 (9.1) | <0.001 |
| Indeterminate for UIP | 11 (7.1) | 0 (0.0) | 1 (9.1) | 3 (20.0) | 1 (6.7)# | 1 (14.3) | 4 (17.4) | 1 (9.1) | 0.037 |
| Other** | 26 (16.9) | 11 (15.5) | 3 (27.3) | 5 (33.3) | 0 (0.0)¥,¤,§ | 3 (42.9) | 3 (13.0) | 0 (0.0) | 0.043 |
Data are presented as median (range) or n (%). The patients who fulfilled the diagnostic criteria of two different CTDs are presented as a distinct subgroup. Seven patients with IIM-ILD fulfilled diagnostic criteria of two different CTDs. Thus, the number of IIM-ILD patients in this table is 15. One patient with systemic lupus was excluded from the comparative analysis between the subgroups. *, missing data: smoking from 4 subjects, baseline FVC from 14 subjects, baseline DLco from 22 subjects, cough data from 32, dyspnea from 13, inspiratory crackles from 2, fever from 54. **, unspecific changes (n=23), DIP (n=1), or DAD (n=2). #, a statistically significant difference (P<0.05) between IIM-ILD and RA-ILD; ¥, P<0.05 between IIM-ILD and Sjs-ILD; ¤, P<0.05 between IIM-ILD and Ssc-ILD; ǂ, P<0.05 between IIM-ILD and MCTD-ILD; §, P<0.05 between IIM-ILD and UCTD-ILD. CTD, connective tissue disease; ILD, interstitial lung disease; RA, rheumatoid arthritis; SjS, Sjögrens’ syndrome; SSc, systemic sclerosis; IIM, idiopathic inflammatory myositis; MCTD, mixed CTD; UCTD, undifferentiated CTD; FVC, forced vital capacity; DLco, diffusing capacity for carbon monoxide; UIP, usual interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia.
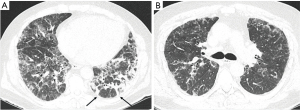
Dichotomous comparison between IIM-ILD patients, including the overlap ones, and other CTD-ILDs is shown in Table 2. The patients with IIM-ILD were younger and more often non-smokers with fever (Table 2). They displayed radiological NSIP/OP pattern more frequently whereas UIP pattern was less common. NSIP/OP overlap pattern showed a 27.3 sensitivity and 98.5 specificity for discriminating IIM-ILD from other CTD-ILDs (Table 2).
Table 2
| Variables | IIM-ILD (n=22) | Other CTD-ILDs (n=132) | P value |
|---|---|---|---|
| Age (y) | 59.7 (35.8–74.8) | 68.0 (26.3–87.0) | 0.023 |
| Male sex | 7 (31.8) | 59 (44.7) | 0.258 |
| Number of deaths | 10 (45.5) | 83 (62.9) | 0.122 |
| Non-smokers* | 15/21 (71.4) | 59/129 (45.7) | 0.029 |
| Fever* | 14/17 (82.4) | 34/83 (41.0) | 0.002 |
| Lung function | |||
| FVC % predicted* | 74.0 (40–116) | 78.5 (31–123) | 0.175 |
| DLco % predicted* | 61.5 (25–87) | 58.5 (16–106) | 0.668 |
| Radiological pattern | |||
| UIP | 3 (13.6)# | 51 (38.6) | 0.023 |
| UIP or probable UIP | 5 (22.7) | 63 (47.7) | 0.029 |
| NSIP | 4 (18.2) | 15 (11.4) | 0.368 |
| OP | 5 (22.7) | 17 (12.9) | 0.222 |
| NSIP/OP overlap | 6 (27.3) | 2 (1.5) | <0.001 |
| Indeterminate for UIP, possible NSIP | 2 (9.1) | 9 (6.8) | 0.702 |
| Other** | 0 (0.0) | 26 (19.7) | 0.022 |
Data were presented as median (range) or n (%). Those IIM-ILD patients that had two CTDs (one of them being IIM), are included in the IIM-ILD-group. *, missing data: smoking data from 1 IIM-ILD subjects and 3 others, baseline FVC from 2 IIM-ILD subjects and 12 others, baseline DLco data from 2 IIM-ILD subjects and 20 others, fever data from 5 IIM-ILD subjects and 49 others. **, unspecific changes (n=23), DIP (n=1), or DAD (n=2). #, concomitant RA in two subjects. ILD, interstitial lung disease; CTD, connective tissue disease; IIM, idiopathic inflammatory myositis; FVC, forced vital capacity; DLco, diffusing capacity for carbon monoxide; UIP, usual interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; RA, rheumatoid arthritis.
Over 90% of the IIM-ILD patients had ILD onset before or simultaneously with CTD presentation, whereas this was the situation in under half of the other CTD-ILDs (Table 3). Every fifth IIM-ILD patient was admitted to intensive care unit (ICU) at the disease onset, which was rare in other CTD-ILDs (22.7% vs. 2.3%, P<0.001). Of the five IIM-ILD patients admitted to ICU, two had CADM with MDA5 antibodies, two had PM + ASyS with positive Jo-1, and one had ASyS with positive PL-12. The median survival between IIM-ILD and other CTD-ILD patients did not differ statistically significantly (101.0 vs. 143.0 months, P=0.663) (Table 3).
Table 3
| Variables | All CTD-ILD (n=154) | IIM-ILD (n=22) | Other CTD-ILDs (n=132) | P value |
|---|---|---|---|---|
| Order of presentation | ||||
| ILD before CTD | 29 (18.8) | 7 (31.8) | 22 (16.7) | 0.092 |
| ILD and CTD simultaneously | 54 (35.1) | 13 (59.1) | 41 (31.1) | 0.011 |
| ILD before CTD or simultaneously | 83 (53.9) | 20 (90.9) | 63 (47.7) | <0.001 |
| CTD before ILD | 71 (46.1) | 2 (9.1) | 69 (52.3) | <0.001 |
| Onset | ||||
| Acute, ICU | 8 (5.2) | 5 (22.7) | 3 (2.3) | <0.001 |
| Acute, pulmonology ward | 33 (21.4) | 7 (31.8) | 26 (19.7) | 0.200 |
| Acute, either ICU or pulmonology ward | 41 (26.6) | 12 (54.5) | 29 (22.0) | 0.001 |
| Chronic/subacute, outpatient clinic | 113 (73.4) | 10 (45.5) | 103 (78.0) | 0.001 |
| Mortality | ||||
| 30 days | 2 (1.3) | 1 (4.5) | 1 (0.8) | 0.146 |
| 90 days | 5 (3.2) | 1 (4.5) | 4 (3.0) | 0.710 |
| 1 year | 11 (7.1) | 2 (9.1) | 9 (6.8) | 0.702 |
| Survival (months) | 101.0 (0.0–436.0) | 143.0 (0.0–191.0) | 100.0 (0.0–436.0) | 0.663 |
Results were expressed as median (range) and n (%). IIM, idiopathic inflammatory myositis; ILD, interstitial lung disease; CTD, connective tissue disease; ICU, intensive care unit.
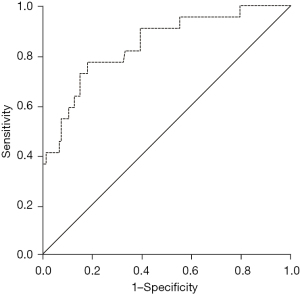
In multivariate analysis, NSIP/OP overlap pattern, acute onset disease treated in ICU and ILD preceding or presenting simultaneously with CTD were significantly associated with IIM-ILD (Table 4). This model, supplemented with age, had excellent diagnostic performance identifying IIM-ILD [area under curve (AUC) 0.845] (Figure 3). The analyses were repeated utilizing the Solomon criteria for ASyS, which did not change the main results (data not shown).
Table 4
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age | 0.964 | 0.923–1.006 | 0.095 |
| NSIP/OP overlap pattern in HRCT | 22.2 | 2.348–210.291 | 0.007 |
| Acute onset, ICU | 19.350 | 1.838–203.735 | 0.014 |
| ILD before CTD or simultaneously | 14.317 | 2.447–83.766 | 0.003 |
IIM, idiopathic inflammatory myositis; ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; HRCT, high-resolution computed tomography; ICU, intensive care unit; OR, odds ratio; CI, confidence interval.
In sensitivity analysis omitting the patients with RA, the IIM-ILD patients were still statistically significantly more likely to develop an acute onset of the ILD simultaneously or prior to CTD with more overlap NSIP/OP radiological appearance (Table 5). Among all subjects with acute onset ILD the most common radiological patterns were OP (41.5%), UIP/probable UIP (19.5%), NSIP (12.2%), and NSIP/OP overlap (12.2%).
Table 5
| Variables | All CTD-ILD (n=76) | IIM-ILD (n=18) | Other CTD-ILDs (n=58) | P value |
|---|---|---|---|---|
| Age | 64.5 (26.3–84.0) | 61.2 (48.2–74.8) | 65.5 (26.3–84.0) | 0.169 |
| Fever* | 33/42 (78.6) | 13/14 (92.9) | 20/28 (71.4) | 0.111 |
| ILD before CTD or simultaneously | 63 (82.9) | 18 (100.0) | 45 (77.9) | 0.021 |
| Onset | ||||
| Acute, ICU | 5 (6.6) | 5 (27.8) | 0 (0.0) | <0.001 |
| Acute, ICU or pulmonology ward | 26 (34.2) | 12 (66.7) | 14 (24.1) | 0.001 |
| Radiological pattern | ||||
| UIP or probable UIP | 22 (28.9) | 3 (16.7) | 19 (32.8) | 0.188 |
| NSIP | 10 (13.2) | 3 (16.7) | 7 (12.1) | 0.614 |
| OP | 13 (17.1) | 5 (27.8) | 8 (13.8) | 0.169 |
| NSIP/OP overlap | 6 (7.9) | 6 (33.3) | 0 (0.0) | <0.001 |
*, data missing form 4 IIM-ILD patients and 30 others. IIM, idiopathic inflammatory myositis; ILD, interstitial lung disease; CTD, connective tissue disease; RA, rheumatoid arthritis; ICU, intensive care unit; UIP, usual interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia.
Detailed data of each IIM-ILD patients diagnostics, treatment, outcome and given medications is described in supplementary Tables S2-S4.
Discussion
To our knowledge, this is the first study to directly compare IIM-ILD with other CTD-ILDs confirming that an acute disease onset was particularly associated with IIM-ILD. Further, NSIP/OP overlap pattern in HRCT, the simultaneous onset of both ILD and CTD symptoms, and younger age were associated with IIM-ILD. These findings are in line with the previous, but mostly uncontrolled studies.
An acute presentation of ILD have been observed in many uncontrolled IIM cohorts. For example, Obert et al. reported acute presentation of ILD in over half of the 48 IIM-ILD patients, defined as the presence of symptoms for less than 2 months (32). Fujisawa et al. examined 114 IIM-ILD patients and reported acute/subacute clinical presentation in 52% of the patients. The definition used for acute presentation was determined as rapidly progressive ILD with deterioration within 3 months (33). A high proportion (>50%) of acute disease onset has been described in many other IIM-ILD cohorts and case reports also (7,8,10), while others have reported smaller percentages of 9–30% (6,34). These controversies probably rise from the different patient selection, as some studies include also asymptomatic patients with an incidental finding of interstitial lung abnormalities (34). In our study, 96% of the patients suffered from either dyspnea or cough. Moreover, the definition of an acute onset varies in different studies as no official definition exists. In the present study, acute onset was based on the need for hospitalization.
We observed a high percentage (91%) of IIM-ILD patients in whom ILD had either preceded or developed simultaneously with IIM symptoms/signs. Almost as high percentages (75–78%) have been reported in many uncontrolled IIM-ILD studies (32,33). Another cohort of 33 IIM-ILD patients reported a simultaneous onset in 67% and ILD being first in 18% (6). In a Danish population-based CTD cohort however, ILD was diagnosed before CTD in only 39% of the IIM-ILD patients similarly as in other CTDs. A simultaneous onset was not reported (35). In that study, the order of presentations was derived from a national registry data without access to individual patient data, which may have affected this result (35).
Based on the previous literature and our results, it seems very common, that in IIM-ILD the respiratory and myositis-related symptoms and signs develop rather simultaneously. Therefore, it is crucial that pulmonologists are familiar with typical IIM features so that the necessary investigations are performed. The pulmonologists should look for the presence of symmetrical muscle weakness, myalgia, Gottron papules/sign, distal digital ulceration, nail fold bleeding, dysphagia, skin rashes, or Raynaud’s symptom (36,37).
Currently, autoimmune serology testing is recommended for all ILD patients (29) although it is unclear what auto-antibodies should be investigated (38,39). Universal screening for myositis-specific or myositis-associated antibodies in newly diagnosed ILD is controversial and currently suggested on a case-by-case consideration (29). However, especially those patients who present an acute/subacute respiratory failure with lung infiltrates, should be thoroughly examined keeping the possible IIM-ILD in mind, as stated by others also (4,38,40). Though myositis-specific and myositis-associated antibodies are useful in the diagnostics of these disorders (14), the delay of their laboratory analysis often necessitates the diagnosis on clinical grounds. Unfortunately it seems that critically ill patients are not commonly evaluated for IIM-ILD, since only 5% were tested for autoantibodies in the multinational Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE) cohort with acute respiratory distress syndrome without an identified etiology (41).
According to previous literature, the most common HRCT patterns in IIM-ILD are NSIP or NSIP with OP (42), in line with our findings. In a study of 33 patients with ASyS, NSIP or mixed NSIP/OP patterns were seen in approximately 70% of subjects (43). In two recent studies of 52 PM/DM and 84 ASyS patients NSIP pattern was observed in 32–44%, OP pattern in 19–25% and NSIP/OP overlap pattern in 9-19% of the subjects (44,45). In a study of Enomoto et al., the HRCT patterns of 444 biopsy-proven patients with IIPs were re-assessed. They reported that the cumulative incidence of newly-developed CTD was similar in 21 (4.7%) patients with NSIP/OP overlap pattern and 44 (9.9%) with NSIP pattern, but the developing CTD was diagnosed as IIM in 100% of the patients with NSIP/OP overlap pattern and in 56% in those with NSIP pattern (46). One previous study has compared the radiological patterns of 12 IIM-ILD and 235 non-IIM-ILD subjects, the control group consisting of CTD-ILDs (n=52), IIPs (n=115) and other ILDs (n=68). They reported a statistically significant difference in the presence of NSIP/OP pattern between the groups (14). In our study, the NSIP/OP pattern was not sensitive, but highly specific to identify IIM-ILD patients. Combining this HRCT pattern to age, order of ILD and CTD presentations and an acute onset disease treated in ICU, we observed an excellent diagnostic performance (47) separating IIM-ILD from other CTD-ILDs. The best model of Jee et al. (14) with the highest AUC (0.96) consisted of myositis autoantibodies line immunoblot assay combined with age, gender, CTD-features, and radiological NSIP/OP pattern supporting our findings despite of the different control group.
Most of the limitations of the study rise from its retrospective nature. In the past, all autoantibodies including myositis-panel were neither yet available in clinical practice nor routinely investigated from all ILD patients, which probably has been the situation all over the world (41). Thus, there is some missing laboratory data. In addition, different symptoms and signs were not available from all patients, which may lead to some inaccuracies. However, gathering equally large CTD-ILD cohort prospectively would take several years given the rarity of the diseases. Another limitation is the fact that the IIM-ILD subgroup is fairly small and somewhat skewed towards ASyS and CADM, which might affect the results. A high proportion of RA-ILD patients could have caused a bias, but the sensitivity analysis omitting them did not change the main results. The classification criteria for ASyS are variable (3) and it is not clear which should be used in studies. We chose to use the Connors criteria, but the main results remained statistically significant using the stricter Solomon criteria.
The strengths of this rather large CTD-ILD cohort is that the data is collected comprehensively and that both pulmonologist, rheumatologist and two radiologists have re-analyzed the data.
Conclusions
In conclusion, IIM-ILD can be distinguished from other CTD-ILDs by clinical and radiological presentation. Compared to other CTD-ILDs, the patients with IIM-ILD are younger, they more often develop an acute onset dyspnoea and fever with simultaneous onset of the systemic disease, and display radiological NSIP, OP or NSIP/OP overlap pattern. When encountering this kind of patients, the clinician should suspect IIM-ILD and promptly test muscle enzymes and comprehensive autoantibody tests including myositis-panel.
Acknowledgments
Funding: This work was supported by Foundation of the Finnish Anti-Tuberculosis Association and the North Savo Regional Fund of the Finnish Cultural Foundation to HN.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1219/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1219/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1219/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1219/coif). HMN reports grants from the Foundation of the Finnish Anti-Tuberculosis Association and the North Savo Regional Fund of the Finnish Cultural Foundation during the current work. She also reports a grant from Duodecim for attending a congress; lecturers’ fees from Boehringer Ingelheim Finland Ltd. and Roche Ltd.; congress/travel support from Boehringer-Ingelheim Ltd., Sanofi-Genzyme Ltd. and Chiesi Ltd., all outside the submitted work. PKE reports a consulting fee from Abbvie Ltd.; congress/travel support form Mylan Ltd. and Pfizer Ltd.; participation on an Advisory Board for Astra Zeneca Ltd., all outside the submitted work. SKS owns stocks of CRISPR Therapeutics AG, Faron Pharmaceuticals Ltd. and Merck&Company, Inc. outside the submitted work. MKP reports lecturers’ fees from Boehringer-Ingelheim Finland Ltd. and Roche Ltd.; congress/travel support from Boehringer-Ingelheim Ltd. and Orion Pharma Ltd.; participation on an Advisory Board of Boehringer-Ingelheim Ltd.; personal grants from the Foundation of the Finnish Anti-Tuberculosis Association, the Jalmari and Rauha Ahokas foundation and the Kuopio region respiratory Foundation, all outside the submitted work. RLK reports lecturers’ fees from Boehringer-Ingelheim Ltd. and Roche Ltd.; congress/travel support from Roche Ltd. and Novartis Ltd.; participation on an Advisory Board of Boehringer-Ingelheim Ltd. and MSD Ltd.; being the President of the Finnish Respiratory Society between march 2017 and march 2020; grants for the study group from the Foundation of the Finnish Anti-Tuberculosis Association, the Research Foundation of the Pulmonary Diseases, the Research Foundation of North Finland, the Jalmari and Rauha Ahokas Foundation, and a state subsidy of the Oulu University Hospital, all outside the submitted work. HOK reports lecturers’ fee from Boehringer Ingelheim Finland Ltd.; congress/travel support from Astra Zeneca Ltd.; owning shares of Orion Pharma Ltd., all outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). According to the Finnish legislation, consents to participate are not required in retrospective register-based studies. The study protocol was approved by the Ethics Committee, Hospital District of Northern Savo (statement No. 151/2015 (17/2013)). In addition, organizational permission of KUH was obtained, which enabled data collection from the hospital registries.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeganathan N, Sathananthan M. Connective Tissue Disease-Related Interstitial Lung Disease: Prevalence, Patterns, Predictors, Prognosis, and Treatment. Lung 2020;198:735-59. [Crossref] [PubMed]
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955-64. [Crossref] [PubMed]
- Marco JL, Collins BF. Clinical manifestations and treatment of antisynthetase syndrome. Best Pract Res Clin Rheumatol 2020;34:101503. [Crossref] [PubMed]
- Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016;352:h6819. [Crossref] [PubMed]
- Marie I, Hatron PY, Dominique S, et al. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum 2011;63:3439-47. [Crossref] [PubMed]
- Won Huh J, Soon Kim D, Keun Lee C, et al. Two distinct clinical types of interstitial lung disease associated with polymyositis-dermatomyositis. Respir Med 2007;101:1761-9. [Crossref] [PubMed]
- Suda T, Fujisawa T, Enomoto N, et al. Interstitial lung diseases associated with amyopathic dermatomyositis. Eur Respir J 2006;28:1005-12. [Crossref] [PubMed]
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest 2009;136:1341-7. [Crossref] [PubMed]
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol 2007;26:1647-54. [Crossref] [PubMed]
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008;63:53-9. [Crossref] [PubMed]
- Enomoto N, Egashira R, Tabata K, et al. Analysis of systemic lupus erythematosus-related interstitial pneumonia: a retrospective multicentre study. Sci Rep 2019;9:7355. [Crossref] [PubMed]
- Furukawa H, Oka S, Higuchi T, et al. Biomarkers for interstitial lung disease and acute-onset diffuse interstitial lung disease in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2021;13:1759720X211022506.
- Roca F, Dominique S, Schmidt J, et al. Interstitial lung disease in primary Sjögren's syndrome. Autoimmun Rev 2017;16:48-54. [Crossref] [PubMed]
- Jee AS, Parker MJS, Bleasel JF, et al. Diagnosis of myositis-associated interstitial lung disease: Utility of the myositis autoantibody line immunoassay. Respir Med 2021;187:106581. [Crossref] [PubMed]
- Nurmi HM, Purokivi MK, Kärkkäinen MS, et al. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med 2016;16:107. [Crossref] [PubMed]
- Nurmi HM, Purokivi MK, Kärkkäinen MS, et al. Are risk predicting models useful for estimating survival of patients with rheumatoid arthritis-associated interstitial lung disease? BMC Pulm Med 2017;17:16. [Crossref] [PubMed]
- Nurmi HM, Kettunen HP, Suoranta SK, et al. Several high-resolution computed tomography findings associate with survival and clinical features in rheumatoid arthritis-associated interstitial lung disease. Respir Med 2018;134:24-30. [Crossref] [PubMed]
- Connors GR, Christopher-Stine L, Oddis CV, et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 2010;138:1464-74. [Crossref] [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. [Crossref] [PubMed]
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747-55. [Crossref] [PubMed]
- LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202-5.
- Alarcón-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol 1989;16:328-34.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151-9. [Crossref] [PubMed]
- Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum 1999;42:1785-96. [Crossref] [PubMed]
- Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren's Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol 2017;69:35-45. [Crossref] [PubMed]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554-8. [Crossref] [PubMed]
- Antunes M, Scirè CA, Talarico R, et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open 2018;4:e000786. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Zuo Y, Ye L, Liu M, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology (Oxford) 2020;59:2829-37. [Crossref] [PubMed]
- Obert J, Freynet O, Nunes H, et al. Outcome and prognostic factors in a French cohort of patients with myositis-associated interstitial lung disease. Rheumatol Int 2016;36:1727-35. [Crossref] [PubMed]
- Fujisawa T, Hozumi H, Kono M, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS One 2014;9:e98824. [Crossref] [PubMed]
- Shi J, Li S, Yang H, et al. Clinical Profiles and Prognosis of Patients with Distinct Antisynthetase Autoantibodies. J Rheumatol 2017;44:1051-7. [Crossref] [PubMed]
- Hyldgaard C, Bendstrup E, Pedersen AB, et al. Interstitial Lung Disease in Connective Tissue Diseases: Survival Patterns in a Population-Based Cohort. J Clin Med 2021;10:4830. [Crossref] [PubMed]
- Waseda Y. Myositis-Related Interstitial Lung Disease: A Respiratory Physician's Point of View. Medicina (Kaunas) 2021;57:599. [Crossref] [PubMed]
- Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: A concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol 2018;78:776-85. [Crossref] [PubMed]
- Bahmer T, Romagnoli M, Girelli F, et al. The use of auto-antibody testing in the evaluation of interstitial lung disease (ILD)--A practical approach for the pulmonologist. Respir Med 2016;113:80-92. [Crossref] [PubMed]
- Alsumrain M, De Giacomi F, Mirza S, et al. Utility of autoimmune serology testing in the assessment of uncharacterized interstitial lung disease: a large retrospective cohort review. Respir Res 2017;18:161. [Crossref] [PubMed]
- Jablonski R, Bhorade S, Strek ME, et al. Recognition and Management of Myositis-Associated Rapidly Progressive Interstitial Lung Disease. Chest 2020;158:252-63. [Crossref] [PubMed]
- de Prost N, Pham T, Carteaux G, et al. Etiologies, diagnostic work-up and outcomes of acute respiratory distress syndrome with no common risk factor: a prospective multicenter study. Ann Intensive Care 2017;7:69. [Crossref] [PubMed]
- Long K, Danoff SK. Interstitial Lung Disease in Polymyositis and Dermatomyositis. Clin Chest Med 2019;40:561-72. [Crossref] [PubMed]
- Debray MP, Borie R, Revel MP, et al. Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings. Eur J Radiol 2015;84:516-23. [Crossref] [PubMed]
- Vojinovic T, Cavazzana I, Ceruti P, et al. Predictive Features and Clinical Presentation of Interstitial Lung Disease in Inflammatory Myositis. Clin Rev Allergy Immunol 2021;60:87-94. [Crossref] [PubMed]
- Jiang M, Dong X, Zheng Y. Clinical characteristics of interstitial lung diseases positive to different anti-synthetase antibodies. Medicine (Baltimore) 2021;100:e25816. [Crossref] [PubMed]
- Enomoto N, Sumikawa H, Sugiura H, et al. Clinical, radiological, and pathological evaluation of "NSIP with OP overlap" pattern compared with NSIP in patients with idiopathic interstitial pneumonias. Respir Med 2020;174:106201. [Crossref] [PubMed]
- Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010;5:1315-6. [Crossref] [PubMed]


