
Global trends in the burden of esophageal cancer, 1990−2019: results from the Global Burden of Disease Study 2019
Highlight box
Key findings
• This study found a decreasing global burden of esophageal cancer. However, the trends differed across countries and territories. A nonlinear but generally inverse correlation was observed between age-standardized disability-adjusted life-years (DALYs) and sociodemographic index (SDI).
What is known and what is new?
• Previous studies have reported various health metrics of esophageal cancer in different countries and regions around the world.
• This cross-sectional study provides the most up-to-date estimates of esophageal cancer incidence, deaths, and DALYs and their temporal trends from 204 countries and territories, with significant differences by gender, region, country, age, and SDI. In addition, the proportion of DALYs attributable to several recognized risk factors for esophageal cancer was presented.
What is the implication, and what should change now?
• Esophageal cancer is a highly fatal disease that requires effective early screening and primary prevention efforts, especially in some high-risk areas.
Introduction
Esophageal cancer is one of the most common causes of cancer-related mortality worldwide (1-3). Esophageal cancer, along with other types of gastrointestinal cancers, accounts for roughly one-third of all cancer-related disability-adjusted life-years (DALYs) (1,3). Notably, the economic burden of esophageal cancer continues to increase worldwide (4), highlighting the need to summarize the global burden of esophageal cancer to provide theoretical evidence for health care planning and resource allocation.
Esophageal cancer can be classified as squamous cell carcinoma and adenocarcinoma according to histological type. Squamous cell carcinoma is the most prevalent subtype worldwide, accounting for more than 85% of all cases (5). Given the absence of early symptoms before local advancement or metastatic deposit (6), more than half of esophageal cancer patients are diagnosed with distant metastases or irreversible lesions (7). Despite advances in diagnosis and treatment, the overall 5-year survival rate for esophageal cancer is less than 20% (8).
Previous epidemiological studies have reported the regional or country-specific burden of esophageal cancer, including its incidence, mortality, DALY rates, and risk factors (1,9,10). However, compared with other malignancies, there are relatively few studies devoted to the comprehensive assessment of the burden of esophageal cancer. In addition, global trends have not been updated since 2017 (11). Therefore, an updated report based on the latest data for esophageal cancer incidence, deaths, and DALYs is needed.
The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 database provides the latest comprehensive and comparable data on esophageal cancer incidence, death, and DALY rates at the global, regional, and country-specific level. This study is based on data from GBD 2019 and presents the updated burden of esophageal cancer and its attributable risk factors for 204 countries and territories between 1990 and 2019. Understanding cancer burden patterns may provide further insights into the specific etiology and management of esophageal cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-856/rc).
Methods
Overview
Estimates on the incidence, mortality, and DALY rates of esophageal cancer were extracted from GBD 2019, which includes data on 369 diseases and injuries and 87 risk factors across 204 countries and territories and 21 GBD regions. The GBD methodology in detail and changes to GBD 2019 have been summarized in previous papers (12-14). Cancers in GBD 2019 have been classified into 30 groups according to the International Classification of Diseases 10th edition (ICD-10). The rates per 100,000 population were age-standardized by GBD standard population. Considering the uncertainty arising from measurement error, potential biases, and modeling, 95% uncertainty intervals (UIs) were employed for all estimates. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since this study is based on public data and does not involve any individual information, ethical approval is not required.
Data sources
Data sources for GBD 2019 included vital registration systems (22,020 site-years), sample vital registration systems (825 site-years), verbal autopsy reports (514 site-years), and cancer registries (5,288 site-years) (14). Among the data sources, only cancer registries provided both mortality and incidence data, while vital registration and verbal autopsy only provided mortality data. A site-year is a geographic unit that combines location with the calendar year and contributes data for a specific year. The ICD-10 classification codes for esophageal cancer are C15.0−C15.9, D00.1, and D13.0 (14).
Statistical analysis
Mortality estimation
Since data coverage and quality differed across regions, combining the existing data and modeling to estimate incidence and mortality was critical. Mortality-to-incidence ratios (MIRs) were first calculated in regions where incidence and mortality data were available for the same year. For other regions, a linear-step mixed-effects model with logit link functions was utilized to estimate MIRs, with the Healthcare Access and Quality (HAQ) Index, age, and sex as covariates. The estimates generated by this model were smoothed over time and space and further adjusted by spatiotemporal Gaussian process regression (14). The estimated mortality data for each region were calculated by multiplying the incidence data by the MIRs. The observed and estimated mortality data from MIRs were then fed into the Cause of Death Ensemble model (CODEm) to generate final mortality estimates (15). CODEm developed different personalized models with the highest predictive validity by integrating all available data and covariates to obtain the best fit.
Incidence, prevalence, and disability estimation
Estimates of esophageal cancer incidence were calculated by dividing mortality estimates by MIRs. To determine the DALYs for esophageal cancer, the total prevalence was divided into 4 sequelae: diagnosis and primary therapy phase, controlled phase, metastatic phase, and terminal phase (Table S1). DALYs were estimated by summing years lived with disability (YLDs) and years of life lost (YLLs). The YLDs were determined by multiplying sequel-specific prevalence by the corresponding disability weights, and the YLLs were obtained through the multiplication of the estimated number of deaths by the standard life expectancy for that age (14). Details of estimation methods and data sources have been published in a previous study (16).
Sociodemographic index (SDI) and risk factors
Risk factor quantification was based on the GBD 2019 comparative risk assessment (17). The SDI is a comprehensive indicator that investigates the relationship between the burden of esophageal cancer and a country’s socioeconomic development status. SDI ranges from 0 (worst) to 1 (best) and consists of the total fertility rate under the age of 25, the average education level for those aged 15 years and older, and the lag-distributed income per capita (12). The correlation between SDI level and the incidence, mortality, and DALY rates of esophageal cancer was then determined using linear correlation and fitted regression curves. We also presented the proportion of DALYs attributable to 6 recognized risk factors for esophageal cancer: smoking, alcohol use, high body-mass index (BMI), diet low in fruits, diet low in vegetables, and chewing tobacco.
Results
Global level
In 2019, nearly 535,000 (95% UI: 467,000−595,000) new cases of esophageal cancer were identified, with an age-standardized incidence rate of 6.5 (95% UI: 5.7−7.3) per 100,000 population. Global esophageal cancer-related deaths were 498,000 (95% UI: 438,000−551,000) in 2019, with an age-standardized mortality rate of 6.1 (95% UI: 5.4−6.8). Esophageal cancer was responsible for 11.7 million (95% UI: 10.4−12.9 million) DALYs in 2019, with an age-standardized rate of 139.8 (95% UI: 124.4−155.0) (Table 1).
Table 1
| GBD regions | Incidence (95% UI) | Deaths (95% UI) | DALYs (95% UI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (95% UI) | ASRs per 100,000 (95% UI) | Percentage change in ASRs per 100,000 | No. (95% UI) | ASRs per 100,000 (95% UI) | Percentage change in ASRs per 100,000 | No. (95% UI) | ASRs per 100,000 (95% UI) | Percentage change in ASRs per 100,000 | |||
| Global | 534,563 (466,513, 595,343) |
6.5 (5.7, 7.3) |
−0.2 (−0.3, 0) |
498,067 (438,411, 551,462) |
6.1 (5.4, 6.8) |
−0.3 (−0.3, −0.1) |
11,666,017 (10,378,747, 12,938,949) |
139.8 (124.4, 155.0) |
−0.3 (−0.4, −0.1) |
||
| Andean Latin America | 827 (670, 1,021) |
1.5 (1.2, 1.9) |
−0.2 (−0.4, 0) |
889 (723, 1,091) |
1.6 (1.3, 2.0) |
−0.2 (−0.4, 0) |
18,839 (15,081, 23,638) |
101.1 (94.4, 108.2) |
−0.3 (−0.4, −0.1) |
||
| Australasia | 2,192 (1,767, 2,707) |
4.4 (3.5, 5.5) |
0 (−0.2, 0.2) |
2,035 (1,830, 2,218) |
4.0 (3.6, 4.4) |
−0.1 (−0.1, 0) |
39,885 (36,426, 43,385) |
61.7 (52.7, 72.4) |
−0.1 (−0.2, 0) |
||
| Caribbean | 1,920 (1,641, 2,200) |
3.7 (3.2, 4.2) |
0 (−0.2, 0.1) |
1,923 (1,649, 2,197) |
3.7 (3.2, 4.2) |
−0.1 (−0.2, 0.1) |
47,316 (40,107, 54,718) |
88.3 (85.0, 91.4) |
0 (−0.2, 0.1) |
||
| Central Asia | 4,834 (4,274, 5,679) |
6.7 (6.0, 7.8) |
−0.5 (−0.6, −0.4) |
4,924 (4,359, 5,769) |
7.1 (6.3, 8.2) |
−0.5 (−0.6, −0.4) |
129,818 (114,167, 153,552) |
85.2 (78.0, 92.4) |
−0.5 (−0.6, −0.5) |
||
| Central Europe | 5,853 (5,109, 6,664) |
2.9 (2.5, 3.3) |
0 (−0.1, 0.1) |
5,856 (5,124, 6,670) |
2.9 (2.5, 3.2) |
0 (−0.2, 0.1) |
143,701 (124,717, 164,319) |
75.8 (70.6, 82.6) |
0 (−0.2, 0.1) |
||
| Central Latin America | 3,869 (3,281, 4,511) |
1.7 (1.4, 1.9) |
−0.3 (−0.4, −0.2) |
4,021 (3,391, 4,707) |
1.7 (1.5, 2.0) |
−0.3 (−0.4, −0.2) |
90,775 (76,781, 107,044) |
88.6 (84.3, 92.9) |
−0.3 (−0.4, −0.2) |
||
| Central Sub-Saharan Africa | 4,431 (2,378, 6,020) |
8.4 (4.5, 11.6) |
−0.2 (−0.4, 0) |
4,509 (2,426, 6,141) |
9.0 (4.8, 12.4) |
−0.2 (−0.4, 0) |
127,510 (68,514, 172,764) |
67.8 (51.5, 81.6) |
−0.2 (−0.4, 0) |
||
| East Asia | 284,908 (220,166, 338,886) |
13.7 (10.6, 16.3) |
−0.3 (−0.5, −0.1) |
263,307 (209,014, 314,860) |
13.0 (10.2, 15.4) |
−0.4 (−0.5, −0.2) |
5,922,865 (4,733,467, 7,156,234) |
33.4 (26.9, 42.0) |
−0.4 (−0.6, −0.2) |
||
| Eastern Europe | 11,086 (9,669, 12,604) |
3.2 (2.8, 3.7) |
−0.2 (−0.3, −0.1) |
10,655 (9,298, 12,077) |
3.1 (2.7, 3.5) |
−0.3 (−0.4, −0.2) |
277,541 (240,922, 316,272) |
83.5 (72.6, 95.1) |
−0.3 (−0.4, −0.2) |
||
| Eastern Sub-Saharan Africa | 16,391 (12,431, 20,713) |
10.0 (7.7, 12.6) |
−0.1 (−0.2, 0.1) |
16,940 (12,941, 21,344) |
10.8 (8.3, 13.5) |
−0.1 (−0.2, 0.1) |
476,744 (361,802, 608,685) |
267.1 239.2, 310.8) |
−0.1 (−0.3, 0.1) |
||
| High-income Asia Pacific | 25,159 (21,213, 29,616) |
5.7 (4.8, 6.8) |
−0.1 (−0.3, 0) |
16,337 (14,651, 17,793) |
3.5 (3.2, 3.8) |
−0.3 (−0.3, −0.2) |
306,118 (281,921, 333,763) |
53.6 (40.4, 71.7) |
−0.4 (−0.4, −0.3) |
||
| High-income North America | 26,162 (22,461, 30,594) |
4.2 (3.6, 5.0) |
0.1 (−0.1, 0.3) |
24,152 (22,876, 25,147) |
3.8 (3.7, 4.0) |
0.1 (0, 0.1) |
524,630 (503,915, 544,030) |
275.4 (221.9, 331.8) |
0 (0, 0) |
||
| North Africa and Middle East | 10,024 (7,415, 11,436) |
2.4 (1.8, 2.7) |
−0.1 (−0.2, 0.1) |
9,968 (7,385, 11,383) |
2.4 (1.9, 2.7) |
−0.1 (−0.2, 0.1) |
259,488 (183,343, 301,673) |
55.6 (40.5, 63.8) |
−0.1 (−0.3, 0) | ||
| Oceania | 147 (110, 196) |
2.2 (1.7, 2.9) |
0 (−0.2, 0.2) |
147 (111, 197) |
2.3 (1.8, 3.0) |
0 (−0.2, 0.2) |
4,213 (3,133, 5,650) |
131.0 (124.3, 137.7) |
0 (−0.2, 0.2) |
||
| South Asia | 53,488 (46,152, 72,051) |
3.8 (3.3, 5.1) |
−0.2 (−0.3, 0) |
54,161 (46,992, 72,771) |
3.9 (3.4, 5.2) |
−0.2 (−0.3, 0) |
1,476,590 (1,282,692, 1,962,191) |
98.3 (85.5, 131.1) |
−0.2 (−0.3, 0) |
||
| Southeast Asia | 15,543 (13,193, 18,202) |
2.5 (2.2, 3.0) |
−0.1 (−0.2, 0.1) |
15,330 (13,164, 17,964) |
2.6 (2.2, 3.1) |
−0.1 (−0.3, 0.1) |
403,725 (342,843, 472,284) |
38.0 (32.1, 44.8) |
−0.1 (−0.3, 0) |
||
| Southern Latin America | 3,945 (3,158, 4,943) |
4.7 (3.8, 5.9) |
−0.4 (−0.5, −0.2) |
4,067 (3,769, 4,359) |
4.8 (4.5, 5.2) |
−0.4 (−0.4, −0.3) |
83,206 (77,617, 89,099) |
74.7 (64.5, 85.3) |
−0.4 (−0.5, −0.4) |
||
| Southern Sub-Saharan Africa | 5,942 (5,316, 6,943) |
10.7 (9.6, 12.3) |
−0.2 (−0.3, 0.1) |
6,095 (5,489, 7,002) |
11.3 (10.2, 12.8) |
−0.2 (−0.3, 0.1) |
159,882 (142,561, 188,482) |
215.2 (115.5, 294.0) |
−0.3 (−0.4, 0) |
||
| Tropical Latin America | 12,684 (11,993, 13,294) |
5.2 (4.9, 5.4) |
−0.2 (−0.3, −0.2) |
12,767 (11,996, 13,448) |
5.2 (4.9, 5.5) |
−0.2 (−0.3, −0.2) |
328,430 (311,722, 345,187) |
90.7 (76.9, 104.8) |
-0.2 (-0.3, -0.2) |
||
| Western Europe | 40,174 (35,133, 45,706) |
4.6 (4.1, 5.3) |
0 (−0.2, 0.1) |
34,847 (32,416, 36,620 |
3.9 (3.6, 4.1) |
−0.2 (−0.2, −0.1) |
706,817 (669,630, 741,655) |
164.8 (145.6, 193.1) | −0.2 (−0.2, −0.2) |
||
| Western Sub-Saharan Africa | 4,986 (3,776, 5,992) |
2.7 (2.1, 3.2) |
0.3 (0, 0.5) |
5,135 (3,916, 6,146) |
2.9 (2.2, 3.4) |
0.3 (0, 0.5) |
137,923 (104,912, 167,521) |
263.4 (200.5, 332.9) |
0.2 (−0.1, 0.5) |
||
DALY, disability-adjusted life-year; GBD, Global Burden of Diseases, Injuries, and Risk Factors Study; UI, uncertainty interval; ASR, age-standardized rate.
Between 1990 and 2019, the global age-standardized incidence rate had decreased by 0.2% (95% UI: −0.3 to 0), mortality rate had decreased by 0.3% (95% UI: −0.3 to −0.1), and DALYs had decreased by 0.3% (95% UI: −0.4 to −0.1). During the same period, new cases of esophageal cancer had increased by 67.1%, from 320,000 (95% UI: 253,000−351,000) to 535,000 (95% UI: 467,000−595,000). The number of deaths had increased by 56.0%, from 319,000 (95% UI: 249,000−351,000) to 498,000 (95% UI: 438,000−551,000). The total DALYs had increased by 42.1%, from 8.2 million (95% UI: 6.3−9.1 million) to 11.7 million (95% UI: 10.4−12.9 million) (Table 1).
Regional level
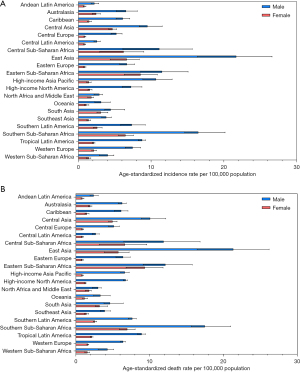
The age-standardized incidence and mortality rate of esophageal cancer in 2019 varied considerably across the 21 GBD regions, with the highest age-standardized incidence rate observed in East Asia [13.7 (95% UI: 10.6−16.3)] per 100,000 population, followed by Southern Sub-Saharan Africa [10.7 (95% UI: 9.6−12.3)] and Eastern Sub-Saharan Africa [10.0 (95% UI: 7.7–12.6)]. In contrast, Andean Latin America [1.5 (95% UI: 1.2−1.9)], Central Latin America [1.7 (95% UI: 1.4−1.9)], and Oceania [2.2 (95% UI: 1.7−2.9)] had the lowest age-standardized incidence rates. Likewise, East Asia [13.0 (95% UI: 10.2−15.4)], Southern Sub-Saharan Africa [11.3 (95% UI: 10.2−12.8)], and Eastern Sub-Saharan Africa [10.8 (95% UI: 8.3−13.5)] were the top 3 regions for the highest age-standardized mortality rate, while Andean Latin America [1.6 (95% UI: 1.3−2.0)], Central Latin America [1.7 (95% UI: 1.5−2.0)], and Oceania [2.3 (95% UI: 1.8−3.0)] were the top 3 regions for lowest age-standardized mortality rate (Table 1). The regional-level age-standardized incidence and mortality rate estimates for all GBD regions in 2019 are depicted by sex in Figure 1A,1B, respectively.
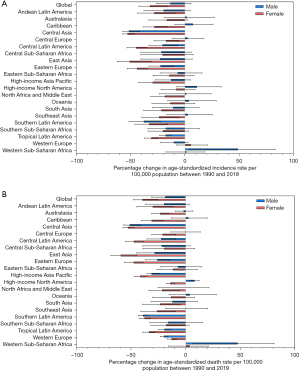
The percentage change in age-standardized incidence rate of esophageal cancer between 1990 and 2019 differed across the 21 GBD regions, with Central Asia [−51.5% (95% UI: −56.6% to −44.1%)], Southern Latin America [−36.7% (95% UI: −50.0% to −20.8%)], and East Asia [−33.0% (95% UI: −45.7% to −12.2%)] experiencing the sharpest drop. By contrast, upward trends were observed in Western Sub-Saharan Africa [28.8% (95% UI: −4.8% to 53.8%)] and High-income North America [9.4% (95% UI: −6.5% to 28.0%)]. With minor changes in rank order, regions with significant decreasing trends in age-standardized mortality rate of esophageal cancer throughout the study period included Central Asia [−51.3% (95% UI: −56.2% to −44.1%)], East Asia [−39.9% (95% UI: −51.2% to −19.9%)], and Southern Latin America [−39.0% (95% UI: −43.2% to −34.5%)], while increasing trends were found in Western Sub-Saharan Africa [29.3% (95% UI: −2.7% to 54.0%)] and High-income North America [6.0% (95% UI: 2.3−10.3%)] (Table 1). Figure 2A,2B show the percentage change in age-standardized incidence and mortality rate at the regional level by sex for all GBD regions from 1990 to 2019.
National level
From among 204 countries and territories, the 3 highest age-standardized incidence rates of esophageal cancer were in Malawi [24.5 (95% UI: 18.7−32.5)], Mongolia [21.9 (95% UI: 14.5−28.0)], and Uganda [15.6 (95% UI: 12.1−19.5)]. In contrast, the lowest rates were seen in Nigeria [0.9 (95% UI: 0.6−1.6)], Syrian Arab Republic [0.9 (95% UI: 0.7−1.2)], and Tunisia [1.0 (95% UI: 0.7−1.3)] (Figure 3A & website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf). Similar results were obtained in age-standardized death rates, with Malawi [25.8 (95% UI: 19.8−33.9)], Mongolia [24.5 (95% UI: 15.3−31.3)], and Uganda [16.5 (95% UI: 12.8−20.6)] showing the highest death rates and Syrian Arab Republic [1.0 (95% UI: 0.7−1.2)], Tunisia [1.0 (95% UI: 0.7−1.3)], and Nigeria [1.0 (95% UI: 0.7−1.8)] showing the lowest rates (Figure 3B & website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf). Malawi [651.6 (95% UI: 481.6−882.9)], Mongolia [486.7 (95% UI: 364.2−628.7)], and Eswatini [409.4 (95% UI: 259.2−568.2)] had the 3 highest age-standardized DALY rates in 2019. By contrast, countries with the lowest DALY rates were Tunisia [21.4 (95% UI: 14.7−29.2)], Syrian Arab Republic [21.7 (95% UI: 16.1−28.5)], and Nigeria [21.9 (95% UI: 14.9−40.0)] (Figure 3C & website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf).
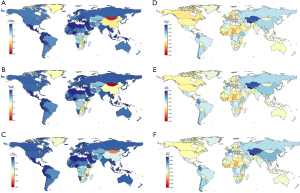
Between 1990 and 2019, the estimated annual percentage change in age-standardized incidence rates of esophageal cancer varied substantially among countries and territories, with Northern Mariana Islands [88.6% (95% UI: −9.9% to 152.7%)], Taiwan (Province of China) [85.2% (95% UI: 40.7−148.5%)], and The Netherlands [84.5% (95% UI: 41.5−135.2%)] experiencing the most significant growth. In contrast, Turkmenistan [−70.1% (95% UI: −76.4% to −62.7%)], Kazakhstan [−63.2% (95% UI: −68.5% to −57.2%)], and Puerto Rico [−60.3% (95% UI: −70.0% to −48.4%)] had the sharpest decline (Figure 3D & website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf). The largest increase in age-standardized death rate was shown in Northern Mariana Islands [83.6% (95% UI: −12.5% to 143.1%)], followed by Sao Tome and Principe [69.7% (95% UI: 12.8−121.2%)] and Cabo Verde [66.5% (95% UI: 35.5−100.6%)]. By contrast, the strongest decreasing trends were observed in Turkmenistan [−70.2% (95% UI: −76.4% to −62.9%)], Singapore [−67.7% (95% UI: −72.1% to −62.4%)], and Kazakhstan [−63.3% (95% UI: −68.6% to −57.3%)] (Figure 3E & website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf). For age-standardized DALY rate, the most significant increases were found in Northern Mariana Islands [80.7% (95% UI: −15.8% to 145.4%)], Taiwan (Province of China) [75.5% (95% UI: 33.1−136.2%)], and Romania [70.6% (95% UI: 34.1−108.4%)], while Turkmenistan [−70.3% (95% UI: −76.7% to −62.8%)], Singapore [−69.3% (95% UI: −73.5% to −64.4%)], and Kazakhstan [−64.8% (95% UI: −69.8% to −58.8%)] showed the largest downward trends (Figure 3F & website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf).
Burden of esophageal cancer by SDI
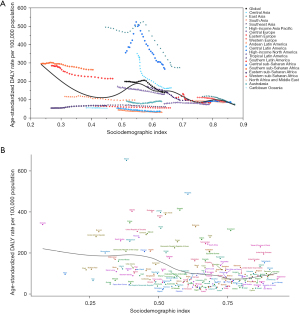
The correlation between regional and national age-standardized DALY rates of esophageal cancer and SDI, together with the expected DALY rate based on SDI, are depicted in Figure 4. The expected pattern represented the average relationship between SDIs and age-standardized rates for esophageal cancer based on values from all countries from 1990 to 2019. The high-SDI regions, including Australasia, Central Europe, Eastern Europe, Western Europe, Southern Latin America, High-income North America, and High-income Asia Pacific, closely followed the expected patterns throughout the study period. However, the actual rates differed substantially among the middle-SDI regions. Some regions were still far below the expected DALY rate with little change, while others remained well above the expected level with fluctuating or declining age-standardized rates (Figure 4A). Similarly, the national-level analysis in 2019 found an overall negative nonlinear correlation between age-standardized DALY rates of esophageal cancer and SDI (Figure 4B). Identical trends were observed in the age-standardized incidence and mortality rates in relation to SDI (Figures S1,S2).
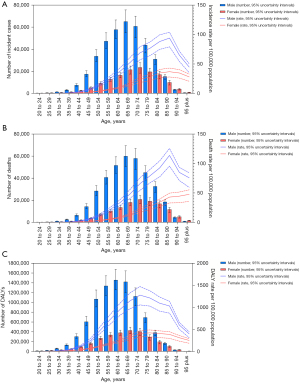
Age and sex patterns
Incidence, mortality, and DALY rates differed by age and sex in 2019. The global incidence and mortality rates of esophageal cancer began to rise at the age of 50, while DALY rates started climbing at the age of 40, and both had a falling trend in the oldest age groups. Generally, males had higher incidence, mortality, and DALY rates than females in all age groups (Figure 5). Comparable findings were obtained in the age-standardized incidence, mortality, and DALY rates, with 3.0 times higher incidence rates, 3.2 times higher mortality rates, and 3.4 times higher DALY rates in males than in females (website: https://cdn.amegroups.cn/static/public/jtd-22-856-01.pdf).
Risk factors
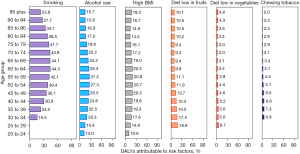
The percentage of DALYs attributable to risk factors in 2019 demonstrated various patterns among regions and age groups. Globally, the following 6 risk factors were responsible for a substantial proportion of DALYs, including 40.7% (95% UI: 36.6−44.5%) attributable to smoking, 24.1% (95% UI: 18.4−29.7%) to alcohol use, 18.9% (95% UI: 6.0−36.0%) to high BMI, 10.7% (95% UI: 3.4−22.6%) to a diet low in fruits, 3.6% (95% UI: 0.6−7.1%) to a diet low in vegetables, and 4.1% (95% UI: 2.8−5.5%) to use of chewing tobacco (Figure 6). The impact of these risk factors differed across regions. The proportion of age-standardized DALYs attributable to smoking was highest in Eastern Europe (49.7%) and East Asia (48.5%) and lowest in Andean Latin America (17.2%). Similarly, the impact of alcohol use was greatest in Central Europe (37.7%) and Western Europe (37.1%) and lowest in North Africa and the Middle East (3.6%). Moreover, the influence of these risk factors varied across age groups. The highest percentage of attributable DALYs for smoking and alcohol consumption was observed in populations aged 60−64 and 50−54, respectively (Figure 7). The sex-specific estimates of DALYs attributable to risk factors are shown in Figures S3-S6.

Discussion
Esophageal cancer is a significant contributor to the global cancer burden. In this study, we reported on GBD 2019 estimates of the latest trends and patterns of the incidence, mortality, and DALYs of esophageal cancer and further identified relevant risk factors through gender and age stratification in 204 countries and territories from 1990 to 2019. Overall, the total number of cases, deaths, and DALYs has been increasing worldwide. Fortunately, the age-standardized incidence, mortality, and DALY rates have steadily declined globally, which may be attributable to improvements in socioeconomic level, prevention and treatment strategies, and population health awareness. Our findings are generally consistent with the Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) project, although our estimates were slightly lower than theirs, with an estimated 535,000 new cases and 498,000 deaths in GBD 2019, versus 604,000 new cases and 544,000 deaths in GLOBOCAN 2020 (18). These discrepancies could be attributed to the different datasets and methodology used in each study. Specifically, since data in GBD is weighted based on the level of completeness, more weight might have been given to data from high-income countries, which have more complete data and lower esophageal cancer incidence rates.
GBD estimates revealed a geographic variation in age-standardized incidence rates of esophageal cancer. Specifically, the estimates showed a decrease in most parts of the world, especially in regions with squamous cell carcinoma as the major histologic subtype, but there was an increase in regions with adenocarcinoma as the predominant subtype, emphasizing the necessity of distinguishing between these 2 common histologic subtypes and further investigating the etiologies of high-risk areas.
The highest age-standardized incidence, mortality, and DALY rates were observed in some regions of East and Central Asia, as well as in Southern, Eastern, and Central sub-Saharan Africa. In Asia, the region with the highest risk of esophageal cancer, extending from Northern Iran through Central Asia to Mongolia and North-Central China, is dubbed the so-called “Asian esophageal cancer belt” (19), with 90% of cases being squamous cell carcinoma (20-22). This area is consistent with the ancient Silk Road established by China 2000 years ago (23), which may be related to some shared environmental risks or genetic structures along this route (21,24). However, as yet, the contributing risk factors for the high incidence and mortality rates in the “Asian esophageal cancer belt” have not been thoroughly investigated, although they are thought to be associated with nutritional deficiencies (25), low intake of fruits and vegetables (26), poor oral hygiene (27), chewing betel quid (28), as well as consumption of pickled vegetables (29) and high-temperature beverages and foods (30-33). In Africa, the area with a high incidence of esophageal cancer is referred to as the “African esophageal cancer corridor”, which runs from Ethiopia and Kenya to South Africa (34,35). Similarly, esophageal squamous cell carcinoma comprises the majority of cases in this region. So far, major risk factors in the “African esophageal cancer corridor” have yet to be elucidated, although tobacco use (36), alcohol consumption (36,37), dental fluorosis (35), hot beverage consumption (37), and exposure to biomass smoke (38,39) have been suspected. The incidence of esophageal squamous cell carcinoma is steadily decreasing in most parts of the world. For certain high-risk areas in Asia, economic development and diet quality improvement may have had some influence on the decline of esophageal cancer incidence (40). Since approximately 90% of esophageal squamous cell carcinoma cases in the United States and several other western countries are caused by tobacco use and excessive drinking (41), the decline is thought to be primarily due to long-term smoking cessation and reduction in alcohol consumption (40), which corroborates with the fact that males had a higher incidence of esophageal cancer than females (36).
Adenocarcinoma accounts for nearly two-thirds of esophageal cancer cases in high-income countries, with smoking (42,43), obesity (44), gastroesophageal reflux disease (GERD) (45), and Barrett’s esophagus (46,47) being the main risk factors. Over the last 3 decades, the incidence rate of esophageal adenocarcinoma has risen rapidly, surpassing that of squamous cell carcinoma in several western countries, and more recently, in some eastern countries (8,48). The underlying causes may be the increasing incidence of overweight and GERD, as well as the declining incidence of Helicobacter pylori infection, which has been proven to be inversely associated with esophageal adenocarcinoma (49). Given the rising prevalence of obesity, these trends are likely to continue for years to come, and being overweight may become an increasingly important contributor in the future burden of esophageal cancer (5,50).
In this study, smoking, chewing tobacco, alcohol use, high BMI, and low fruit and vegetable intake were identified as important risk factors attributable to esophageal cancer. It is worthwhile mentioning that some specific risk factors may differ in the 2 major histologic types, for instance, obesity was correlated with a higher risk of esophageal adenocarcinoma but did not appear to increase the risk of squamous cell carcinoma (51). In addition, smoking was another critical risk factor contributing to esophageal cancer. A meta-analysis assessed the risk of esophageal cancer after smoking cessation by histologic type, demonstrating no significant reduction in the risk of adenocarcinoma compared with squamous cell carcinoma (52). The discrepancies in the risk factors suggested the different pathogenesis of the 2 subtypes. To address the increasing global burden of esophageal cancer, the above-mentioned risk factors should be closely monitored and controlled in the future. For instance, both smoking cessation and control of alcohol consumption are recommended to reduce the risk of squamous cell carcinoma, especially for populations in western countries. For adenocarcinoma, it is critical to slow down the growing rates of obesity and metabolic syndrome, which are also important risk factors for other common chronic illnesses such as cardiovascular disease and other cancers.
The current study found an inverse association between age-standardized DALY rates of esophageal cancer and SDI at the regional and national levels. These results were in line with prior epidemiological studies that have reported a negative correlation between esophageal squamous cell carcinoma and socioeconomic status (21,53-55). Although the incidence of squamous cell carcinoma has decreased in recent years, it still comprises the vast majority of esophageal cancer cases globally. Hence, an inverse association between esophageal cancer and SDI was observed on a global scale, which may be attributed to several correlated and interconnected variables. Specifically, low SDI is a proxy for lower intake of certain nutrients and exposure to certain environmental risk factors associated with esophageal cancer. Previous studies have identified that, in Malawi, the country with the highest incidence of esophageal cancer, iron (Fe), magnesium (Mg), zinc (Zn), and selenium (Se) intake were generally lower, which might have been related to the local low-pH soils (56,57). Moreover, low SDI was associated with unimproved water sources, poor oral health, consumption of hot beverages, air pollution, and exposure to biomass smoke, which have been proven to be well-established risk factors for esophageal squamous cell carcinoma (32,37,53). In addition, the lack of effective diagnostic tools in low-income countries may have given rise to the high incidence rate of esophageal cancer (58,59). These findings might help us understand the substantial decline in the incidence of esophageal squamous cell carcinoma, which may be related to improved socioeconomic status worldwide (60).
GBD estimates have provided high-quality data on global cancer burden, yet the outcomes of our study were affected by several limitations. The major limitation of the current study was the lack of high-quality data in certain regions, especially in low-income countries, leading to biased estimates of esophageal cancer incidence and mortality. For example, in some low- or middle-SDI regions, morbidity or mortality data were either scarce or unavailable. In addition, ascertainment bias, detection bias, and inaccuracy in diagnosis may have further reduced the accuracy of the estimates. Hence, a model based on predictive covariates and nearby locations was utilized to estimate the outcomes. However, this modeling may have introduced greater bias for esophageal cancer than for other cancers as the incidence of esophageal squamous cell carcinoma may vary significantly within a short geographic distance. An additional limitation of this study was the paucity of independent data for the 2 subtypes. As mentioned above, the epidemiologic features are drastically different for the 2 predominant histological subtypes of esophageal cancer (21), but data for these 2 subtypes have not yet been separately obtained in GBD. Further studies using esophageal cancer data stratified by histologic subtypes could shed more light on how genetic susceptibility and environmental exposure contribute to increased risk of esophageal cancer.
Conclusions
In conclusion, esophageal cancer incidence, deaths, and DALYs appeared to be increasing globally, suggesting a greater burden on the global health care system, especially among males and countries with lower SDI. However, age-standardized incidence, deaths, and DALY rates for esophageal cancer have declined, which may be related to the intensive reduction of major known and potential risk factors such as smoking and alcohol consumption. The burden of esophageal cancer was largely heterogeneous in geographical distributions, reflecting differences in the corresponding genetic and environmental risk factors, as well as levels of socioeconomic status, lifestyle, and resources for early screening and therapeutic care. Understanding these trends is critical for developing and implementing appropriate preventive and treatment strategies.
Acknowledgments
We highly appreciate the works by the Global Burden of Disease Study 2019 collaborators. Besides, we thank the language editing work from AME language editing service.
Funding: This work was supported by China National Science Foundation (Grant Nos. 82022048 and 81871893) and the Key Project of Guangzhou Scientific Research Project (Grant No. 201804020030).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-856/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-856/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-856/coif). Jianxing He serves as Executive Editor-in-Chief of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since this study is based on public data and does not involve any individual information, ethical approval is not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fitzmaurice C, Abate D, Abbasi N, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Casamayor M, Morlock R, Maeda H, et al. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience 2018;12:883. [Crossref] [PubMed]
- Arnold M, Laversanne M, Brown LM, et al. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol 2017;112:1247-55. [Crossref] [PubMed]
- Veugelers PJ, Porter GA, Guernsey DL, et al. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus 2006;19:321-8. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) 2021;41:1037-48. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:582-97. [Crossref] [PubMed]
- Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1160-203. [Crossref] [PubMed]
- Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223-49. [Crossref] [PubMed]
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. [Crossref] [PubMed]
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. [Crossref] [PubMed]
- Kocarnik JM, Compton K, Dean FE, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol 2022;8:420-44. [Crossref] [PubMed]
- The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022;400:563-91. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Codipilly DC, Qin Y, Dawsey SM, et al. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc 2018;88:413-26. [Crossref] [PubMed]
- Islami F, Kamangar F, Aghcheli K, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer 2004;90:1402-6. [Crossref] [PubMed]
- Kamangar F, Chow WH, Abnet CC, et al. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27-57. vii. [Crossref] [PubMed]
- Grille VJ, Campbell S, Gibbs JF, et al. Esophageal cancer: the rise of adenocarcinoma over squamous cell carcinoma in the Asian belt. J Gastrointest Oncol 2021;12:S339-49. [Crossref] [PubMed]
- Yao YG, Kong QP, Wang CY, et al. Different matrilineal contributions to genetic structure of ethnic groups in the silk road region in china. Mol Biol Evol 2004;21:2265-80. [Crossref] [PubMed]
- Kamangar F, Malekzadeh R, Dawsey SM, et al. Esophageal cancer in Northeastern Iran: a review. Arch Iran Med 2007;10:70-82.
- McCormack VA, Menya D, Munishi MO, et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: A review of setting-specific exposures to known and putative risk factors. Int J Cancer 2017;140:259-71. [Crossref] [PubMed]
- Liu J, Wang J, Leng Y, et al. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473-85. [Crossref] [PubMed]
- Dar NA, Islami F, Bhat GA, et al. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer 2013;109:1367-72. [Crossref] [PubMed]
- Akhtar S, Sheikh AA, Qureshi HU. Chewing areca nut, betel quid, oral snuff, cigarette smoking and the risk of oesophageal squamous-cell carcinoma in South Asians: a multicentre case-control study. Eur J Cancer 2012;48:655-61. [Crossref] [PubMed]
- Wang L, Zhu D, Zhang C, et al. Mutations of O6-methylguanine-DNA methyltransferase gene in esophageal cancer tissues from Northern China. Int J Cancer 1997;71:719-23. [Crossref] [PubMed]
- Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491-524. [Crossref] [PubMed]
- Islami F, Pourshams A, Nasrollahzadeh D, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ 2009;338:b929. [Crossref] [PubMed]
- Yu C, Tang H, Guo Y, et al. Hot Tea Consumption and Its Interactions With Alcohol and Tobacco Use on the Risk for Esophageal Cancer: A Population-Based Cohort Study. Ann Intern Med 2018;168:489-97. [Crossref] [PubMed]
- Islami F, Poustchi H, Pourshams A, et al. A prospective study of tea drinking temperature and risk of esophageal squamous cell carcinoma. Int J Cancer 2020;146:18-25. [Crossref] [PubMed]
- Schaafsma T, Wakefield J, Hanisch R, et al. Africa's Oesophageal Cancer Corridor: Geographic Variations in Incidence Correlate with Certain Micronutrient Deficiencies. PLoS One 2015;10:e0140107. [Crossref] [PubMed]
- Menya D, Maina SK, Kibosia C, et al. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer 2019;145:99-109. [Crossref] [PubMed]
- Sewram V, Sitas F, O'Connell D, et al. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol 2016;41:113-21. [Crossref] [PubMed]
- Menya D, Kigen N, Oduor M, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer 2019;144:459-69. [Crossref] [PubMed]
- Mlombe YB, Rosenberg NE, Wolf LL, et al. Environmental risk factors for oesophageal cancer in Malawi: A case-control study. Malawi Med J 2015;27:88-92. [Crossref] [PubMed]
- Kayamba V, Heimburger DC, Morgan DR, et al. Exposure to biomass smoke as a risk factor for oesophageal and gastric cancer in low-income populations: A systematic review. Malawi Med J 2017;29:212-7. [Crossref] [PubMed]
- Blot WJ TR. Esophageal cancer. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Cancer Epidemiology and Prevention. 4th ed. Oxford University Press; 2018:579-593.
- Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003;95:1404-13. [Crossref] [PubMed]
- Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology 2011;22:344-9. [Crossref] [PubMed]
- Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344-53. [Crossref] [PubMed]
- Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol 2012;41:1706-18. [Crossref] [PubMed]
- Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One 2014;9:e103508. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;18:432-43. [Crossref] [PubMed]
- Caspa Gokulan R, Garcia-Buitrago MT, Zaika AI. From genetics to signaling pathways: molecular pathogenesis of esophageal adenocarcinoma. Biochim Biophys Acta Rev Cancer 2019;1872:37-48. [Crossref] [PubMed]
- Rokkas T, Pistiolas D, Sechopoulos P, et al. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol 2007;5:1413-7, 1417.e1-2.
- Lin L, Li Z, Yan L, et al. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990-2019. J Hematol Oncol 2021;14:197. [Crossref] [PubMed]
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- Wang QL, Xie SH, Li WT, et al. Smoking Cessation and Risk of Esophageal Cancer by Histological Type: Systematic Review and Meta-analysis. J Natl Cancer Inst 2017;
- Sheikh M, Poustchi H, Pourshams A, et al. Individual and Combined Effects of Environmental Risk Factors for Esophageal Cancer Based on Results From the Golestan Cohort Study. Gastroenterology 2019;156:1416-27. [Crossref] [PubMed]
- Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009;38:978-88. [Crossref] [PubMed]
- Dar NA, Shah IA, Bhat GA, et al. Socioeconomic status and esophageal squamous cell carcinoma risk in Kashmir, India. Cancer Sci 2013;104:1231-6. [Crossref] [PubMed]
- Chasimpha SJD, Parkin DM, Masamba L, et al. Three-year cancer incidence in Blantyre, Malawi (2008-2010). Int J Cancer 2017;141:694-700. [Crossref] [PubMed]
- Hurst R, Siyame EW, Young SD, et al. Soil-type influences human selenium status and underlies widespread selenium deficiency risks in Malawi. Sci Rep 2013;3:1425. [Crossref] [PubMed]
- Gowing LR, Ali RL, Allsop S, et al. Global statistics on addictive behaviours: 2014 status report. Addiction 2015;110:904-19. [Crossref] [PubMed]
- Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 2011;105:S77-81. [Crossref] [PubMed]
- Khademi H, Kamangar F. Esophageal cancer incidence trends in northeastern Iran: comparing rates over 36 years. Arch Iran Med 2012;15:194-5.
(English Language Editors: A. Muijlwijk)


