Postperfusion lung syndrome and related sequelae
Introduction
Postperfusion lung syndrome, i.e., acute respiratory distress syndrome (ARDS) secondary to cardiopulmonary bypass, is rare with an incidence of only 1–2% (1). Mortality is as high as 91.6%, with 70% of patients ultimately progressing to multiorgan failure (2). Besides, mixed acid-base disturbances often develop as a sequel of ARDS. These two sequelae of ARDS can be the direct causes of death rather than postperfusion lung syndrome itself. Triple acid-base disturbances (TABDs) in association with ARDS remain a topic of interest in terms of the complexities of the diagnostic criteria and management strategies. Discussions on TABDs are usually confined to high-anion gap (AG) type TABD; whereas hyperchloride TABDs are rarely encountered (3). This article presents a complicated postperfusion lung syndrome with high-AG and hyperchloride TABDs following cardiac surgery.
Case presentation
A 69-year-old female complained of a four-year exertional chest distress, dyspnea and dizziness. Echocardiography in a local hospital was suggestive of a hyperdense echo mass on the anterior mitral leaflet. On admission, her vital signs were normal. Electrocardiogram showed sinus bradycardia (heart rate 54/min) and first degree atrioventricular block (P-R interval 0.23 s) with an axis deviation of −13°. Echocardiography revealed a hyperdense echo mass 1.6 cm × 1.2 cm attaching to the atrial septum (Figure 1A). Pulmonary function tests and coronary angiography revealed normal results.
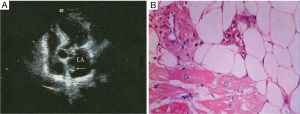
She was diagnosed with a left atrial myxoma and was scheduled for a surgical resection. The neoplasm was approached via a right atrium-atrial septum approach and was resected en bloc. After crossclamp removal, the heart recovered to automatic rhythm with a heart rate of 20–30/min. The right ventricular epicardial pacing lead was secured and a temporary pacemaker was connected with a setup of heart rate of 90–100/min, an output of 5 mV and a sensitivity of 2.0. Her blood pressure and heart rate gradually became normal after 3.5-hour pacemaking and inotropic infusions (adrenaline 5 mg/50 mL, dopamine 160 mg + dobutamine 160 mg/50 mL, infused at 13 mL/hour). The rhythm was predominantly sinus with intermittent ventricular pacing. The cardiopulmonary bypass and crossclamp times were 105 and 56 minutes, respectively. She was sent to the Intensive Care Unit. Histology suggested a cardiac lipoma (Figure 1B).
In Intensive Care Unit, Acute Physiology and Chronic Health Evaluation (APACHE) II scored 17. With continuous inotropic infusions, her blood pressure was 88–118/58–79 mmHg and central venous pressure was 16–26 cmH2O. The ventilatory modes were synchronized intermittent mandatory ventilation-pressure controlled (SIMV-PC) with a respiratory rate 12–16/min, tidal volume 6 mL/kg, peak inspiratory pressure (PIP) 12 cmH2O, positive end-expiratory pressure (PEEP) 3 cmH2O, pressure support ventilation (PSV) 12 cmH2O, inspiratory to expiratory time (I:E) ratio 1:1.9–2.4 and fraction of inspiration O2 (FiO2) 0.45–0.50.
Blood-gas analysis revealed metabolic acidosis at postoperative hours (POHs) one and five and then a mixed respiratory alkalosis with metabolic acidosis, with high chlorine and AG thereafter. A low dose of sodium bicarbonate was alternatively given based on blood-gas analyses. Calculations of the metabolic parameters revealed a separation of ∆AG + ∆Cl− and ∆HCO3−, outlining a sustained increase of ∆AG + ∆Cl− over ∆HCO3− (Figure 2A).
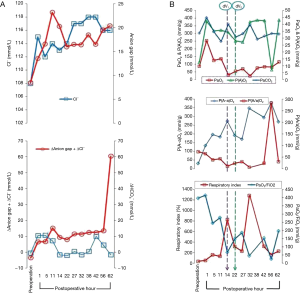
PaO2/FiO2 showed significant negative correlations with alveolar-arterial oxygen gradient [P(A-a)O2] (Y=−0.6829X + 357.84, r=−0.8837, P=0.0003) and with respiratory index (Y=−1.8937X + 711.3, r=−0.6499, P=0.0304). No significant correlation was found between PaO2/FiO2 and alveolar-arterial oxygen tension ratio [P(A/a)O2] (Y=−0.111X + 79.019, r=−0.1860, P=0.5840).
At POH 14, deventilation failed due to hypoxemia after a 30-minute attempt. Continued ventilation seemed to be mandatory with increased respiratory parameters: PIP 18 cmH2O, PEEP 5–10 cmH2O, PSV 15 cmH2O, I:E ratio 1:1.5 and FiO2 0.60. At POH 22, a second attempt of deventilation for only five minutes failed due to more severe hypoxemia (Figure 2B).
Multiorgan failure developed early involving the heart (lactate dehydrogenase 9,622 U/L, creatine kinase 8,199 U/L, creatine kinase isoenzyme MB 132 U/L, troponin I 16 µg/L and atrial natriuretic peptide 1,510 pg/mL), liver (alanine aminotransferase 1,045 U/L, aspartate aminotransferase 2,488 U/L, total protein 54.9 g/L, albumin 27.8 g/L and albumin/globulin ratio 1.03), kidney (creatinine 250 µmol/L), pancreas (amylase 177 U/L and glucose 28.3 µmol/L), and hematological (hemoglobin 76 g/L and platelet 39×109/L) and blood coagulation systems (prothrombin time 19.1 s, activated partial thromboplastin time 42.8 s, thrombin time 29.9 s, fibrinogen 1.69 and D-dimer 1.01 mg/L). However, bedside echocardiography at POH 48 revealed no significant structural or functional cardiac abnormalities with an ejection fraction of 66%. Unfortunately, oliguria and anuria occurred at POHs 64 and 69, respectively.
She developed postperfusion lung syndrome, high-AG and hyperchloride TABD and multiorgan failure. Her gradually decreasing blood pressure showed poor responses to augmenting inotropic doses and all supportive treatments. She was moribund. Her family declined further treatment including intraaortic balloon pump support and brought her home.
Discussion
Postperfusion lung syndrome is rare but sometimes lethal. The underlying mechanisms remain uncertain. In cardiac surgery, systemic inflammatory response induced by contact between the blood components and the artificial surface of the circuit, ischemia-reperfusion injury, endotoxemia and surgical trauma have been considered predisposing risk factors (2).
The duration of the heart operation is an important factor for mechanical ventilation. Longer operation time may be associated with high dose of anesthetics and severe corporal disturbances. Colloidal osmotic pressure decreases remarkably and the lung water content increases. Additionally, prolonged aspiration of dry cold pure oxygen is prone to alveolar collapse, diffuse atelectasis, oxygen exchange disorder and ventilation/perfusion mismatch, all of which may require mechanical ventilation for rectification. Plasma albumin, which reflects organ protein synthesis and the severity and prognosis of the disease, shows a significant relation with postoperative mechanical ventilation. Low plasma albumin levels decrease colloidal osmotic pressure, increase lung water content and decrease PaO2/FiO2. Therefore, plasma albumin levels of the first day of Intensive Care Unit stay have become an independent predictor for hypoxemia leading to prolonged ventilation and even failed extubation (4).
Patient’s age, cardiac function impairment, high creatinine level, pulmonary hypertension, prolonged bypass time, postoperative dopamine and milrinone requirements were significant risk factors for the development of postperfusion lung syndrome. Prolonged crossclamp and cardiopulmonary bypass durations could be the leading causes of systemic inflammatory reactions and pulmonary ischemia-reperfusion injury (5). APACHE II ≥19 points may indicate poor prognosis of ARDS patients on PEEP therapy (6). The higher APACHE II scores may predict a lower extubation rate (7).
PEEP can increase functional residual capacity, reexpand the atelectatic alveoli, ameliorate gas exchange and lessen intrapulmonary shunting and therefore lower the respiratory index. PEEP of as high as 15 cmH2O during mechanical ventilation in anesthetic pigs caused a significant reduction of mean arterial pressure, cardiac index, stroke volume index and intrathoracic blood volume index (8). During cardiopulmonary bypass, diffuse atelectasis, alveolar collapse and ventilation/perfusion mismatch develop easily (9). An animal experiments revealed that high tidal volume ventilation (25 mL/kg) was associated with significant pulmonary vascular dysfunction characterized by enhanced pulmonary vasoconstriction and reduced vasodilatation (10). Early application of proper PEEP (10 cmH2O) and low tidal volume (6 mL/kg) may reduce extravascular lung water in porcine pulmonary edema models (11). A smaller tidal volume (≤6 mL/kg) with PEEP may protect the lung tissues from ventilation-related injuries, by which the pulmonary alveoli are kept open (12).
When ΔCl− is constant and ΔAG↑ is > ΔHCO3−↓, there might be an “overshoot alkalosis”, i.e., high-AG type metabolic acidosis associated with metabolic alkalosis. When ΔAG is constant and ΔCl−↑ is > ΔHCO3−↓, a hyperchloride type metabolic acidosis is accompanied by a metabolic alkalosis. When ΔCl− and ΔAG are apart, HCO3− may remain unchanged, increase or decrease; whereas when ΔCl− and ΔAG change in the same direction, there must be a significant decrease of ΔHCO3−. At the very beginning, there should be ΔAG↑ = ΔHCO3−↓. Further ΔHCO3−↓ would be compensated by ΔCl−↑, i.e., concurrent hyperchloride metabolic acidosis with high-AG metabolic acidosis (mixed metabolic acidosis). If ΔAG↑ + ΔCl−↑ is > ΔHCO3−↓, it indicates a metabolic alkalosis with mixed metabolic acidosis (3). Hyperchloride TABD is rare. Sun et al. (3) reported hyperchloride TABDs in nine burn patients, with eight being of respiratory alkalosis type and one of respiratory acidosis type TABD. All nine patients had an elevated plasma aldosterone, implicating aldosterone secretion in regulating acid-base balance.
PaO2/FiO2 is also termed oxygenation capacity index. It is a common indicator of pulmonary gas exchange and a major diagnostic index of ARDS with a normal range of 430–560. The dynamic monitoring of this index is very helpful in anticipating the disease advances and the response to treatment (13). Furthermore, respiratory index ≥250% is a high risk indicator for ARDS. A respiratory index ≥300% is a criterion for early diagnosis of ARDS. A respiratory index <250% by repeated blood-gas analysis within 24 hours is one of the safe indicators for tracheal extubation (14). Respiratory index decrease after treatment indicates amelioration of the patient‘s condition. If a respiratory index remains high or even increases, it may herald a critical condition of the patient (15). This patient had an extremely low PaO2/FiO2 and an extremely high respiratory index at POH 32 (10 hours after second attempt of deventilation), indicating her critical condition and poor response to treatments.
Conclusions
The prognosis of postperfusion lung syndrome is often poor. Debates remain on management strategies for its critical and refractory sequelae. It is recommended that a proper PEEP (5–10 cmH2O) with low tidal volume (6 mL/kg) be applied in order to minimize cardiac function impairment following cardiac surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the kin of the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Yuan SM. Postperfusion lung syndrome: Respiratory mechanics, respiratory indices and biomarkers. Ann Thorac Med 2015;10:151-7. [Crossref] [PubMed]
- Asimakopoulos G, Smith PL, Ratnatunga CP, et al. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg 1999;68:1107-15. [Crossref] [PubMed]
- Sun YX, Zhang ZF, Li SS, et al. Diagnosis of hyperchloremic triple acid-base disorders in burn patients and its mechanisms. Chin J Pathophysiol 2000;16:744-8.
- Meng L, Zhao WJ, Liu GJ. Clinical analysis of factors related to the length of postoperative mechanical ventilation in ICU. Acta Acad Med Xuzhou 2004;24:490-2.
- Li X, Wang H, Guo Z, et al. A clinical study on risk factors of post extracorporeal circulation hypoxemia. Chin J Extracopor Circ 2006;4:90-3.
- Zhao JR. Analysis on prognostic indications of acute respiratory distress syndrome. Pract J Cardiac Cerebr Pneum Vasc Dis 2010;18:314-5.
- Lin M, Xie JQ, Wu LX, et al. The Treatment of PEEP in Acute respiratory distress syndrome. Med Inform 2011;24:3928-9.
- Liu N, Gu Q, Yu JF. The influence of positive end-expiratory pressure on stroke volume variation for the accuracy of evaluating volume. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2012;24:419-22. [PubMed]
- Polese G, Lubli P, Mazzucco A, et al. Effects of open heart surgery on respiratory mechanics. Intensive Care Med 1999;25:1092-9. [Crossref] [PubMed]
- Menendez C, Martinez-Caro L, Moreno L, et al. Pulmonary vascular dysfunction induced by high tidal volume mechanical ventilation. Crit Care Med 2013;41:e149-55. [Crossref] [PubMed]
- Colmenero-Ruiz M, Fernández-Mondéjar E, Fernández-Sacristán MA, et al. PEEP and low tidal volume ventilation reduce lung water in porcine pulmonary edema. Am J Respir Crit Care Med 1997;155:964-70. [Crossref] [PubMed]
- Krishnan JA, Brower RG. High-frequency ventilation for acute lung injury and ARDS. Chest 2000;118:795-807. [Crossref] [PubMed]
- Han SZ, Wang LX, Zhang LY. A comparative research on pulmonary dynamic compliance and oxygenation index in patients with acute respiratory distress syndrome. Chin J Respir Crit Care Med 2003;2:95-6.
- Yu T, Zheng XF, Xie DX. Clinical implication of respiratory index in the diagnosis and treatment of ARDS. Gansu Sci Tech 2003;19:119-21.
- Hu L, Zhou SM, Zhao X. Clinical significance of respiratory index in hypoxemia. Mod Diagn Treat 1996;7:15-7.


