
Comparison of lymph node metastasis pattern from esophagogastric junction adenocarcinoma versus very low thoracic esophageal squamous cancer: a propensity-matched analysis
Highlight box
Key findings
• Patients with very low thoracic esophageal squamous cell carcinoma (ESCC) exhibit stronger metastatic ability in the lower mediastinal and paracardial nodes than Siewert I-II esophagogastric junction adenocarcinoma (AEG).
• The median survival was poor for AEG patients with lower mediastinal LNM.
What is known and what is new?
• Squamous carcinoma around esophagogastric junction (EGJ) exhibit stronger locoregional lymph metastatic ability than adenocarcinoma.
• The lower mediastinal lymph nodes metastasis in AEG patients was associated with poor survival outcomes.
What is the implication, and what should change now?
• Complete lower mediastinal lymph node dissection should be performed in locally advanced AEG patients.
Introduction
The incidence of esophagogastric junction adenocarcinoma (AEG) has recently increased worldwide, especially in Western countries (1). Siewert classification is universally employed to group AEG into three types according to the location of the tumor epicenter (2). For Siewert type I–II AEG, transthoracic surgery is used by an increasing number of surgeons due to the possibility of mediastinal lymph nodal metastasis. Based on the eighth edition of the TNM classification system, Siewert I–II AEG is staged as the adenocarcinoma of esophagus stage manual (3). However, the optimal extent of lymphadenectomy in this group remains uncertain.
In Eastern countries, esophageal squamous cell carcinoma (ESCC) is the most common histological type among esophageal cancers. Lymphadenectomy of the mediastinum, particularly upper mediastinum lymph node dissection, has been regarded as a standard procedure. Many studies are found to focus on the lymph node metastasis (LNM) pattern of ESCC (4-7). However, very few studies have focused on the LNM pattern of ESCC involving the esophagogastric junction. In this study, we selected 120 very low thoracic ESCC patients, whose tumor proximal edge location was within 5 cm from the esophagogastric junction (EGJ), for LNM pattern analysis from 1,455 ESCC patients in our hospital. The anatomical location of very low thoracic ESCC was similar to that of Siewert I–II AEG. We aimed to investigate whether the lymph node metastasis capability and distribution of these two groups of patients differed depending on the histology.
Mine et al. (8) recently compared the distributions of mediastinal and abdominal LNM in patients with AEG versus those with squamous cell carcinoma (SCC) of the esophagogastric junction. However, the tumor characteristics of these two groups in Mine et al.’s study were significantly different, which may lead to selection bias. In our study, propensity score matching was conducted to explore the differences in LNM distribution between Siewert I–II AEG and very low thoracic ESCC patients. Additionally, the survival impact of significant lymph node station metastasis was further investigated. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1028/rc).
Methods
Patients, eligibility criteria and follow-up
In this study, very low thoracic esophageal squamous cell carcinoma (vlESCC) was defined as a tumor whose proximal edge was located within 5 cm of the EGJ. Among 1,455 patients with esophageal squamous cell carcinoma undergone curative esophagectomy classified as R0 at the Cancer Institute and Hospital of Tianjin Medical University from January 2007 to December 2017, 120 very low thoracic ESCC patients were selected. A total of 156 Siewert I–II type adenocarcinoma of esophagogastric junction cases were chosen from 202 AEG patients who underwent curative surgery classified as R0 between January 2010 and December 2014 in our department. The flow chart of selection of patients is shown in Figure 1.
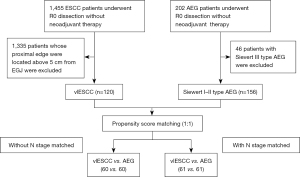
The exclusion criteria were as follows: (I) patients who had a history of malignant disease; (II) patients who had received preoperative treatment (chemotherapy and/or radiotherapy); and (III) patients who had other malignant tumors simultaneity.
After curative resection, except for 5 T1b patients, AEG patients received systematic fluoropyrimidine-based chemotherapy after the operation. R0 resection ESCC patients with lymph node metastasis received systematic fluoropyrimidine-based chemotherapy. All patients were monitored every 3 months for the first 2 years, every 6 months during the third to fifth years, and then every year until death or the last follow-up. The follow-up was completed in January 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Review Board of the Tianjin Medical University Cancer Institute and Hospital (No. bc2022047), and the requirement for informed consent of patients was waived.
Surgical procedures
All eligible ESCC patients underwent esophagectomy via Ivor-Lewis or McKeown procedures with standard 2-field lymphadenectomy. All Siewert AEG procedures were performed via the Ivor-Lewis procedure or combined thoracoabdominal approach. Proximal gastrectomy was routinely performed in AEG patients. Patients with a combined thoracoabdominal approach did not have esophageal involvement of more than 3.0 cm, and upper mediastinal lymph node metastasis was evaluated by preoperative CT scan. Due to the surgical approach, these AEG patients did not undergo complete upper mediastinal lymphadenectomy. In this study, the mediastinal lymph nodes were divided into upper and lower areas. The upper mediastinal lymph area included the left and right recurrent laryngeal nerve, upper thoracic paraesophageal, paratracheal, and subcarinal lymph nodes. The depth of the primary tumor, grade of the tumor, degree of lymph node, and TNM staging were defined according to the Union Internationale Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) TNM classification (8th edition) (3).
Propensity score matching
To balance the potential baseline characteristic differences of patients between AEG and ESCC patients and reduce selection bias, a nearest-neighbor propensity score matching was performed using a 1:1 ratio (9,10). The caliper definition was set at 0.01. For the analysis of differences in LNM capability, the vlESCC and AEG groups were matched according to 5 baseline variables (age, sex pathological T stage, grade, and tumor length). 60 pairs of patients were matched (dataset 1). With dataset 1, the capacity of each station’s lymph node metastasis was analyzed. To compare the LNM distributions, the pathological N stage, as a new covariate, was further entered into the previous propensity score matching (PSM) model, and 61 new well-balanced pairs were created (dataset 2).
Statistical analysis
The statistical analyses were performed with SPSS 21.0 software (ver. 21 SPSS Inc., Chicago, IL, USA). The chi-squared test or Fisher’s exact test was employed to compare the proportions of the patients. The overall cumulative probability of survival was calculated by the Kaplan-Meier method, and the difference was assessed by the log-rank test. P<0.05 was considered statistically significant.
Results
Patient demographics
Prior to the PSM procedure, 120 very low thoracic ESCC and 156 Siewert I–II AEG patients were eligible for inclusion. In 120 vlESCC patients, the median age was 60 years (range, 37 to 81 years). The mean number of totally dissected lymph nodes per patient was 18.6. In 156 AEG patients, the median age was 67 years (range, 38 to 84 years). The mean number of totally dissected lymph nodes per patient was 14.1. Significant differences were found between these two groups in age, pathological T stage, tumor grade, N stage, and tumor length (all P<0.05).
To explore differences in LNM capability, PSM analysis was conducted with age, sex, pathological T stage, grade, and tumor length (dataset 1). Since the number of LNM might influence the analysis of LNM distribution, the pathological N stage was further added to the aforementioned PSM model, thus dataset 2 was yielded (age, sex, pathological T stage, grade, tumor length, and N stage were matched). All variables were well-balanced in both dataset 1 (60 ESCC patients and 60 AEG patients) and dataset 2 (61 ESCC patients and 61 AEG patients). The baseline patient disease characteristics are shown in Table 1.
Table 1
| Characteristics | Original dataset | Matched dataset 1 | Matched dataset 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| vlESCC | AEG | P | vlESCC | AEG | P | vlESCC | AEG | P | |||
| Age, years, n (%) | 0.000 | 0.346 | 0.700 | ||||||||
| ≤60 | 67 (55.8) | 36 (21.3) | 25 (41.7) | 20 (33.3) | 42 (68.9) | 40 (65.6) | |||||
| >60 | 53 (44.2) | 120 (76.9) | 35 (58.3) | 40 (66.7) | 19 (31.1) | 21 (34.4) | |||||
| Sex, n (%) | 0.657 | 0.306 | 0.769 | ||||||||
| Male | 106 (88.3) | 135 (86.5) | 49 (81.7) | 53 (88.3) | 55 (90.2) | 54 (88.5) | |||||
| Female | 14 (11.7) | 21 (13.5) | 11 (18.3) | 7 (11.7) | 6 (9.8) | 7 (11.5) | |||||
| Pathological T status, n (%) | 0.000 | 0.310 | 0.267 | ||||||||
| T1 | 13 (10.8) | 5 (3.2) | 1 (1.7) | 5 (8.3) | 2 (3.3) | 5 (8.2) | |||||
| T2 | 16 (13.3) | 14 (9.0) | 10 (16.7) | 10 (16.7) | 8 (13.1) | 7 (11.5) | |||||
| T3 | 46 (38.3) | 16 (10.3) | 14 (23.3) | 10 (16.7) | 20 (32.8) | 12 (19.7) | |||||
| T4 | 45 (37.5) | 121 (77.6) | 35 (58.3) | 35 (58.3) | 31 (50.8) | 37 (60.7) | |||||
| Tumor grade, n (%) | 0.000 | 0.835 | 0.548 | ||||||||
| Grade 1 | 2 (1.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Grade 2 | 99 (82.5) | 80 (51.3) | 44 (73.3) | 45 (75.0) | 45 (73.8) | 42 (68.9) | |||||
| Grade 3 | 19 (15.8) | 76 (48.7) | 16 (26.7) | 15 (25.0) | 16 (26.2) | 19 (31.1) | |||||
| N stage, n (%) | 0.017 | 0.056 | 0.465 | ||||||||
| N0 | 60 (50.0) | 64 (41.0) | 27 (45.0) | 35 (58.3) | 27 (44.3) | 35 (57.4) | |||||
| N1 | 32 (26.7) | 33 (21.2) | 19 (31.7) | 8 (13.3) | 17 (27.9) | 15 (24.6) | |||||
| N2 | 26 (21.7) | 44 (28.2) | 13 (21.7) | 13 (21.7) | 16 (26.2) | 10 (16.4) | |||||
| N3 | 2 (1.7) | 15 (9.6) | 1 (1.7) | 4 (6.7) | 1 (1.6) | 1 (1.6) | |||||
| LNM, n (%) | 0.137 | 0.144 | 0.147 | ||||||||
| Negative | 60 (50.0) | 64 (41.0) | 27 (45.0) | 35 (58.3) | 27 (44.3) | 35 (57.4) | |||||
| Positive | 60 (50.0) | 92 (59.0) | 33 (55.0) | 25 (41.7) | 34 (55.7) | 26 (42.6) | |||||
| Tumor length (mean ± SD), cm | 4.1±1.9 | 5.1±2.3 | 0.000 | 4.3±2.0 | 4.3±2.2 | 0.949 | 4.3±2.0 | 4.2±2.1 | 0.964 | ||
Surgical procedure: the ESCC patients underwent esophagectomy via Ivor-Lewis or McKeown procedures with standard 2-field lymphadenectomy. All AEG procedures were performed via the Ivor-Lewis procedure or combined thoracoabdominal approach. For AEG patients, at least lower mediastinal lymphadenectomy and D1+ lymph node dissection were performed. vlESCC, very low thoracic esophageal squamous cell carcinoma; AEG, esophagogastric junction adenocarcinoma; LNM, lymph node metastasis; SD, standard deviation.
Lymph node metastasis capability and distribution in vlESCC and AEG
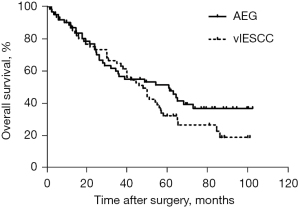
In dataset 1, the incidence of nodal metastasis in vlESCC seemed higher than that of AEG patients, although the difference was nonsignificant (55% vs. 41.7%, P=0.144) (Table 1). Compared with AEG, the metastatic rate of vlESCC thoracic lymph node (LN), especially in the lower mediastinum, was significantly higher (all P<0.05). No significant difference was found between these two groups at other LN stations. In contrast, in addition to the lower mediastinum LN, the paracardial LNM rate in dataset 2 also differed significantly between vlESCC and AEG (36.1% vs. 19.7%, P=0.043) (Table 2). We also compared the overall survival differences between vlESCC and AEG in dataset 2. No significant survival difference was found (P=0.225). The median survival rates of vlESCC and AEG were 48.1 months and 60.8 months respectively (Figure 2).
Table 2
| Lymph nodes stations metastasis | Matched dataset 1 | Matched dataset 2 | |||||
|---|---|---|---|---|---|---|---|
| vlESCC (%) | AEG (%) | P value | vlESCC (%) | AEG (%) | P value | ||
| Upper mediastinum | 5/60 (8.3) | 0/60 (0) | 0.057 | 4/61 (6.6) | 0/61 (0) | 0.119 | |
| Subcarinal | 4/60 (6.7) | 0/60 (0) | 0.119 | 3/61 (4.9) | 0/61 (0) | 0.244 | |
| Middle and lower thoracic paraesophageal | 8/60 (13.3) | 2/60 (3.3) | 0.099 | 12/61 (19.7) | 2/61 (3.3) | 0.011 | |
| Right and left tracheobronchial | 2/60 (3.3) | 0/60 (0) | 0.496 | 2/61 (3.3) | 0/61 (0) | 0.496 | |
| Lower mediastinum | 11/60 (18.3) | 2/60 (3.3) | 0.019 | 15/61 (24.6) | 2/61 (3.3) | 0.002 | |
| Total thoracic | 15/60 (25.0) | 2/60 (3.3) | 0.002 | 18/61 (29.5) | 2/61 (3.3) | 0.000 | |
| Paracardial | 20/60 (33.3) | 12/60 (20.0) | 0.099 | 22/61 (36.1) | 12/61 (19.7) | 0.043 | |
| Lesser curvature | 3/60 (5.0) | 6/60 (10.0) | 0.488 | 4/61 (6.6) | 6/61 (9.8) | 0.741 | |
| Left gastric artery | 16/60 (26.7) | 18/60 (30.0) | 0.685 | 16/61 (26.2) | 18/61 (29.5) | 0.686 | |
| Total abdominal | 28/60 (46.7) | 23/60 (38.3) | 0.356 | 29/61 (47.5) | 25/61 (41.0) | 0.466 | |
vlESCC, very low thoracic esophageal squamous cell carcinoma; AEG, esophagogastric junction adenocarcinoma.
Correlation between characteristics and LNM of locoregional nodes of the EGJ
The lower mediastinum and paracardial LN could be regarded as locoregional nodes of the EGJ according to anatomical area (11). The relationship between clinical characteristics and locoregional lymph node metastasis of the EGJ was analyzed in 120 vlESCC and 156 AEG patients (Original Dataset). No significant difference was found between lower mediastinum LNM and clinical characteristics (age, sex, pathological T stage, grade, tumor length) in either vlESCC or AEG patients (Table 3). However, paracardial LNM was associated with pathological T stage (P=0.002) in vlESCC patients and correlated with pathological T stage (P=0.000) and tumor length (P=0.030) in AEG patients (Table 4).
Table 3
| Characteristics | vlESCC | AEG | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | P value | Negative | Positive | P value | ||
| Age, years, n (%) | 0.059 | 0.627 | |||||
| ≤60 | 52 (52.0) | 15 (75.0) | 34 (23.9) | 2 (14.3) | |||
| >60 | 48 (48.0) | 5 (25.0) | 108 (76.1) | 12 (85.7) | |||
| Sex, n (%) | 1.000 | 1.000 | |||||
| Male | 88 (88.0) | 18 (90.0) | 123 (86.6) | 12 (85.7) | |||
| Female | 12 (12.0) | 2 (10.0) | 19 (13.4) | 2 (14.3) | |||
| Pathological T status, n (%) | 0.056 | 0.051 | |||||
| T1–2 | 28 (28.0) | 1 (5.0) | 19 (13.4) | 0 (0) | |||
| T3–4 | 72 (72.0) | 19 (95.0) | 123 (86.6) | 14 (100.0) | |||
| Tumor grade, n (%) | 1.000 | 0.509 | |||||
| Grade 1–2 | 84 (84.0) | 17 (85.0) | 74 (52.1) | 6 (42.9) | |||
| Grade 3 | 16 (16.0) | 3 (15.0) | 68 (47.9) | 8 (57.1) | |||
| Tumor length (mean ± SD), cm | 4.0±2.0 | 4.2±1.4 | 0.749 | 5.1±2.4 | 5.1±1.3 | 0.824 | |
vlESCC, very low thoracic esophageal squamous cell carcinoma; AEG, esophagogastric junction adenocarcinoma.
Table 4
| Characteristics | vlESCC | AEG | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | P value | Negative | Positive | P value | ||
| Age, years, n (%) | 0.861 | 0.903 | |||||
| ≤60 | 49 (56.3) | 18 (54.5) | 23 (22.8) | 13 (23.6) | |||
| >60 | 38 (43.7) | 15 (45.5) | 78 (77.2) | 42 (76.4) | |||
| Sex, n (%) | 0.679 | 0.350 | |||||
| Male | 78 (89.7) | 28 (84.8) | 85 (84.2) | 50 (90.9) | |||
| Female | 9 (10.3) | 5 (15.2) | 16 (15.8) | 5 (9.1) | |||
| Pathological T status, n (%) | 0.002 | 0.000 | |||||
| T1–2 | 28 (32.2) | 1 (3.0) | 19 (18.8) | 0 (0) | |||
| T3–4 | 59 (67.8) | 32 (97.0) | 82 (81.2) | 55 (100.0) | |||
| Tumor grade, n (%) | 0.900 | 0.081 | |||||
| Grade 1–2 | 73 (83.9) | 28 (84.8) | 57 (56.4) | 23 (41.8) | |||
| Grade 3 | 14 (16.1) | 5 (15.2) | 44 (43.6) | 32 (58.2) | |||
| Tumor length (mean ± SD), cm | 4.0±2.0 | 4.2±1.7 | 0.604 | 4.8±2.6 | 5.5±1.7 | 0.030 | |
vlESCC, very low thoracic esophageal squamous cell carcinoma; AEG, esophagogastric junction adenocarcinoma.
Prognostic impact of locoregional node metastasis of the EGJ in AEG
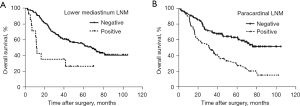
The survival impacts of lower mediastinum and paracardial LNM were investigated in 156 patients with AEG. The median survival rate of patients with metastatic lower mediastinum LNs was worse than that of patients without metastasis (12.0 vs. 68.1 months, P=0.001). Compared with the group without paracardial LNM, patients with paracardial LNM had a worse 5-year overall survival rate (62.0% vs. 27.6%, P=0.000) (Figure 3).
Discussion
To the best of our knowledge, this study is the first to compare lymph node metastasis capacity and distribution between AEG and ESCC with the involvement of the esophagogastric junction using the PSM method. Previous studies (8,12) argued that the distribution of mediastinal and abdominal lymph node metastasis between esophagogastric junction adenocarcinoma and squamous carcinoma was not significantly different. However, the baseline clinical characteristics of these two groups in these previous studies were not completely matched, which may contribute to selection bias and various outcomes. In our research, we found that the metastatic rate of locoregional nodes of the EGJ in very low thoracic ESCC patients was higher than that of the AEG group. AEG patients with metastatic lower mediastinum and paracardial lymph nodes had poor survival outcomes.
As the number of LNMs would have some influence on LNM distribution, we balanced clinical covariates with or without pathological N stage in the PSM model separately. After matching without pathological N stage, the comparison of lymph node metastatic rate between the vlESCC and AEG group was more likely to represent the capability of LNM. Although no significant difference for the total LNM rate between these two groups was found, this rate in vlESCC patients was higher than that in AEG patients (55% vs. 41.7%, P=0.144). These data showed that SCC exhibited a stronger lymph node metastasis ability. Yabusaki et al. (13) found that SCC patients had more advanced stage disease after the exploration of 72 patients with Siewert type II ADC and 51 patients with SCC in the same area, which was consistent with our results.
The distribution of lymph node metastasis was not only associated with tumor invasion, but also influenced by the total metastatic rate and lymph node number. Therefore, we chose the pathological N stage and entered it into the PSM model to investigate the differences in LNM distribution between AEG and vlESCC patients. In the matched dataset 2, vlESCC patients exhibited more aggressive features in the lower mediastinum and paracardial lymph nodes than patients with AEG. Nishiwaki et al. also argued that histological squamous type was a risk factor for mediastinal LNM of Siewert I–II esophagogastric junction carcinomas (14). However, our findings are not consistent with the results of previous studies (8,12), which indicated that the lymph nodal metastasis rates of different types of EGJ carcinoma were similar, regardless of histology. As we mentioned above, the baseline clinical characteristics of these two groups in these studies differed significantly, and this was an obvious limitation. In fact, the lower mediastinum and paracardial lymph nodes could be regarded as locoregional nodes of the EGJ and these sites were the first station of LNM for EGJ carcinoma. The metastasis rate of locoregional lymph nodes of the EGJ could be different since the tumor spread ability of vlESCC was higher than that of Siewert I–II type AEG.
According to previous studies, the rates of metastasis among lower mediastinal LNs were 21.1% to 25.0% for lower thoracic ESCC (15-17), but no more than 10% for Siewert AEG (11,18). The difference between the vlESCC group and the AEG group was also noticeable even in our unmatched data (16.7% vs. 9.0%). The incidence rate of mediastinal lymph node metastasis is strongly associated with the esophageal invasion length of tumors from the esophagogastric junction (19). In our study, the average proximal edge of the tumor in the vlESCC patients was higher than that of the AEG group, even though both the vlESCC and Siewert I–II AEG were located in the range of the EGJ area according to the Siewert classification. Interestingly, the paracardial LN metastatic rate of vlESCC was still higher than that of Siewert I–II AEG in matched dataset 2 (36.1% vs. 19.7%). In other studies, the LNM rate of paracardial was 18.9% to 53.8% (15-17) for lower thoracic ESCC, and 26.1–32.9% for Siewert II AEG (11,18,20). The paracardial LNM rate in our unmatched series (35.3%) was consistent with the previous studies mentioned above. However, after using PSM to analyze the paracardial LNM feature, our results revealed that SCC of the esophagogastric junction metastasized more easily than adenocarcinoma in a similar area.
For lower thoracic ESCC, two field lymphadenectomy is the standard surgical procedure (21). However, the surgical approach to Siewert II type AEG is still under debate (22). A previous study showed that the therapeutic value of lower mediastinal node dissection was relatively high in EGJ carcinoma (23). In the AEG group of our study, positive lymph nodes of the lower mediastinal region were correlated with poor survival outcomes. All patients with metastatic lower mediastinal LN belonged to the pathological T3–4 stage, which was consistent with Shiraishi’s results (24). Considering that paracardial LNM was also associated with the T stage, complete lower mediastinal and abdominal lymph node dissection should be performed in advanced AEG patients.
One limitation of the present study is the retrospective, single center design. After PSM was performed, the number of patients was relatively small and may not be sufficient to draw a firm conclusion on the basis of statistical evidence. Not all AEG patients in our study underwent complete mediastinal lymphadenectomy because of the surgical approach selection, especially for upper mediastinal nodes dissection. In this study, we only balanced some already known tumor characteristics, but other variates that may affect lymph node metastasis. We did not put them into the PSM model, which caused selection bias and uncertain results. For the analysis of the lymph node distribution differences, we put N-stage as a covariate in the PSM model, which may also introduce selection bias.
In conclusion, through PSM, we found that patients with very low thoracic ESCC exhibit stronger metastatic ability in lower mediastinal and paracardial nodes than Siewert I–II AEG. The pathological metastasis of AEG to these locoregional lymph nodes was associated with poor survival outcomes. Complete lower mediastinal and abdominal lymph node dissection should be performed in locally advanced AEG patients.
Acknowledgments
Funding: This work was supported by the fund from the Fundamental Research Funds for Universities in Tianjin (No. 2020KJ134).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1028/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1028/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1028/coif). All authors report that this work was supported by the fund from the Fundamental Research Funds for Universities in Tianjin (No. 2020KJ134). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Review Board of the Tianjin Medical University Cancer Institute and Hospital (No. bc2022047), and informed consent of patients was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Wen J, Chen J, Chen D, et al. Comprehensive analysis of prognostic value of lymph node classifications in esophageal squamous cell carcinoma: a large real-world multicenter study. Ther Adv Med Oncol 2021;13:17588359211054895. [Crossref] [PubMed]
- Harada H, Hosoda K, Moriya H, et al. Optimized lymph node dissection range during progression of lower thoracic esophageal squamous cell carcinoma in the latest therapeutic surgical strategy: A retrospective analysis. Oncol Lett 2018;16:3281-9. [Crossref] [PubMed]
- Tokairin Y, Nakajima Y, Kawada K, et al. A feasibility study of mediastinoscopic radical esophagectomy for thoracic esophageal cancer from the viewpoint of the dissected mediastinal lymph nodes validated with thoracoscopic procedure: a prospective clinical trial. Esophagus 2019;16:214-9. [Crossref] [PubMed]
- Defize IL, Gorgels SMC, Mazza E, et al. The Presence of Metastatic Thoracic Duct Lymph Nodes in Western Esophageal Cancer Patients: A Multinational Observational Study. Ann Thorac Surg 2022;113:429-35. [Crossref] [PubMed]
- Mine S, Watanabe M, Kumagai K, et al. Comparison of mediastinal lymph node metastases from adenocarcinoma of the esophagogastric junction versus lower esophageal squamous cell carcinoma with involvement of the esophagogastric junction. Dis Esophagus 2019;32:doz002. [Crossref] [PubMed]
- Xu ZJ, Zhuo ZG, Song TN, et al. Role of nodal skip metastasis in patients with mid-thoracic oesophageal squamous cell carcinoma: a propensity score matching study. Eur J Cardiothorac Surg 2021;59:799-806. [Crossref] [PubMed]
- Liu J, Chen Y, Zhan X, et al. Effect of prior cancer history on survival of patients with esophageal carcinoma: a propensity score matching, population-based study. J Thorac Dis 2022;14:979-94. [Crossref] [PubMed]
- Kurokawa Y, Takeuchi H, Doki Y, et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann Surg 2021;274:120-7. [Crossref] [PubMed]
- Yoshikawa T, Takeuchi H, Hasegawa S, et al. Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction. Gastric Cancer 2016;19:143-9. [Crossref] [PubMed]
- Yabusaki H, Nashimoto A, Matsuki A, et al. Comparison of the surgical treatment strategies for Siewert type II squamous cell carcinoma in the same area as esophagogastric junction carcinoma: data from a single Japanese high-volume cancer center. Surg Today 2014;44:1522-8. [Crossref] [PubMed]
- Nishiwaki N, Noma K, Matsuda T, et al. Risk factor of mediastinal lymph node metastasis of Siewert type I and II esophagogastric junction carcinomas. Langenbecks Arch Surg 2020;405:1101-9. [Crossref] [PubMed]
- Li B, Chen H, Xiang J, et al. Pattern of lymphatic spread in thoracic esophageal squamous cell carcinoma: A single-institution experience. J Thorac Cardiovasc Surg 2012;144:778-85; discussion 785-6. [Crossref] [PubMed]
- Kosugi SI, Ichikawa H, Hanyu T, et al. Appropriate extent of lymphadenectomy for squamous cell carcinoma of the esophagogastric junction. Int J Surg 2017;44:339-43. [Crossref] [PubMed]
- Yang Y, Li Y, Qin J, et al. Mapping of Lymph Node Metastasis From Thoracic Esophageal Cancer: A Retrospective Study. Ann Surg Oncol 2022;29:5681-8. [Crossref] [PubMed]
- Yamashita H, Seto Y, Sano T, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer 2017;20:69-83. [Crossref] [PubMed]
- Koyanagi K, Kato F, Kanamori J, et al. Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: A retrospective single-institution study. Ann Gastroenterol Surg 2018;2:187-96. [Crossref] [PubMed]
- Cai MZ, Lv CB, Cai LS, et al. Priority of lymph node dissection for advanced esophagogastric junction adenocarcinoma with the tumor center located below the esophagogastric junction. Medicine (Baltimore) 2019;98:e18451. [Crossref] [PubMed]
- Li B, Zhang Y, Miao L, et al. Esophagectomy With Three-Field Versus Two-Field Lymphadenectomy for Middle and Lower Thoracic Esophageal Cancer: Long-Term Outcomes of a Randomized Clinical Trial. J Thorac Oncol 2021;16:310-7. [Crossref] [PubMed]
- Hölscher AH, Law S. Esophagogastric junction adenocarcinomas: individualization of resection with special considerations for Siewert type II, and Nishi types EG, E=G and GE cancers. Gastric Cancer 2020;23:3-9. [Crossref] [PubMed]
- Yura M, Takeuchi H, Fukuda K, et al. High-risk group of upper and middle mediastinal lymph node metastasis in patients with esophagogastric junction carcinoma. Ann Gastroenterol Surg 2018;2:419-27. [Crossref] [PubMed]
- Shiraishi O, Yasuda T, Kato H, et al. Risk Factors and Prognostic Impact of Mediastinal Lymph Node Metastases in Patients with Esophagogastric Junction Cancer. Ann Surg Oncol 2020;27:4433-40. [Crossref] [PubMed]


