
Contemporary real-world radiotherapy outcomes of unresected locally advanced non-small cell lung cancer
Highlight box
Key findings
• A population-based analysis of treatment outcomes from the province of Ontario, Canada, in the period of 2007–2017, shows that in patients with unresectable LA-NSCLC treated with RT, dose of chest RT and utilization of staging FDG-PET are associated with improved survival.
What is known and what is new?
• It is unclear whether curative doses of chest RT or use of FDG-PET can help improve outcomes in patients with LA-NSCLC unfit to receive the standard of care treatment of concurrent chemo-radiotherapy. As a result, poor performance status patients are frequently treated with low or palliative dose RT. This study provides strong retrospective evidence that the two parameters are independently associated with improved survival in this group of patients.
What is the implication, and what should change now?
• This work provides guidance for clinicians and a valid basis for prospective investigation of curative dose RT in well-staged patients unable to receive systemic therapy.
Introduction
Lung cancer is a leading cause of cancer death worldwide (1). Non-small cell lung cancer (NSCLC) accounts for about 85% of newly diagnosed lung cancer (2). Approximately one third of NSCLC patients present with locally advanced (LA) NSCLC (corresponding to AJCC 8th ed. stage III), and most are not amenable to surgical resection (3). Until the 1990s, the standard of care (SοC) for unresectable NSCLC was radiotherapy (RT) alone. At that time, studies reported median overall survival (OS) and 5-year OS rates of 10 months and 7%, respectively (4). Successive trials in the past 30 years introduced initially sequential chemo-radiation (sCRT) and, subsequently, concurrent chemo-radiotherapy (cCRT) without or with consolidation chemotherapy as SοC in stage III eligible patients. In RTOG-9410 cCRT improved median and 5-year OS to 17 months and 16%, respectively, compared to 14.6 months and 10%, with sCRT (5). In recent years, the addition of anti-Programmed Death Ligand 1 (PD-L1) immunotherapy (Durvalumab) as consolidation treatment after cCRT was shown to improve further median OS in unresectable LA-NSCLC further (47.5 vs. 29.1 months) (6). Currently, cCRT in combination with consolidation anti-PD-L1 therapy are considered SοC. However, not all patients are able to receive this lengthy treatment. Examples include those with contraindications for chemotherapy, which precludes them from receiving Durvalumab also.
In recent years, population studies (1,7-9) suggested that 39–52% of LA-NSCLC patients may be treated with RT alone (1,8,9). Although, the contribution of modern RT techniques to cCRT outcomes has been explored (10), there is a need to understand better the impact of modern RT when used as monotherapy. A study from Ontario, Canada, suggested that in the period of 2010–2015 only 22.1% of patients with stage III LA-NSCLC received cCRT, while 41% of patients received RT alone (1).
The above data indicate the clinical importance of understanding well contemporary real-world outcomes of RT alone. This is of increased value since outcomes of cCRT in unresected LA-NSCLC improve over time while the dose of chest RT and chemotherapy agents used in cCRT have not changed substantially. In 2011 Curran et al. (RTOG 9410) (5) reported a median OS of 17 months for cCRT but this increased to about 29 months in 2020 in RTOG-0617 (11). Similarly, RT alone yielded median OS of 10 months in historical trials [e.g., CALGB 8433, (1990) (12)], yet more recent, real-world data suggests median OS can be as high as 17 months (1,7). The etiology of these apparent improvements is unclear. Utilization of 18F-deoxy-glucose-positron emission tomography (FDG-PET) for staging and improvements in RT delivery techniques are suggested as potential reasons (13,14). Although, studies observe trends for improved survival outcomes, their association with the dose of chest RT or use of FDG-PET is not frequently examined.
Here, we pursued a population-based analysis of clinical treatment utilization data in the province of Ontario to obtain a contemporary view of management of unresectable LA-NSCLC. Our aim was to explore real-world outcomes of modern RT in stage III NSCLC patients and explore the association of RT dose and utilization of FDG-PET with patient survival outcomes. We focused on outcomes of RT as monotherapy and contrasted them to those of patients receiving SoC cCRT. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-925/rc).
Methods
Patient population
A population-based retrospective search of Ontario health information data was conducted through the Institute of Clinical Evaluative Sciences (IC/ES) to identify patients with stage III NSCLC (AJCC8th edition) that received chest RT in the period of 2007 to 2017. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Hamilton integrated ethics board (HiREB #10652). Individual consent for this retrospective analysis was waived.
Most Ontario residents are insured through the Ontario Health Insurance Plan (OHIP), and health administrative data on the services these residents receive can be accessed. Provincial databases were searched using International Classification of Disease for Oncology morphology codes. Patients with stage III NSCLC who received at least one dose of RT within 180 days following diagnosis, with or without chemotherapy, were included. The choice of 180 days was selected as most patients would be expected to receive curative treatment within six months of diagnosis. This approach would exclude patients that received consolidation or palliative RT at later stages. Exclusion criteria included histology other than NSCLC, stage other than III, prior cancer less than 5 years from the NSCLC diagnosis, RT or chemotherapy prior to diagnosis, multiple cancers on the same day, or cancer surgery within 90 days of diagnosis. To distinguish between treatment regimen types and reduce survivorship bias, patients were included only if they had follow-up of 60 days or more after initial RT dose. Curative regimens of RT are typically six weeks (42 days) in duration, and the use of 60 days cut off ensured that most patients could have completed RT, including a possible delay, and then started chemotherapy, as per standard of care, if that was the regimen prescribed.
Analyses and patient categories
Patients were categorized into one of three treatment modalities: RT alone, cCRT or sCRT. A patient was defined to have received cCRT if at least one chemotherapy dose was administered between the first and last RT fraction, or at least one RT fraction occurred between the first and last dose of chemotherapy, within 180 days of RT. A patient was defined as having sCRT if they received chemotherapy within 180 days of RT but did not receive cCRT by the definition above. A patient was defined as having received RT only if they did not receive any chemotherapy within 180 days of RT.
We grouped patients into three RT dose categories of <40, 40–55.9 and ≥56 Gy. With an α/β ratio of 10 for lung cancer, these categories include RT schemas with BED <50, 50–65 and >65 Gy and encompass well schemas typically given for palliation, short-term local control or definitive treatment, respectively (Table S1). Finally, patients were separated into groups that did or did not undergo staging FDG-PET.
Since income, distance from a cancer care facility and performance status can influence treatment selection and overall outcomes, we also included in our analysis models income quintile, rurality, distance from a regional cancer center (RCC) and reported Charlson’s score.
Statistical analysis
Descriptive statistics were used to summarize the patient population and outcomes. The primary outcome of interest in this study was overall survival (OS), defined from the date of first treatment with RT to the date of death. The Kaplan-Meier method was used to estimate the OS outcomes, and patients not known to be deceased were censored on the last date they had contact with the provincial health care system prior to 31 March 2019. Univariable Cox proportional hazards regression was used to explore the effect of selected prognostic factors on OS. An a priori selected subgroup analysis was performed exploring the effect of PET utilization within each RT dose group. A multivariable model was constructed based on the full model, i.e., including all factors explored in the univariable model. The only factor not included was distance to the nearest cancer center as it was confounded with rurality. Interactions were explored between PET utilization, radiotherapy dose and treatment modality. Confidence intervals (CI) were constructed for outcomes of interest. All tests and CI were two-sided and statistical significance was defined at the α=0.05 level.
Results
Patient characteristics and utilization patterns
Between January 2007 to March 2017, 110,690 individuals were diagnosed with lung cancer in Ontario. After exclusion of patients with non-NSCLC histology, stage other than III, those with multiple cancers on the same day, and patients with prior cancer, 14,802 patients were found to have stage III NSCLC. After applying the remaining exclusion criteria, summarized in Figure 1, 5,577 individuals were identified and were included in this analysis. The baseline characteristics of patients analyzed are presented in Table 1. Slightly more than half of population consisted of males (53.5%; n=2,985). Due to privacy concerns, the ICES database does not permit extraction of individual age information. However, distribution of age groups (in 10-year groupings) was obtained and just over half the patients (50.2.%; n=2,801) were 70 years or older.
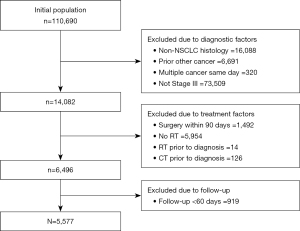
Table 1
| Characteristics and outcomes | N | Variable | Statistic |
|---|---|---|---|
| Patient variables, n (%) | |||
| Year of diagnosis | 5,577 | 2007–2010 | 2,009 (36.0) |
| 2011–2014 | 2,071 (37.1) | ||
| 2015–2017 | 1,497 (26.8) | ||
| Sex | 5,577 | Male | 2,985 (53.5) |
| Age groups | 5,577 | ≤59 | 1,083 (19.4) |
| 60–69 | 1,693 (30.4) | ||
| 70–79 | 1,901 (34.1) | ||
| 80+ | 900 (16.1) | ||
| Income quintile | 5,565 | 1 | 1,386 (24.9) |
| 2 | 1,258 (22.6) | ||
| 3 | 1,061 (19.1) | ||
| 4 | 1,028 (18.5) | ||
| 5 | 832 (15.0) | ||
| Rural patient | 5,575 | Yes | 958 (17.2) |
| Distance to nearest cancer centre, in km | 5,573 | Median (range) | 17 (0, 653) |
| Known Charlson Score | 5,577 | Median (range) | 0 (0, 8) |
| ≥1 | 677 (12.1) | ||
| Treatments within 180 days of diagnosis | |||
| PET prior to RT treatment | 5,577 | Yes | 2,308 (41.4) |
| Radiotherapy | 5,577 | Days to radiotherapy, median (IQR) | 55 (37, 82) |
| Radiotherapy dose | 5,577 | Median (IQR) | 34 (20, 60) |
| <40 | 3,015 (54.1) | ||
| 40 to 55.9 | 586 (10.5) | ||
| ≥56 | 1,976 (35.4) | ||
| Chemotherapy modality | 5,577 | Concurrent | 2,645 (47.4) |
| Sequential | 707 (12.7) | ||
| No chemo | 2,225 (39.9) | ||
| Chemotherapy | 3,352 | Prior to RT | 1,176 (35.1) |
| Outcomes | |||
| Overall survival, from date of RT, median (95% CI) | 5,577 | Deaths, n (%) | 4,564 (81.8) |
| Months | 12.4 (11.9, 12.9) | ||
| 1-year | 51.1 (49.8, 52.5) | ||
| 2-year | 28.8 (27.6, 30.1) | ||
| 5-year | 12.2 (11.2, 13.2) | ||
| Overall survival, of Pts who received 40 Gy+ radiation, median (95% CI) | 2,562 | Deaths, n (%) | 1979 (77.2) |
| Months | 17.8 (16.7, 18.7) | ||
| 1-year | 63.5 (61.5, 65.3) | ||
| 2-year | 39.1 (37.2, 41.1) | ||
| 5-year | 18.2 (16.6, 20.0) |
PET, positron emission tomography; RT, radiotherapy; IQR, interquartile range; 95% CI, 95% confidence interval.
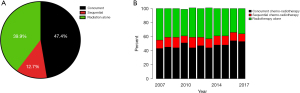
RT was utilized as monotherapy in 2,225 (39.8%) patients, while cCRT and sCRT were utilized in 2,645 (47.4%) and 707 (12.6%) patients, respectively (Figure 2A). Use of cCRT appeared to increase slightly over time but use of sCRT remained relatively constant in this population (Figure 2B). Within the group treated with RT alone, the majority of patients (1,611, 72.4%) were treated with low-dose RT (<40 Gy), while 292 (13.1%) and 322 (14.5%) received 40–55.9 and ≥56 Gy, respectively. Conversely, within the cCRT group, 857 (32.4%) received <40 Gy, 208 (7.9%) received 40–55.9 Gy and 1,580 (59.7%) received ≥56 Gy (Table S2).
Overall, 2,308 (41.4%) patients had a PET scan prior to RT. There were 1,315 (49.7%), 207 (29.3%) and 786 (35.3%) patients who had a PET scan amongst patients receiving cCRT, sCRT and RT alone, respectively. Alternatively, of all patients receiving <40, 40–55.9 and ≥56 Gy, 1,057 (35.1%), 217 (37.0%) and 1,034 (52.3%) underwent imaging with FDG-PET.
Outcomes
Median OS of the entire cohort was 12.4 months (95% CI: 11.9–12.9). Factors associated with survival are shown in Table 2. In univariate analysis year of diagnosis, sex, age, income quintile, Charlson score, use of chemotherapy and increasing dose of RT were associated with improved survival. However, only gender, use of chemotherapy, higher dose RT and staging with FDG-PET maintained significant in multivariable analyses. Males (HR =1.18, 95% CI: 1.12–1.25) and patients who received cCRT (HR =0.51, 95% CI: 0.48–0.56) or sCRT (HR =0.80, 95% CI: 0.72–0.88) had improved survival relative to patients who received RT alone. Increasing dose of RT was also associated with improved survival (HR =0.85, 95% CI: 0.77–0.93 for 40–55.9 Gy and HR =0.70, 95% CI: 0.65–0.75 for ≥56 Gy versus patients who received <40 Gy). Patients with baseline PET imaging also had significantly improved survival (HR =0.87, 95% CI: 0.81–0.93). Survival estimates by chemotherapy modality, RT dose and PET utilization are given in Table 3 (sCRT outcomes in Table S3). Figure 3A,3B illustrates Kaplan-Meier survival curves for patients in the RT alone and cCRT groups in the described RT-dose and PET utilization categories (sCRT in Figure S1).
Table 2
| Variables | N | HR (95% CI) | P value |
|---|---|---|---|
| Univariable analysis | |||
| Year of diagnosis | 5,577 | 0.99 (0.98, 1.00) | 0.006 |
| Sex (male vs. female) | 5,577 | 1.19 (1.12, 1.26) | <0.001 |
| Age groups | 5,577 | 1.10 (1.08, 1.12) | <0.001 |
| Income quintile | 5,565 | 0.97 (0.95, 0.99) | 0.005 |
| Rurality (yes vs. no) | 5,575 | 1.06 (0.98, 1.14) | 0.17 |
| RCC distance, km | 5,573 | 1.00 (1.00, 1.00) | 0.76 |
| Known Charlson Score (≥1 vs. 0) | 5,577 | 1.20 (1.10, 1.31) | <0.001 |
| Chemotherapy (yes vs. no) | 5,577 | 0.49 (0.46, 0.52) | <0.001 |
| PET prior to RT (yes vs. no) | 5,577 | 0.77 (0.73, 0.82) | <0.001 |
| Chemotherapy | |||
| Concurrent | 5,577 | 0.43 (0.40, 0.46) | <0.001 |
| Sequential | 0.81 (0.74, 0.89) | ||
| None | Reference | ||
| RT dose | |||
| <40 | 5,577 | Reference | <0.001 |
| 40–55.9 | 0.84 (0.76, 0.92) | ||
| 56+ | 0.52 (0.49, 0.55) | ||
| PET prior to RT, by RT dose (yes vs. no) | |||
| <40 | 3,015 | 0.78 (0.72, 0.85) | <0.001 |
| 40–55.9 | 586 | 1.02 (0.85, 1.23) | 0.80 |
| 56+ | 1,976 | 0.88 (0.79, 0.97) | 0.012 |
| Multivariable analysis | 5,565 | ||
| Year of diagnosis | 0.99 (0.98, 1.00) | 0.12 | |
| Sex (male vs. female) | 1.18 (1.12, 1.25) | <0.001 | |
| Age groups | 1.02 (1.00, 1.03) | 0.097 | |
| Income quintile | 0.99 (0.97, 1.01) | 0.18 | |
| Rurality (yes vs. no) | 1.05 (0.97, 1.14) | 0.21 | |
| Known Charlson Score (≥1 vs. 0) | 0.96 (0.88, 1.05) | 0.40 | |
| Chemotherapy | <0.001 | ||
| Concurrent | 0.51 (0.48, 0.56) | ||
| Sequential | 0.80 (0.72, 0.88) | ||
| None | Reference | ||
| RT dose | <0.001 | ||
| <40 | Reference | ||
| 40–55.9 | 0.85 (0.77, 0.93) | ||
| 56+ | 0.70 (0.65, 0.75) | ||
| PET prior to RT (yes vs. no) | 0.87 (0.81, 0.93) | <0.001 |
Interaction tests: PET with chemotherapy modality P value =0.043; PET with RT dose P value =0.20; RT dose with chemotherapy modality P value <0.001. RT, radiotherapy; RCC, regional cancer center; PET, positron emission tomography.
Table 3
| Treatment modality | RT dose (Gy) | PET | Median (95% CI) | 1-year (95% CI) | 2-year (95% CI) | 5-year (95% CI) |
|---|---|---|---|---|---|---|
| Radiation alone | <40 | All (n=1,611) | 7.2 (6.9, 7.6) | 31 (29, 34) | 12 (10, 14) | 2 (2, 3) |
| No PET (n=1,109) | 6.6 (6.2, 7.0) | 28 (25, 30) | 10 (8, 12) | 2 (1, 3) | ||
| PET (n=502) | 8.8 (7.8, 9.7) | 39 (35, 43) | 16 (13, 19) | 2 (1, 5) | ||
| 40–55.9 | All (n=292) | 8.5 (7.3, 10.6) | 39 (33, 45) | 21 (16, 26) | 7 (4, 10) | |
| No PET (n=173) | 7.5 (6.6, 9.7) | 37 (30, 44) | 21 (15, 28) | 7 (4, 12) | ||
| PET (n=119) | 10.3 (7.6, 12.2) | 42 (33, 51) | 20 (13, 29) | 6 (2, 12) | ||
| 56+ | All (n=322) | 13.3 (11.2, 15.7) | 53 (47, 58) | 30 (25, 35) | 7 (4, 10) | |
| No PET (n=157) | 10.8 (8.5, 13.8) | 47 (38, 54) | 26 (19, 34) | 7 (3, 12) | ||
| PET (n=165) | 15.4 (12.3, 19.2) | 59 (51, 66) | 34 (26, 41) | 7 (3, 13) | ||
| Concurrent-chemo-radiotherapy | <40 | All (n=857) | 16.5 (14.9, 18.4) | 60 (57, 63) | 37 (34, 41) | 17 (14, 20) |
| No PET (n=451) | 15.8 (13.6, 17.9) | 58 (53, 62) | 37 (32, 42) | 17 (13, 22) | ||
| PET (n=406) | 17.8 (15.2, 20.6) | 63 (58, 68) | 37 (32, 42) | 17 (12, 22) | ||
| 40–55.9 | All (n=208) | 15.8 (12.6, 19.2) | 61 (54, 67) | 33 (27, 40) | 16 (11, 22) | |
| No PET (n=138) | 15.4 (12.0, 19.1) | 58 (50, 66) | 31 (23, 39) | 15 (9, 21) | ||
| PET (n=70) | 16.8 (12.2, 24.3) | 65 (53, 75) | 39 (27, 51) | 19 (10, 31) | ||
| 56+ | All (n=1,580) | 22.0 (21.0, 23.8) | 72 (70, 74) | 47 (44, 50) | 24 (22, 26) | |
| No PET (n=741) | 21.4 (19.5, 23.8) | 71 (67, 74) | 46 (43, 50) | 21 (18, 24) | ||
| PET (n=839) | 23.0 (21.1, 25.0) | 74 (71, 77) | 48 (44, 51) | 28 (24, 31) |
PET, positron emission tomography; RT, radiotherapy.

Interactions between PET utilization and chemotherapy modality and between RT dose and chemotherapy modality were both statistically significant. Thus, interpretation should be performed separately for each RT/cCRT/sCRT and dose group, and not as an additive effect. Follow-up was a minimum of 1.25 years (end of 2017 was last patients diagnosis date, end of follow up was 31 march 2019).
Discussion
The aim of this analysis was to evaluate patterns of care and RT outcomes of unresected LA-NSCLC in Ontario, Canada, in recent years. We report on real-world survival outcomes of modern RT used as monotherapy. We included in the analysis utilization of FDG-PET to help understand better the potential impact of RT dose in well-staged patients. We need to emphasize that, given the reasons for a patient to receive RT as monotherapy vs. cCRT, performance status is an unmeasurable confounder, the effect sizes observed cannot be assumed to be causal. Results in this report should therefore be used solely to improve our understanding of RT alone outcomes in a contemporary real-world North American setting.
Concordant with previous reports (1,7-9), we found that a significant proportion of patients in our cohort from Ontario were treated with RT alone (39.8%) (Figure 2A). Trends of treatment use (RT alone vs. cCRT or sCRT) have changed slightly over the years in favor of cCRT, but overall remained similar in the period 2007–2017 (Figure 2B). Importantly, 27.6% of patients that received RT alone were treated with RT doses higher than those typically used for palliation (>40 Gy), indicating that RT is perceived as a potentially useful tool for local disease control also.
Figure 3 illustrates, (I) the average performance of RT alone treatment in LA-NSCLC patients receiving contemporary chest RT in Ontario, (II) how OS relates to RT dose and FDG-PET utilization and (III) how these compare with outcomes of good performance status patients receiving SoC treatment.
The higher OS observed in the RT alone groups managed with increased RT dose and PET utilization are indeed important. Although increasing RT dose did not translate to substantial absolute improvements in long-term (i.e., 5-year) OS in this group, median, 1- and 2-year OS show significant improvements with use of high-dose RT. We believe that short-term outcomes are more reliable evaluators of potential treatment benefit in patients receiving RT alone. The group of patients treated with RT alone is characterized by poor performance status and comorbidities, which determine long-term survival. Nevertheless, higher dose of chest RT appears feasible and effective in selected patients.
In our cohort, patients that received curative dose RT as monotherapy (≥56 Gy) had a median OS rate of 13.3 months, which further increased to 15.4 months in PET-staged patients. While not formally comparable, these values are higher than historical clinical trials [such as CALGB 8433 (12) and RTOG 8808 (15); median OS of 10 and 11 months, respectively]. Discrepancies between values from historical trials versus modern studies may be due to various factors including, FDG-PET based staging, improved RT planning and RT delivery with intensity-modulated-(IMRT) and image-guided RT (IGRT) (1,11,13). There is limited contemporary randomized clinical trial data on OS achieved with curative dose RT alone. Recently, a phase III randomized trial that accrued patients in US centers between 2012 and 2018 reported RT-alone outcomes in patients treated with either conventional (60 Gy in 30 fractions) or hypo-fractionated RT (60 Gy in 15 fractions) (16). FDG-PET imaging was optional in that study. Hypo-fractionated RT did not offer overall benefit and conventional RT showed 1- and 2-year OS of 44% and <30%, respectively. In comparison, 1- and 2-year OS in our cohort with RT-alone of ≥56 Gy were 53% and 30% for the entire group, increasing to 59% and 34%, respectively, for those staged with FDG-PET, indicating the value of on-going investigation of RT-alone outcomes in LA-NSCLC.
In our cohort OS of patients treated with cCRT is lower compared to those reported in recent landmark trials of cCRT without or with consolidation immunotherapy, such as RTOG 0617 (11) and PACIFIC (17), respectively. These trials showed median and 2-year OS of 29 months and 55–57.65% for standard cCRT vs. 21–23.0 months and 46–48% in this study. Further, very recently reported phase II randomized trials that accrued stage IIIA-B NSCLC patients, staged with FDG-PET, in the US and Canada in the past 7 years, reported higher 2-year OS of 65–66% with standard cCRT alone (18,19). SoC therapy outcomes may be improving over time and this will, likely, be reflected in future reports of population outcomes.
In this study, FDG-PET use was associated with higher median OS, regardless of RT dose. The contribution of FDG-PET was not analyzed in prior population studies of RT-alone outcomes but other reports in LA-NSCLC suggested a positive correlation of PET use with improved OS. In a secondary analysis of the PROCLAIM trial (20), patients staged with FDG-PET showed trends for longer median OS vs. those who were not (median OS 27.2 vs. 20.8 months; non-significant). While their data did not reach statistical significance for OS, median progression-free survival (mPFS) was significantly longer in the PET-staged group (11.3 vs. 9.2 months). A frequently-cited reason for the benefit of baseline FDG-PET is the effective exclusion of metastatic patients resulting in stage migration. However, PET may also contribute to improved outcomes by improving tumor delineation during RT planning (14,21-27), leading to improvements in tumor targeting and reduced toxicity through sparing of organs at risk.
This study did not aim to analyze the impact of chemotherapy on outcomes. Therefore, sCRT outcomes are not discussed in detail here (see Table S2 and Figure S1). Expectantly, use of sCRT is associated with improved OS compared to RT monotherapy but inferior compared to cCRT, in agreement with other studies (5,12). However, it should be noted that the parameters set to select this group of patients aimed to be more inclusive of patients treated with both radiotherapy and chemotherapy, but not cCRT, and were not designed to select patients that are typically planned to receive sCRT.
Other factors, such as access to cancer care due to income inequalities and distance from a cancer care facility, are often described to potentially influence outcomes in lung cancer patients in North America and worldwide (28). We found that such trends may exist in patients receiving care in Ontario centers; however, these factors did not predict OS independently. Future studies should investigate these factors further as they relate to specific treatment centers in Ontario or other jurisdictions.
Our study has several shortcomings. Population-based evidence is retrospective and limited by the detail and quality of data. While IC/ES provides reliable access to health service utilization in Ontario, the database does not include information on the intent of the RT treatment regimen or the intent of staging investigation used. Further, databases accessed by IC/ES have limited information on the cause of death. We attempted to reduce effects of survivorship bias by selecting for patients with available follow-up of greater than 60 days. However, this type of “landmark analysis” does not completely eliminate all potential bias within the data. Lack of PET utilization in some patients may have been due to limited use of FDG-PET in the early years of its introduction into clinical practice as well as wait times or patient specific factors. It should also be recognized that, apart from FDG-PET, more systematic use of brain magnetic resonance imaging (MRI) and mediastinal staging over the past 15 years, have likely contributed to further improvement of outcomes in patients with LA-NSCLC through stage migration. Future population studies may be able to analyze the impact of these factors. Finally, SoC in unresected LA-NSCLC is evolving rapidly. The data presented in this study illustrate outcomes of RT or CRT alone, as consolidation anti-PD-L1 therapy (Durvalumab) was not approved by Health Canada until May 2018. Given the results of the PACIFIC trial (17), patients receiving SoC treatment today are expected to show improved OS rates that may be detected in future analyses.
Conclusions
This real-world data analysis from the province of Ontario illustrates that a large number of patients with LA-NSCLC continues to be managed with RT alone. In this understudied population, we find that higher chest RT dose and utilization of staging FDG-PET are associated with improved OS. These results provide important information to support clinical practice and future prospective clinical trials in this group of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-925/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-925/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-925/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-925/coif). Greg Pond declares receiving consulting fees from profound Medical and Merck. He also received honoraria from Astra-Zeneca for educational events; received honorarium from Takeda for DSMB (Data Safety Monitoring Board or Advisory Board) membership. He has a close family member who is an employee of Roche Canada, and who owns stock in Roche Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Hamilton integrated ethics board (HiREB #10652). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seung SJ, Hurry M, Walton RN, et al. Retrospective cohort study of unresectable stage III non-small-cell lung cancer in Canada. Curr Oncol 2020;27:e354-60. [Crossref] [PubMed]
- Ganti AK, Klein AB, Cotarla I, et al. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol 2021;7:1824-32. [Crossref] [PubMed]
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13:5-13. [Crossref] [PubMed]
- Trovó MG, Minatel E, Franchin G, et al. Radiotherapy versus radiotherapy enhanced by cisplatin in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1992;24:11-5. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. [Crossref] [PubMed]
- Moore S, Leung B, Wu J, et al. Real-World Treatment of Stage III NSCLC: The Role of Trimodality Treatment in the Era of Immunotherapy. J Thorac Oncol 2019;14:1430-9. [Crossref] [PubMed]
- Vinod SK, Wai E, Alexander C, et al. Stage III non-small-cell lung cancer: population-based patterns of treatment in British Columbia, Canada. J Thorac Oncol 2012;7:1155-63. [Crossref] [PubMed]
- Yusuf D, Walton RN, Hurry M, et al. Population-based Treatment Patterns and Outcomes for Stage III Non-Small Cell Lung Cancer Patients: A Real-world Evidence Study. Am J Clin Oncol 2020;43:615-20. [Crossref] [PubMed]
- Peng J, Pond G, Donovan E, et al. A Comparison of Radiation Techniques in Patients Treated With Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2020;106:985-92. [Crossref] [PubMed]
- Bradley JD, Hu C, Komaki RR, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:706-14. [Crossref] [PubMed]
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940-5. [Crossref] [PubMed]
- Cheng M, Jolly S, Quarshie WO, et al. Modern radiation further improves survival in non-small cell lung cancer: an analysis of 288,670 patients. J Cancer 2019;10:168-77. [Crossref] [PubMed]
- Taus Á, Aguiló R, Curull V, et al. Impact of 18F-FDG PET/CT in the treatment of patients with non-small cell lung cancer. Arch Bronconeumol 2014;50:99-104. [Crossref] [PubMed]
- Sause W, Kolesar P, Taylor S IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358-64. [Crossref] [PubMed]
- Iyengar P, Zhang-Velten E, Court L, et al. Accelerated hypofractionated image-guided vs conventional radiotherapy for patients with stage II/III non-small cell lung cancer and poor performance status: a randomized clinical trial. JAMA Oncol 2021;7:1497-505. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Skinner H, Hu C, Tsakiridis T, et al. Addition of Metformin to Concurrent Chemoradiation in Patients With Locally Advanced Non-Small Cell Lung Cancer: The NRG-LU001 Phase 2 Randomized Clinical Trial. JAMA Oncol 2021;7:1324-32. [Crossref] [PubMed]
- Tsakiridis T, Pond GR, Wright J, et al. Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: the OCOG-ALMERA randomized clinical trial. JAMA Oncol 2021;7:1333-41. [Crossref] [PubMed]
- Vokes EE, Govindan R, Iscoe N, et al. The Impact of Staging by Positron-Emission Tomography on Overall Survival and Progression-Free Survival in Patients With Locally Advanced NSCLC. J Thorac Oncol 2018;13:1183-8. [Crossref] [PubMed]
- Berberoğlu K. Use of Positron Emission Tomography/Computed Tomography in Radiation Treatment Planning for Lung Cancer. Mol Imaging Radionucl Ther 2016;25:50-62. [Crossref] [PubMed]
- Bradley J, Thorstad WL, Mutic S, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;59:78-86. [Crossref] [PubMed]
- Nestle U, De Ruysscher D, Ricardi U, et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol 2018;127:1-5. [Crossref] [PubMed]
- van Der Wel A, Nijsten S, Hochstenbag M, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys 2005;61:649-55. [Crossref] [PubMed]
- Ceresoli GL, Cattaneo GM, Castellone P, et al. Role of computed tomography and [18F] fluorodeoxyglucose positron emission tomography image fusion in conformal radiotherapy of non-small cell lung cancer: a comparison with standard techniques with and without elective nodal irradiation. Tumori. 2007;93:88-96.
- Faria SL, Menard S, Devic S, et al. Impact of FDG-PET/CT on radiotherapy volume delineation in non-small-cell lung cancer and correlation of imaging stage with pathologic findings. Int J Radiat Oncol Biol Phys 2008;70:1035-8. [Crossref] [PubMed]
- Yin LJ, Yu XB, Ren YG, et al. Utilization of PET-CT in target volume delineation for three-dimensional conformal radiotherapy in patients with non-small cell lung cancer and atelectasis. Multidiscip Respir Med 2013;8:21. [Crossref] [PubMed]
- Ambroggi M, Biasini C, Del Giovane C, et al. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist 2015;20:1378-85. [Crossref] [PubMed]


