
Cardioprotective medication in Duchenne muscular dystrophy: a single-centre cohort study
Highlight box
Key findings
• Long-term use of ACE-inhibitors and beta-blockers is associated with a reduced decline in the LVEF in patients with DMD, and may be protective of adverse cardiovascular ill health.
What is known and what is new?
• Patients with DMD typically have life-limiting complications due to cardiorespiratory complications, including the developing feature of DMD-associated cardiomyopathy.
• It is important to notice that continuous protection of cardiomyopathic progression can be delivered by ongoing prescription of ACE-inhibitors and betablockers.
What is the implication, and what should change now?
• The long-term care of patients with complex needs due to DMD requires the coordinated guidance by a multidisciplinary team, including the respiratory physician and the cardiologist to guarantee optimal treatment and preserve cardiac function.
Introduction
Duchenne muscular dystrophy (DMD) is an X-chromosome linked neuromuscular disorder affecting one in 3,600–6,000 live male births (1), resulting in progressive skeletal muscle failure with generalised effects on the muscles in the entire body, including the cardiovascular and the respiratory system.
DMD typically presents with first clinical signs before the age of five with gait disturbances and difficulty in climbing stairs, with a full loss of ambulation by the age of 13 years (2). Eventually, deterioration of the skeletal muscles involved in the respiratory and cardiac system leads to respiratory failure and, commonly, dilated cardiomyopathy (3-5). Cardiorespiratory complications are the leading cause of mortality in DMD patients (6). However, with advancing respiratory support, such as home mechanical ventilation (HMV), respiratory failure can be better controlled. This development shifts the focus towards cardiovascular causes as the life-limiting factor in DMD (7,8), and left ventricular function is an important clinical marker for the DMD associated cardiomyopathy (9).
Studies have suggested the prophylactic use of angiotensin-converting enzyme inhibitors (ACE-I) (10,11), and the 2010 DMD care considerations support the use of ACE-I or angiotensin receptor blocker (ARB) by the age of ten (12), although there is no mentioning on the effects of beta-blockers.
This cohort study sought to investigate the effect of ACE-I and beta-blockers on clinical progression of the cardiomyopathy of DMD patients during their follow up in a tertiary referral centre, with specific focus on the left ventricular function. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1528/rc).
Methods
This was a retrospective, single-centre cohort study of patients with DMD who required HMV with follow up period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Guy’s & St Thomas’ NHS Foundation Trust as service review (reference No: 2021/12469) and individual consent for this retrospective analysis was waived. The local electronic patient records (EPRs) were screened to identify patients with DMD between 1st January 1993 and 15th June 2021. Patients were selected from the Lane Fox Unit, a large tertiary referral centre for HMV, for more information on the protocol please refer to the online supplement.
In- and exclusion criteria
Patients with a diagnosis of DMD clearly stated in the records and under the Lane Fox service were selected for analysis, excluding any intermediate phenotypes. Patients of any age were included (excluding patients <18 years). Due to the nature of DMD (X-chromosome recessive), patients were male.
Outcome parameters
The primary outcome parameter was decline in the left ventricular ejection fraction (LVEF; %). Secondary outcomes parameters included morbidity, HMV, hospitalisations and mortality.
Statistical analysis
Data were initially collected in a spreadsheet on MS Excel (version 16.54, Microsoft, Seattle, WA, USA). The Kolmogorov-Smirnov test was used to tested for normality using IBM SPSS Statistics for Macintosh (version 27.0, IBM Corp., Armonk, NY, USA). Mean and standard deviation (SD) were reported for normally distributed data, while median and interquartile range (IQR) was stated for non-normally distributed data. Unpaired two-tailed t-tests were used to compare the body mass index (BMI), HMV settings, arterial blood gasses (ABGs), the LVEF (% change), and differences in LVEF change when taking ACE-I and beta-blockers between different patient groups. The group change in the observed LVEF was tested using paired t-tests, and LVEF decline was further described using the 95% confidence intervals (CIs). The Mann-Whitney U test was used to assess the difference in severity measures and hospitalisations between survivors and deceased patients. The level of significance was defined as P<0.05.
Results
Baseline demographics
A total of 68 patients were included in this study aged 27.4 (6.6; range, 18–46) years, 76.5% were still alive {28 [7] years}; 23.5% of the cohort had died with an average age of 26 [6] years. The BMI for the entire cohort was 22.7 (5.6) kg/m2, while survivors had a higher BMI than deceased patients [23.8 (5.9) vs 19.9 (3.8) kg/m2, P=0.03]. At the time of the initial diagnosis, patients were 11 [7] years old, and they were seen in regular follow up intervals of 6 [1] months in the HMV services; the total follow up period in the Lane Fox service was on average 133.7 (31.1) months. A DMD mutation type was identified in 54.4% patients (Table S1). A total of 69.1% patients had ABGs at the last follow up, of which 70.2% were still alive and 29.8% had died (Table 1). For further information on hospitalisations please refer to Appendix 1.
Table 1
| ABG analysis | Survivors | Deceased | Combined | P value (survivors vs. deceased) |
|---|---|---|---|---|
| pH | 7.41 (0.07) | 7.34 (0.21) | 7.39 (0.13) | 0.07 |
| pO2 (mmHg) | 10.15 (3.59) | 8.91 (3.73) | 9.79 (3.63) | 0.31 |
| pCO2 (mmHg) | 6.26 (1.93) | 7.21 (2.95) | 6.55 (2.3) | 0.20 |
| HCO3− (meq/L) | 26.43 (4.08) | 27.80 (5.47) | 26.85 (4.54) | 0.35 |
| BE | 1.37 (4.12) | 3.82 (5.17) | 2.21 (4.59) | 0.12 |
Data are presented as mean (SD). The P value was derived from an unpaired t-test. ABG, arterial blood gas; pO2, partial pressure of oxygen; pCO2, partial pressure of carbon dioxide; HCO3−, bicarbonate; BE, base excess; SD, standard deviation.
HMV
A total of 89.7% of the patients had been established on HMV for an average of 94 [79] months. Hypercapnic respiratory failure had been diagnosed 95 [77] months after initially being diagnosed with DMD (Table S2). There were no significant differences in the ventilator settings or devices between the survivors and the deceased patient [P= not significant (NS); Table 2, for devices refer to Table S3]. In patients established on HMV, overnight monitoring confirmed sufficient respiratory control with the average percutaneous arterial oxygen saturation (SpO2) at 97.0% (2.1%), the apnoea-hypopnoea index (AHI) was 8.5 (5.8)/hour, the 4% oxygen desaturation index (ODI) was 2.2 (0.8–8.3)/hour, and the time below an SpO2 of 90% (T<90) was 6.0% (0.0–11.0%) of the night.
Table 2
| NIV settings | Survivors | Deceased | All patients | P value (survivors vs. deceased) |
|---|---|---|---|---|
| IPAP (cmH2O) | 20.0 (5.0) | 21.6 (5.0) | 20.4 (5.2) | 0.29 |
| EPAP (cmH2O) | 5.2 (2.0) | 4.9 (2.0) | 5.0 (2.0) | 0.71 |
| BUR (breaths/min) | 16.3 (2.9) | 16.7 (2.3) | 16.4 (2.7) | 0.58 |
| Ti (ms) | 1.3 (0.1) | 1.3 (0.1) | 1.3 (0.1) | 0.4 |
Data are presented as mean (SD). The P value was derived from an unpaired t-test. NIV, non-invasive ventilation; IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure; BUR, back-up rate; Ti, inspiratory time; SD, standard deviation.
Morbidity status
All patients were non-ambulatory, and were under joint respiratory and cardiology follow up. 68.8% patients used a mechanical insufflation-exsufflation (MIE) device, 44.8% had a feeding tube [percutaneous endoscopic gastrostomy (PEG)] for an average of 75 [50] months, and 40.0% had undergone spinal surgery. The associated cardiomyopathy was developed after 102 [76] months (Table S1). The morbidity severity score was 1.4 (1.0), with 23.5% of the patients scoring zero points, 27.9% scoring one point, 29.4% with two points, and 19.1% of the patients had the highest score of three points. The mean morbidity score for alive patients was 1.3 (1.1) points, and 1.8 (0.8) points for deceased patients (P=0.11). Following comparison of the initial and the final electrocardiograms (ECGs) for each patient, there was in increase in pathological findings over time (Table S4).
Medication
A total of 89.7% of patients took ACE-I at some point in the follow up period for 96 [40] months (Table 3), although 25.0% patients did not remain on the medication due to side effects with hypotension, cough, renal failure, angioedema, headache, and rash. A total of 91.2% of patients were established on beta-blockers at some point in the follow up period for 87 [39] months (Table 3), with 10.3% coming off/never starting the medication due to wheeze/asthma, hypotension, and peripheral circulation problems; some patients had multiple reasons for not taking the above medications (more details on medication are listed in Table 4).
Table 3
| Medication | No. of patients at final follow up | Dosage (mg), mean (SD) |
|---|---|---|
| ACE-I | ||
| Lisinopril | 12 | 10.2 (4.5) |
| Ramipril | 28 | 4.6 (3.0) |
| Perindopril | 10 | 3.0 (1.1) |
| Captopril | 1 | 25.0 (0.0) |
| Beta-blockers | ||
| Bisoprolol | 55 | 3.6 (2.2) |
| Carvedilol | 6 | 11.1 (7.6) |
ACE-I, angiotensin-converting enzyme inhibitors; SD, standard deviation.
Table 4
| Other key medications | No. of patients | Length of time taken (months), mean [SD] |
|---|---|---|
| Corticosteroids | 11 | 115 [41] |
| Antidiabetic medication | 4 | 104 [69] |
| Mineralocorticoid receptor antagonist | 3 | 44 [8] |
| Ivabradine | 13 | 49 [25] |
| Candesartan | 5 | 50 [50] |
| Other medications | – | |
| Digoxin | 1 | |
| Eplerenone | 2 | |
| PPI | 20 | |
| H2 receptor blocker | 7 | |
| Antihistamines | 5 | |
| Bisphosphonates | 8 | |
| Anticoagulants | 3 | |
| Antiplatelets | 2 | |
| Osteoporosis prophylaxis | 20 |
PPI, protein-protein interaction; H2, histamine type 2; SD, standard deviation.
Echocardiography
Echocardiography was repeatedly recorded in intervals of 84 [48] months. The LVEF at initial presentation was 44.8% (10.6%) and at most recent follow up 41.9% (12.0%) (P=0.002), with a change of −3.3% (95% CI: 0.4% to −7.0%). The proportion of LVEF decline, comparing the final LVEF to the initial recordings, was −10.0% (95% CI: −3.5% to −16.5%). The proportionate decline in LVEF for survivors was −7.1% (95% CI: −0.3% to −13.9%) (P=0.008) and −19.8% (95% CI: −3.8% to −35.8%) (P=0.02) for deceased patients.
ACE-I and LVEF
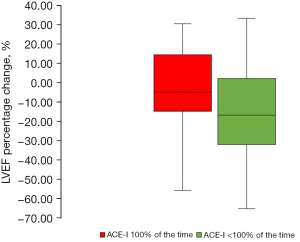
Patients were established on ACE-I during 75.9% (35.1%), or 95.9 (39.6) months of the follow up interval. The proportionate change in LVEF for those established on ACE-I for the entire interval (100%) was −4.3% (95% CI: 4.2% to −12.8%) (P=0.4; n=29). In contrast, there was a significant decline in those taking ACE-I for less than the entire follow up interval (<100%) [LVEF −15.7% (95% CI: −6.2% to −25.2%), P=0.002, n=39; Figure 1].
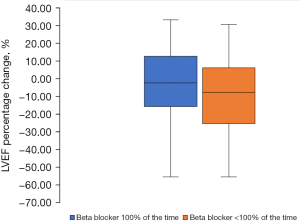
Betablockers and LVEF
Patients were established on beta-blockers for 73.6% (33.5%), or 87.1 (38.8) months of the follow up interval. The proportionate LVEF change for those established on beta-blockers (n=22) for the entire period (100%) was non-significant [LVEF −5.73% (95% CI: 4.97% to −16.73%), P=0.2]. For those taking the medication for less than the entire follow up period (<100%) there was a significant decline [LVEF −7.6% (95% CI: −0.9% to −14.3%), P=0.02; Figure 2].
Discussion
In a cohort of DMD patients who were followed for over a decade in a tertiary referral centre patients developed hypercapnic respiratory failure and cardiomyopathy about 8 years following diagnosis of the disease, requiring joint cardiorespiratory specialist input (HMV/pacemaker). The permanent use of both ACE-I and beta-blockers over the follow up period was associated with preserved left ventricular pump function and protective of progression of the disease-specific cardiomyopathy. In this cohort study, lower body mass was associated with mortality. Morbidity and mortality did not significantly differ between survivors and those who died during the follow up. However, patients with DMD have a high carer burden due to developing comorbidities and other issues.
Clinical significance of the findings
ACE-I usage has previously been reported to prevent cardiomyopathic features in DMD patients (10,11), likely due to an inhibition of the “cardiac remodelling” properties of this drug class. Studies show that beta-blockers alongside ACE-I also have beneficial effects on survival and heart failure progression (1). Consistent with these reports, our study observed a protective inhibition of the LVEF decline when patients were established permanently on both ACE-I and beta-blockers. Following NHS advice the majority of the patients in our cohort study were established on ACE-I and beta-blockers (13). Previous studies look at the effects of maintenance doses of ACE-I and beta-blockers, in the future investigating the effects of higher doses providing tolerability could give more insight into cardiac management.
Furthermore, there were 11 patients who took corticosteroids for 115 [41] months to prevent loss of ambulation and, more recently, corticosteroids have been reported to reduce the progression of the left ventricular dysfunction in DMD (14,15). Furthermore, it has been highlighted that Ivabradine may provide benefits for DMD patients in reducing acute adverse cardiac events (16). In our cohort, 13 patients took Ivabradine, five of whom died during the observation period; it is possible that the use of Ivabradine may be indicated in the more severe cases of DMD with progressing features of cardiomyopathy and, thus, may represent a selection bias. Five other patients were established on Candesartan as an alternative to ACE-I (17). However, these subgroups were small and the data could not be analysed in a meaningful manner to draw further firm conclusions.
There is a so-called “obesity paradox” in patients with heart failure, where cachectic patients are more likely to have a worse prognosis (18). This is also found in patients with chronic obstructive pulmonary disease (COPD) (19). In our study, there was a statistically significant difference in the BMI between survivors and deceased patients, with poorer outcomes associated with lower BMI. In the context of a cohort of patients that frequently requires nutritional support, it is important to highlight the appropriate nutritional intake and, if indicated, the involvement and early consultation of dietitian and the gastrointestinal specialist to decide future treatment (e.g., PEG insertion).
Substantial ventilator settings indicate the need to support ventilator pressures and reduce the risk of hypoventilation in DMD (20). Consistent with these descriptions, the group of deceased patients in our study required higher backup rates and higher inspiratory pressures to achieve sufficient ventilatory control. Furthermore, deceased patients were identified with respiratory acidosis and notably higher partial pressure of carbon dioxide (pCO2) and lower partial pressure of oxygen (pO2) levels during emergency admission, indicating life-limiting hypercapnic respiratory failure. In contrast, relatively normal blood gas samples in the cohort of survivors indicated good respiratory control during regular usage of the non-invasive ventilation (NIV), as previously described in similar cohort studies (21).
A MIE device is indicated for DMD patients with associated respiratory failure, as the respiratory muscle weakness causes hypoinflation of the lungs, leaving the patients to breathe at low lung volume as the disease progresses; this contributes to narrowing of the lower airways, increased airway resistance, hypoinflated and dystelectatic lung regions, and an ineffective cough (22). Due to the progressive muscle weakness, a third of the patients have swallowing difficulties (23), requiring feeding tubes. Spinal surgery is required in patients with scoliosis, with the aim to improve posture, function, balance, and quality of life (24). Ambulation was lost in our cohort at the age of 13 years. The morbidity scores indicated in this study reveal the serious impact of the condition on patients with DMD and highlight the need for a supportive care package to facilitate ambulation, chest clearance, diet, and have a meaningful impact on quality of life for patients who live with a lifelong condition.
Limitations of the study
Due to the relatively small sample size and the retrospective nature of this cohort study there are certain limitations to the generalisability of the data. Two different software systems were used to collect the clinical data but some information was missing, sometimes due to late referral. Incomplete records were passed on during the referral and transitioning process, making it difficult to identify onset of the conditions and rule out that cardiac function had not been assessed earlier on, or medication had been issued at an earlier stage. As a result, we cannot determine the effect of implantable cardioverter defibrillator (ICD)/cardiac resynchronisation therapy (CRT) implantation on cardiac progression. However, given the consistent findings of ACE-I and beta-blockers improving outcomes and comorbidities, such as respiratory failure and cardiomyopathies, being diagnosed the authors feel that the current dataset is a true representation of a clinical cohort sample of DMD patients in a tertiary referral centre.
Conclusions
Long-term follow up of patients with DMD is important, as they develop life-limiting comorbidity with hypercapnic respiratory failure and cardiomyopathy. The permanent use of ACE-I and beta-blockers is important to improve long-term outcomes and may be protective of the cardiac remodelling associated with the development of the disease specific cardiomyopathy. Patients with DMD should be followed up in multidisciplinary settings, involving the respiratory physician, the cardiologist, the dietitian, and gastrointestinal team, as well as a dedicated care coordinator.
Acknowledgments
We gratefully acknowledge the support and comments from the specialist Lane Fox Unit team and outpatient clinic coordinator, as well as the multidisciplinary input from the wider St Thomas’ support network.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “Clinical Update Sleep 2023”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1528/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1528/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1528/coif). The series “Clinical Update Sleep 2023” was commissioned by the editorial office without any funding or sponsorship. JS serves as the unpaid Guest Editor of the series and an unpaid editorial board member of Journal of Thoracic Disease. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Guy’s & St Thomas’ NHS Foundation Trust as service review (reference No: 2021/12469) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77-93. [Crossref] [PubMed]
- Darras BT, Urion DK, Ghosh PS. Dystrophinopathies. In: Adam MP, Everman DB, Mirzaa GM, et al. editors. GeneReviews®. Seattle: University of Washington, 2022.
- Nigro G, Comi LI, Politano L, et al. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 1990;26:271-7. [Crossref] [PubMed]
- Sachdev B, Elliott PM, McKenna WJ. Cardiovascular Complications of Neuromuscular Disorders. Curr Treat Options Cardiovasc Med 2002;4:171-9. [Crossref] [PubMed]
- Kaspar RW, Allen HD, Montanaro F. Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J Am Acad Nurse Pract 2009;21:241-9. [Crossref] [PubMed]
- Van Ruiten HJ, Marini Bettolo C, Cheetham T, et al. Why are some patients with Duchenne muscular dystrophy dying young: An analysis of causes of death in North East England. Eur J Paediatr Neurol 2016;20:904-9. [Crossref] [PubMed]
- Ballard E, Grey N, Jungbluth H, et al. Observation cohort study of cause of death in patients with Duchenne muscular dystrophy (DMD). Eur Respir J 2012;40:1720.
- Nastase L, Desikan M, Price S, et al. Analysis of mortality in a cohort of adult Duchenne muscular dystrophy. Neuromuscular Disorders 2017;27:S101.
- Corrado G, Lissoni A, Beretta S, et al. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol 2002;89:838-41. [Crossref] [PubMed]
- Viollet L, Thrush PT, Flanigan KM, et al. Effects of angiotensin-converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. Am J Cardiol 2012;110:98-102. [Crossref] [PubMed]
- Duboc D, Meune C, Lerebours G, et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol 2005;45:855-7. [Crossref] [PubMed]
- Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018;17:251-67. [Crossref] [PubMed]
- NHS. Muscular dystrophy. 2021. (Cited 2022 Jun 7). Available online: https://www.nhs.uk/conditions/muscular-dystrophy/
- Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2008;CD003725. [Crossref] [PubMed]
- Markham LW, Kinnett K, Wong BL, et al. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord 2008;18:365-70. [Crossref] [PubMed]
- Adorisio R, Calvieri C, Cantarutti N, et al. Heart rate reduction strategy using ivabradine in end-stage Duchenne cardiomyopathy. Int J Cardiol 2019;280:99-103. [Crossref] [PubMed]
- Allen HD, Flanigan KM, Thrush PT, et al. A randomized, double-blind trial of lisinopril and losartan for the treatment of cardiomyopathy in duchenne muscular dystrophy. PLoS Curr 2013;5:ecurrents.md.2cc69a1dae4be7dfe2bcb420024ea865.
- Lavie CJ, De Schutter A, Alpert MA, et al. Obesity paradox, cachexia, frailty, and heart failure. Heart Fail Clin 2014;10:319-26. [Crossref] [PubMed]
- Guo Y, Zhang T, Wang Z, et al. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine (Baltimore) 2016;95:e4225. [Crossref] [PubMed]
- Janssens JP, Adler D, Pasquina P, et al. Contribution of Back-Up Respiratory Rate Setting in Noninvasive Ventilation. In: Esquinas AM. editor. Noninvasive Mechanical Ventilation: Theory, Equipment, and Clinical Applications. Cham: Springer, 2016:673-80.
- Güell MR, Avendano M, Fraser J, et al. Pulmonary and nonpulmonary alterations in Duchenne muscular dystrophy. Arch Bronconeumol 2007;43:557-61. [Crossref] [PubMed]
- Suárez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil 2002;81:506-11. [Crossref] [PubMed]
- Toussaint M, Davidson Z, Bouvoie V, et al. Dysphagia in Duchenne muscular dystrophy: practical recommendations to guide management. Disabil Rehabil 2016;38:2052-62. [Crossref] [PubMed]
- Takaso M, Nakazawa T, Imura T, et al. Surgical management of severe scoliosis with high risk pulmonary dysfunction in Duchenne muscular dystrophy: patient function, quality of life and satisfaction. Int Orthop 2010;34:695-702. [Crossref] [PubMed]


