
Risk factors for late-onset pulmonary fistula after pulmonary segmentectomy
Highlight box
Key findings
• We performed a single-institution retrospective study with 396 patients who underwent pulmonary segmentectomy to identify risk factors for late-onset pulmonary fistula (LOPF).
What is known and what is new?
• To date, no studies have analyzed both the incidence of and risk factors for LOPF after segmentectomy. In this manuscript, we show that LOPF incidence was 4.5% after segmentectomy. The incidence of LOPF after segmentectomy was high compared with lobectomy during the same study period. In addition, we found that surgical procedures with cranial side free space (CSFS) in the intersegmental plane and division of the intersegmental plane using electrocautery were both independent risk factors for LOPF development.
What is the implication, and what should change now?
• Segmentectomy has become very popular in recent years, prolonged air leak as well as LOPF are complications of importance in thoracic surgery and it is vital to accumulate evidence on these pathologies.
Introduction
Prolonged air leak (PAL) is one of the most common postoperative complications in the early phase after pulmonary resection, especially segmentectomy. PAL results in prolonged chest tube drainage, longer length of hospital stay, and an increased risk of other complications, such as empyema (1-8). Late-onset pulmonary fistula (LOPF) is occasionally encountered in the late phase after segmentectomy, but its incidence and risk factors are still unclear.
LOPF development is caused by reopening of a tiny bronchial stump or pulmonary fistula at the divided intersegmental plane, probably due to an intolerable airway pressure in the late postoperative phase. It is speculated that the risk factors for LOPF are similar to those of PAL, including smoking-related comorbidities and the various methods used to divide the intersegmental plane during segmentectomy. Recently, it has been emphasized that methods to divide the intersegmental plane affect the development of LOPF after segmentectomy (9-14), but the actual risk factors for LOPF development have not been clearly defined in a multivariate analysis. This study aimed to assess the incidence of LOPF requiring readmission after segmentectomy and to determine the risk factors for LOPF development based on a multivariate analysis. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1212/rc).
Methods
Patients and identification of LOPF and PAL
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Gunma University Hospital (No. HS2019-279) and individual consent for this retrospective analysis was waived. From January 2010 to April 2021, 1,731 patients underwent segmentectomy or lobectomy at Gunma University Hospital. All analyses were limited to segmentectomy. Subsegmentectomy or segmentectomy with subsegmentectomy was performed according to the extent of tumor invasion. We excluded cases of multiple segmentectomy (i.e., lingulectomy + S6 segmentectomy) and segmentectomy for thoracic trauma, as well as cases of combined chest wall resection. Finally, we performed a retrospective review of 396 patients who underwent pulmonary segmentectomy, and we compared patient groups with and without LOPF or PAL development.
Surgical treatment
We performed segmentectomy by thoracoscopy, as reported previously (15). After April 2015, we performed selective inflation of target segments to determine the pleural intersegmental line. After August 2019, we performed selective jet ventilation combined with intravenous indocyanine green (ICG) injection. Methods of intersegmental plane dissection were classified into three categories: “mainly electrocautery”, “mainly stapler”, and “mainly energy device”. In the mainly electrocautery group, electrocautery alone or electrocautery combined with fewer than two surgical stapler firings was used. In the mainly stapler group, only surgical staplers or electrocautery combined with two or more surgical stapler firings was used. In some analyses, we divided the method of intersegmental plane dissection into four categories (electrocautery alone, surgical stapler alone, electrocautery combined with a surgical stapler, and use of an energy device).
Postoperative management
Complications were classified using the Clavien-Dindo classification (16). We analyzed adverse events of greater than grade II. All patients were followed from the day of discharge and were examined at 1–2-week intervals for 1 month, at 3–6-month intervals for 5 years, and at 1-year intervals thereafter. LOPF was defined as follows: (I) an increased free space when compared with that at discharge on chest roentgenogram and computed tomography and need for readmission; (II) PAL was defined as air leak lasting for more than 7 consecutive days after surgery. Perioperative mortality was defined as death within the first 30 days after surgery.
Surgical procedure classification
Segmentectomy was categorized into several groups based on anatomical features. Previous studies have reported that simple segmentectomies include upper-division segmentectomy (left S1–3), lingulectomy (left S4–5), superior segmentectomy (S6), and basilar segmentectomy (S7–10 for right and S8–10 for left), while complex segmentectomies include resection of individual upper lobe, middle lobe, and basilar segments (17). We further divided segmentectomy based on whether it had an intersegmental plane with cranial side free space (CSFS) or ventral side free space (VSFS) (Table S1). Segmentectomies with CSFS in the intersegmental plane mainly included upper-division, S6, S1, and S1+2 segmentectomies, as well as those combined with adjacent segments or subsegments. Segmentectomies with VSFS mainly included S3, S4, S5, or S8 segmentectomies on the right side and S3, S5, and S8 segmentectomies on the left side. Single or multiple segments were classified according to the number of resected segments. The number of intersegmental planes was classified into single or multiple.
Data collection and statistical analysis
Perioperative variables were retrospectively recorded. Statistical analyses were performed using IBM SPSS software (version 25, Chicago, IL, USA). Continuous variables are reported as mean ± standard deviation (or interquartile range), and categorical variables are expressed as percentages. For comparisons between the two groups, the Mann-Whitney U test was used to compare continuous variables, while Fisher’s exact test was used to compare categorical variables. Factors shown to be significant using a univariate analysis with a P value of <0.05 were included in the multivariate analysis, and independent risk factors were considered significant if they had a P value of <0.05. For the multivariate analysis, a binomial logistic regression analysis was used.
Results
Patient characteristics and LOPF development
Table 1 summarizes the patients’ characteristics. We evaluated a total of 396 patients with a mean age of 68.1 years. Tumor pathologies included primary lung cancer (64.6%), metastatic lung tumor (28.6%), inflammatory disease (3.5%), and others (3.0%). More than half of patients had a smoking history (58.6%), and the mean pack-year was 23.6. The most common respiratory comorbidity was chronic obstructive pulmonary disease (COPD) (n=85, 21.5%).
Table 1
| Characteristic | Mean [range] or n (%) |
|---|---|
| Age, years | 68.1 [26–88] |
| Sex | |
| Male | 221 (55.8) |
| Female | 175 (44.2) |
| Respiratory history | |
| Smoking | 232 (58.6) |
| Pack-year | 23.6 [0–220] |
| COPD | 85 (21.5) |
| History of lung resection | 76 (19.2) |
| IP | 10 (2.5) |
| Asthma | 21 (5.3) |
| Pulmonary function | |
| FVC, L | 3.11 [1.15–5.64] |
| FEV1.0, L | 2.33 [0.69–4.55] |
| Endocrine/metabolic status | |
| BMI, kg/m2 | 22.4 [14.1–31.7] |
| Diabetes mellitus | 62 (15.7) |
| Previous steroid therapy | 13 (3.3) |
| Hemodialysis | 9 (2.3) |
| Histologic diagnosis | |
| Lung cancer | 255 (64.6) |
| Metastatic lung tumor | 113 (28.6) |
| Inflammatory disease | 14 (3.5) |
| Other | 12 (3.0) |
COPD, chronic obstructive pulmonary disease; IP, interstitial pneumonia; FVC, forced vital capacity; FEV1.0, forced expiratory volume in 1 second; BMI, body mass index.
The resected pulmonary segments were located on the right side in 176 of cases and on the left-side in 220 of cases. The most frequent regions of segmentectomy were upper-division (n=63) segments on the left side, and S6 (n=33) segments on the right side. The most frequent method for dividing the intersegmental plane was a surgical stapler alone (45.7%), followed by a combination of electrocautery with a surgical stapler (29.0%), electrocautery alone (19.4%), and an energy device (7.0%) (Table S2). The combination of electrocautery with a surgical stapler involved several methods; thus, we divided this group of patients into three categories to analyze the data: the “mainly electrocautery” group (31.6%), the “mainly surgical stapler” group (62.6%), and the “mainly energy device” group (5.8%).
A summary of postoperative complications is shown in Table 2. The overall morbidity rate was 19.4% (complications greater than or equal to grade II), and there was no mortality in this cohort. The following complications occurred: PAL in 25 patients (6.3%), LOPF in 18 patients (4.5%), pneumonia in 11 patients (2.8%), bloody sputum in 5 patients (1.3%), empyema in 6 patients (1.5%), and arrythmia in 3 patients (0.8%). The most common complication was PAL. Independent risk factors for PAL were pack-year and BMI according to the multivariate analysis (Tables S3 and S4).
Table 2
| Complication* | n | % |
|---|---|---|
| Respiratory | ||
| LOPF | 18 | 4.5% |
| PAL | 25 | 6.3% |
| Pneumonia | 11 | 2.8% |
| Bloody sputum | 5 | 1.3% |
| Other | 3 | 0.8% |
| Surgical | ||
| Empyema | 6 | 1.5% |
| With LOPF | 5 | 1.3% |
| Without LOPF | 1 | 0.3% |
| Cardiac | ||
| Arrhythmias | 3 | 0.8% |
*, Clavien-Dindo classification ≥ grade II. LOPF, late-onset pulmonary fistula; PAL, prolonged air leakage.
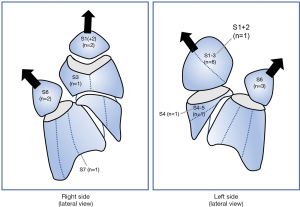
The details of the 18 patients who developed LOPF requiring readmission after segmentectomy are shown in Table 3; 11 were female and 7 were male. The mean period from surgery to LOPF onset was 42.4 days (range 5–190 days) after drain removal. Five cases of LOPF (27.8% of LOPF patients) occurred within 14 postoperative days, while 13 cases (72.2% of LOPF patients) occurred after 14 days. Of the patients with LOPF, none had postoperative PAL. As shown in Figure 1, patients with LOPF underwent segmentectomy of upper-division (n=6) or S6 (n=5) segments. For treatment or LOPF, one patient underwent observation while hospitalized, three were treated with pleural drainage alone, 10 were treated with pleural drainage followed by pleurodesis, and four were surgically treated. Five patients had LOPF associated with pleural empyema. Four patients who underwent reoperation after LOPF had concurrent empyema. The mean length of hospital stay in patients with empyema was longer compared with those without empyema (40.4±24.7 vs. 11.0±4.8 days, respectively; P=0.01). The mean period from appearance of any symptoms to readmission was significantly longer in the empyema group compared with the non-empyema group (14.4±18.6 vs. 3.5±3.5 days, respectively; P=0.049).
Table 3
| Variables | LOPF – (n=378) | LOPF + (n=18) | P value |
|---|---|---|---|
| Preoperative variables | |||
| Age, years | 68.3 | 63.7 | 0.077 |
| Sex | |||
| Male/female | 214/164 | 7/11 | 0.152 |
| Smoking | 223 | 9 | 0.471 |
| Pack-year | 24.0 | 15.5 | 0.247 |
| BMI, kg/m2 | 22.4 | 22.9 | 0.518 |
| Medical history | |||
| Diabetes mellitus | 59 | 3 | 1.000 |
| Hemodialysis | 9 | 0 | 1.000 |
| Previous steroid therapy | 13 | 0 | 1.000 |
| COPD | 84 | 1 | 0.139 |
| History of lung resection | 74 | 2 | 0.544 |
| IP | 10 | 0 | 1.000 |
| Asthma | 20 | 1 | 1.000 |
| Pulmonary function | |||
| FVC, L | 3.1 | 3.3 | 0.290 |
| FEV1.0, L | 2.3 | 2.6 | 0.097 |
| Surgical variables | |||
| Primary site | |||
| Right/left | 170/208 | 6/12 | 0.468 |
| Primary lobe | |||
| Upper, middle/lower | 210/168 | 12/6 | 0.468 |
| Approach | |||
| Open/VATS | 25/353 | 2/16 | 0.351 |
| Segmentectomy type | |||
| Simple/complex | 158/220 | 12/6 | 0.050 |
| Number of resected segments | |||
| Single/multiple | 205/173 | 10/8 | 1.000 |
| Number of intersegmental planes | |||
| Single/multiple | 199/179 | 13/5 | 0.146 |
| Position of intersegmental plane free space | |||
| Cranial side | 165 | 14 | 0.006 |
| Ventral side | 82 | 2 | 0.385 |
| Intersegmental plane dissection method | |||
| Electric scalpel | 114 | 11 | 0.009 |
| Staple | 245 | 3 | 0.000 |
| Energy device | 19 | 4 | 0.015 |
| Covering | 349 | 18 | 0.621 |
LOPF, late-onset pulmonary fistula; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IP, interstitial pneumonia; FVC, forced vital capacity; FEV1.0, forced expiratory volume in 1 second; VATS, video-assisted thoracic surgery.
Univariate and multivariate analysis
We conducted a univariate analysis of risk factors for LOPF. Perioperative variables were considered in the univariate analysis. We examined factors related to surgical procedures, including type of segmentectomy, number of resected segments, number of segmental planes, position of free space according to the intersegmental plane (Table S1), and method used to divide the intersegmental plane. The results are provided in Table 3. Smoking history did not influence LOPF. Both pack-year and BMI, which were independent risk factors for PAL, were not risk factors for LOPF (P=0.247 and 0.518, respectively). Intraoperative variables associated with LOPF development were surgical procedures that demonstrated CSFS in the intersegmental plane (P=0.006), simple segmentectomy (P=0.050), and use of electrocautery to divide the intersegmental plane (P=0.009). Other factors related to the surgical procedure, including the number of resected segments and presence of multiple intersegmental planes, were not risk factors for LOPF. After multivariate analysis, two variables remained in the final model as independent predictors of LOPF development: segmentectomy with CSFS in the intersegmental plane and use of electrocautery to divide the intersegmental plane (Table 4).
Table 4
| Variable | SE | Wald | P | OR |
|---|---|---|---|---|
| Age | 0.021 | 1.603 | 0.206 | 0.973 |
| Position of intersegmental plane free space: cranial side free space | 0.586 | 8.048 | 0.005 | 5.278 |
| Intersegmental plane dissection using an electric scalpel | 0.514 | 6.655 | 0.010 | 3.768 |
SE, standard error; OR, odds ratio.
Discussion
We performed a single-institution retrospective study with 396 patients who underwent pulmonary segmentectomy to identify risk factors for LOPF. To date, no studies have analyzed both the incidence of and risk factors for LOPF after segmentectomy. We found that surgical procedures with CSFS in the intersegmental plane and division of the intersegmental plane using electrocautery were both independent risk factors for LOPF development.
LOPF is a well-described complication in the late phase after segmentectomy, and several small-scale studies have reported an incidence ranging from 1.4% to 18.0% (18-21). In our series, LOPF incidence was 4.5% after segmentectomy. In cases of pulmonary lobectomy, the incidence of readmission for pneumothorax was 0.2–1.6% in large series (22-25). At our institution, the incidence of LOPF after pulmonary lobectomy during the same study period was 0.70%. Therefore, the incidence of LOPF after segmentectomy was high compared with lobectomy.
In general, the risk factors for LOPF are similar to PAL after segmentectomy because a fragile lung parenchyma in patients with a smoking history may affect the development of PAL in the early phase and LOPF in the late phase. PAL is one of the most common early-phase complications after pulmonary segmentectomy. Several risk factors for PAL have been identified, including age, sex, BMI, Brinkman index, surgical site, and procedure (1-8). The incidence of PAL in our series was within the range reported in other large series (6.3%, 25/396) (1-5). We also found that pack-year of smoking and BMI were independent risk factors for PAL using a multivariate analysis. Conversely, no clinicopathological factors were identified as risk factors for LOPF. However, our results revealed that a younger age tended to be a risk factor for LOPF development (P=0.077). This observation may be associated with the fact that younger female patients tended to (I) undergo division of the intersegmental plane by electrocautery based on no previous smoking history and (II) perform intense physical activity after discharge, resulting in enforced stress at the intersegmental plane. However, no clinicopathological factors, such as sex and age, were significant risk factors for LOPF, indicating that other factors caused LOPF. Furthermore, no patients with LOPF had PAL in the early phase. These results suggest that the pathogenesis of LOPF development may differ from that of PAL development after segmentectomy.
The method used to divide the intersegmental plane may be related to LOPF development after segmentectomy. It is controversial whether the use of electrocautery to divide the intersegmental plane affects PAL and LOPF development (9-14). Saito et al. reported that the incidence of both PAL and LOPF development after dividing the intersegmental plane by electrocautery was lower in patients closed using pleural suture compared with mesh (11). However, this study only included a small cohort, and the incidence of LOPF was relatively high (10.9%) in the mesh group. In our series, use of electrocautery to divide the intersegmental plane was not a risk factor for PAL after segmentectomy (P=0.267). We used electrocautery to divide the intersegmental plane in more than 60% of patients with no heavy smoking history or with a definite pleural intersegmental plane detected by selective jet ventilation or intravenous ICG injection. We believe that use of electrocautery to divide the intersegmental plane does not affect the development of PAL in adequately selected patients. Moreover, we found that use of electrocautery to divide the intersegmental plane was an independent risk factor for LOPF after segmentectomy. However, patients who underwent intersegmental plane division by electrocautery included those who underwent electrocautery alone, electrocautery combined with a surgical stapler, and electrocautery combined with an energy device, among others. In the subgroup analysis, use of electrocautery alone was not a risk factor for LOPF development (P=0.059, Table S2). It has been shown that intersegmental plane dissection using an energy device is a risk factor for LOPF, but not for PAL. This may be because air leakage is caused by dehiscence of the shielded bronchus and lung parenchyma in the late phase.13 We also found that intersegmental plane dissection using an energy device was a high-risk factor for LOPF development, even in a small number of patients.
The most common procedures associated with LOPF were upper-division and S6 segmentectomy, which are classed as typical segmentectomy procedures. In general, atypical segmentectomy is a technically complex procedure, but the incidence of LOPF development is lower than for typical segmentectomy. Therefore, the technical difficulty of atypical segmentectomy is not related to the development of LOPF. In the present study, we found that segmentectomy with CSFS in the intersegmental plane is an independent risk factor for LOPF development. The mechanism of LOPF development after segmentectomy with CSFS in the intersegmental plane remains speculative. One possibility is that the pathogenesis is similar to that of spontaneous pneumothorax. In patients with spontaneous pneumothorax, the most common site for bulla formation and rupture is the lung apex, including the S6 segment (26,27). The fragile intersegmental plane created during upper-division and S6 segmentectomy may be affected by the higher negative pressure at the upper side of the thoracic cavity or by the higher alveolar pressure in the airways of the lung apex (28). The second possibility is that the CSFS generated after upper-division and S6 segmentectomy may prevent adhesion of the intersegmental plane with other anatomical structures, including the thoracic wall. Previous studies have reported that thin-walled cavities, which was considered to be one manifestation of a postoperative pulmonary fistula, could be identified at several months after lung resection close to the hilum, including segmentectomy (13,26). Segmentectomies without CSFS in the intersegmental plane have a lower risk of LOPF development due to adhesion between the intersegmental plane and neighboring structures.
In a subgroup analysis on patients who underwent segmentectomy with CSFS in the intersegmental plane, the incidence of LOPF development in those who underwent intersegmental plane dissection using a surgical stapler alone was significantly lower compared with patients in whom electrocautery was used alone (P=0.0083). However, a surgical stapler alone was used to divide the intersegmental plane in two cases with LOPF development. Therefore, use of a surgical stapler alone cannot completely prevent LOPF development after segmentectomy. Although the incidence of LOPF development was relatively high after pulmonary segmentectomy, around 80% of patients with LOPF were treated by drainage and pleurodesis without reoperation. All four patients who underwent reoperation suffered with empyema, which was related to delayed readmission. Based on these results, careful follow-up and rapid treatment are necessary to avoid development of severe empyema in patients with LOPF.
Our study had several limitations. First, it was a retrospective study performed at a single institution. The incidence of LOPF development was not high; thus, a multicenter analysis may be necessary to clarify its pathogenesis. Second, there is no common definition of LOPF. In the present study, we defined LOPF as pulmonary fistula requiring readmission for drainage. Therefore, the period between drain removal and LOPF development has a substantially wide range (5–190 days). LOPF with a relatively early onset after drain removal could have been caused by acute pulmonary fistula. We compared early-onset and later-onset LOPF, but no significant difference was found between the two groups. Third, the surgical method used to divide the intersegmental plane was chosen in accordance with the surgeon’s preference. However, it was assumed that method selection depended on the condition of the lung parenchyma, including smoking history and patients with an unclear intersegmental plane demarcation. Finally, there have been technological advances over the past decade for segmentectomy. Such technological changes could have affected the onset of LOPF.
Conclusions
We performed a large single-institution study with a 4.5% incidence of LOPF after segmentectomy. Segmentectomy with CSFS in the intersegmental plane was an independent risk factor for LOPF development. It is important to consider that both careful follow up and rapid treatment are necessary to avoid LOPF with empyema after segmentectomy with CSFS in the intersegmental plane.
Acknowledgments
We thank Emily Woodhouse, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1212/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1212/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1212/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1212/coif). Kimihiro Shimizu serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Gunma University Hospital (No. HS2019-279) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. [Crossref] [PubMed]
- Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg 2013;17:838-44. [Crossref] [PubMed]
- Orsini B, Baste JM, Gossot D, et al. Index of prolonged air leak score validation in case of video-assisted thoracoscopic surgery anatomical lung resection: results of a nationwide study based on the French national thoracic database, EPITHOR. Eur J Cardiothorac Surg 2015;48:608-11. [Crossref] [PubMed]
- Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2017;51:504-10. [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Ojanguren A, Gossot D, Seguin-Givelet A. Division of the intersegmental plane during thoracoscopic segmentectomy: is stapling an issue? J Thorac Dis 2016;8:2158-64. [Crossref] [PubMed]
- Ohtsuka T, Goto T, Anraku M, et al. Dissection of lung parenchyma using electrocautery is a safe and acceptable method for anatomical sublobar resection. J Cardiothorac Surg 2012;7:42. [Crossref] [PubMed]
- Saito H, Konno H, Atari M, et al. Management of Intersegmental Plane on Pulmonary Segmentectomy Concerning Postoperative Complications. Ann Thorac Surg 2017;103:1773-80. [Crossref] [PubMed]
- Chen X, Jin R, Xiang J, et al. Methods for Dissecting Intersegmental Planes in Segmentectomy: A Randomized Controlled Trial. Ann Thorac Surg 2020;110:258-64. [Crossref] [PubMed]
- Takagi K, Hata Y, Sasamoto S, et al. Late onset postoperative pulmonary fistula following a pulmonary segmentectomy using electrocautery or a harmonic scalpel. Ann Thorac Cardiovasc Surg 2010;16:21-5. [PubMed]
- Miyasaka Y, Oh S, Takahashi N, et al. Postoperative complications and respiratory function following segmentectomy of the lung - comparison of the methods of making an inter-segmental plane. Interact Cardiovasc Thorac Surg 2011;12:426-9. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Ohtaki Y, Shimizu K. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:586-93. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1032-9. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Zheng B, Xu G, Fu X, et al. Management of the inter-segmental plane using the "Combined Dimensional Reduction Method" is safe and viable in uniport video-assisted thoracoscopic pulmonary segmentectomy. Transl Lung Cancer Res 2019;8:658-66. [Crossref] [PubMed]
- Freeman RK, Dilts JR, Ascioti AJ, et al. A comparison of length of stay, readmission rate, and facility reimbursement after lobectomy of the lung. Ann Thorac Surg 2013;96:1740-5; discussion 1745-6. [Crossref] [PubMed]
- Jean RA, Chiu AS, Hoag JR, et al. Identifying Drivers of Multiple Readmissions After Pulmonary Lobectomy. Ann Thorac Surg 2019;107:947-53. [Crossref] [PubMed]
- Bailey KL, Merchant N, Seo YJ, et al. Short-Term Readmissions After Open, Thoracoscopic, and Robotic Lobectomy for Lung Cancer Based on the Nationwide Readmissions Database. World J Surg 2019;43:1377-84. [Crossref] [PubMed]
- Stark P, Pugatch RD, DeCamp M, et al. Precision electrocautery excision of pulmonary lesions (Perelman technique): radiologic features. Radiology 1993;187:43-4. [Crossref] [PubMed]
- Grundy S, Bentley A, Tschopp JM. Primary spontaneous pneumothorax: a diffuse disease of the pleura. Respiration 2012;83:185-9. [Crossref] [PubMed]
- Mendogni P, Vannucci J, Ghisalberti M, et al. Epidemiology and management of primary spontaneous pneumothorax: a systematic review. Interact Cardiovasc Thorac Surg 2020;30:337-45. [Crossref] [PubMed]


