
Neoadjuvant therapy with immunoagent (nivolumab) or placebo plus chemotherapy followed by surgery and adjuvant treatment in subjects with resectable esophageal squamous cell carcinoma: study protocol of a randomized, multicenter, double blind, phase II trial (NATION-2203 trial)
Introduction
Esophageal cancer (EC) is the seventh most common cancer globally, causing more than half a million deaths each year. As a heterogenous disease with a dismal prognosis, EC poses a major health challenge worldwide. In East Asia, EC is one of the important disease burdens and esophageal squamous cell carcinoma (ESCC) accounts for over 90% of all cases. Notably, from both histological and molecular point of view, ESCC and esophageal adenocarcinoma (EAC) stand for two different entities (1), entailing tailored therapeutic approaches (2). Approximately 50% of EC will be locally advanced at diagnosis and thus amenable to potentially curative locoregional therapy. Esophagectomy remains the cornerstone therapy for EC, but surgery alone is far from enough due to the high recurrence or metastasis rates (3), and survival rates barely exceeded 1 year (4). Multidisciplinary collaboration is pivotal to effective combination and sequencing of treatment modalities tailored per patient.
Preoperative chemotherapy is an evidence-based approach for locally advanced potentially resectable EC (5). In the Medical Research Council OEO2 trial, overall survival (OS) benefit was gained after neoadjuvant chemotherapy (nCT) compared with surgery alone (6,7). Similarly, the effectiveness of nCT followed by surgery has also been demonstrated in several other trials (8-10). Our own phase III multicenter prospective CMISG1701 trial showed that despite the better local control and tumor regression achieved by neoadjuvant chemoradiotherapy (nCRT) in locally advanced ESCC as compared with nCT, survival benefit was not significantly different between nCRT and nCT groups (11,12). In East Asia, where ESCC is more prevalent, nCT is widely used in stage II–III EC patients especially with multiple lymph nodes metastasis for inaccessibility of radiotherapy, and is thus considered as an alternative standard of care (13).
Through interruption of tumor immune escape and reactivation of anti-tumor T cell activity, inhibitors of programmed cell death protein 1 (PD-1) and its ligand (PD-L1) have been identified as one of the most efficient and broadly used immunotherapies for cancer (14-16). EC is a tumor with high mutation loads which has attracted considerable attention. In fact, rapid advances in immunotherapy for ESCC have been achieved since numerous recent clinical trials reported positive results. ESCC has emerged as one of the most promising targets for immune checkpoint inhibitor (ICI) treatment.
The ICIs in ESCC were historically evaluated in later lines of treatment of metastatic ESCC. According to the ATTRACTION-3 (17), KEYNOTE-181 (18), ESCORT (19) and RATIONALE 302 trials (20), anti-PD-1 ICIs have emerged as best option in the second-line treatment for patients with metastatic ESCC who have not been treated with immunotherapy. Of note, nivolumab received the approval from US, European Commission, Japan, and China, and considered as novel standard of care in the second-line treatment for metastatic ESCC after a fluoropyrimidine-based chemotherapy, regardless of PD-L1 status. Moving forward, in the first-line treatment of metastatic ESCC, anti-PD-1 ICIs in combination with chemotherapy/anti-CTLA4 agent have also demonstrated exciting results. In several randomized phase III trials, including CheckMate 648 (21), KEYNOTE-590 (22), ESCORT-1 (23), ORIENT-15 (24), JUPITER-06 (25), the survival benefit of combination of anti-PD1 drugs and chemotherapy was confirmed as compared with standard chemotherapy. Based on these trials, anti-PD-1 ICIs have profoundly reshaped the landscape of metastatic ESCC therapy.
Furthermore, the introduction of immunotherapy in the non-metastatic disease setting may have theoretical advantages, especially in the neoadjuvant setting. For instance, neoadjuvant immunotherapy (nIT) may enhance systemic antitumor immunity including regulatory T cells (T-reg) depletion and increase tumor-specific CD8+ T-cells as well as enhance immune surveillance after surgery (26,27). However, the role of immunotherapy in neoadjuvant setting has just begun to emerge. In a small single-arm phase II (PERFECT) trial, nIT (atezolizumab) combined with standard nCRT (CROSS regimen) indicated the manageable safety profile in resectable EAC (28). Also, in a small cohort of 20 patients with locally advanced ESCC (PALACE-1), preoperative pembrolizumab plus CROSS regimen was shown satisfactory short-term outcome with a pathological complete response (pCR) rate of 55.6% (in 18 operated patients), but 5% (1/20) of patients died of massive esophageal hemorrhage during neoadjuvant treatment (29). Similarly, 12.5% mortality was reported in a Korean study using pembrolizumab plus chemoradiotherapy (30), which has raised our concern regarding the safety profile. On the other hand, several pilot clinical trials have demonstrated advantages of neoadjuvant therapy with anti-PD-1 ICI in combination with chemotherapy, including favorable safety profile and efficacy (31-35). However, various ICIs and combination with chemotherapy caused drastically different short-term outcomes (pathological complete regression, pCR, ranging from 18.8–39.2%) and adverse events (AEs) rate (grade 3 or more AEs rate, ranging from 10.7–56.7%), optimal combination of anti-PD-1 ICIs and chemotherapy is yet to be identified. In addition, current studies only provided with short-term outcomes, while survival benefit of anti-PD-1 ICIs is unknown. There is an urgent need to obtain evidence on whether this combination of anti-PD-1 ICI with chemotherapy could exert survival or tumor response in locally advanced resectable ESCC (stage II–III) patients.
On the other hand, CheckMate 577 has convincingly shown a doubling in the median disease-free survival (DFS) in nivolumab group as compared with placebo, thereby establishing the critical role of ICI treatment in the adjuvant settings, for patients who have received nCRT and R0 resection but did not achieve pCR in the resected specimen (36). Nevertheless, the question remains that whether adjuvant immunotherapy offers additional benefit in patients who have received nIT/nCT. Early evaluation and diagnosis of tumor recurrence and micrometastases, as well as the life quality of patients, are also worth exploring to achieve a better survival benefit.
Hence, we launched a two-arm multicenter, randomized, controlled phase II trial (NATION-2203 trial) to assess the superiority of neoadjuvant nivolumab in combination with chemotherapy [immunotherapy plus nCT (IO-nCT)] over nCT in tumor response (pCR) for locally advanced (stage II–III) ESCC patients. Moreover, we also intend to evaluate whether adjuvant administration of nivolumab could impose survival benefit in non-pCR patients after IO-nCT/nCT, as compared with no adjuvant treatment in pCR patients, which will answer the questions that CheckMate 577 did not cover. We present the following article in accordance with the SPIRIT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1789/rc).
Methods
Study design
NATION-2203 trial is a randomized, multicenter, prospective, double-blind, phase II study of neoadjuvant nivolumab or placebo plus chemotherapy followed by surgery in subjects with resectable ESCC and adjuvant nivolumab treatment for non-pCR patients. The study will be conducted in 6 hospitals in China, recruiting 90 patients. The trial will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol has been reviewed and approved by the Institution Review Committee (B2022-004R). In each institution, approval by the institutional review board will be obtained before patient accrual. All patients will provide written informed consent before enrollment. The study has been registered in ClinicalTrials.gov with number NCT05213312. The protocol is designated NATION-2203 (version 1.1; dated January 10, 2022).
The objective of this study is to compare the safety and efficacy of neoadjuvant nivolumab and placebo plus chemotherapy followed by surgery in subjects with resectable ESCC and adjuvant nivolumab treatment in non-pCR patients. Recruitment started in March 2022 and is estimated to continue until December 2022. Patients are randomly allocated in a ratio of 2:1 to receive either nivolumab or placebo plus nCT followed by minimally invasive esophagectomy (MIE) (Figure 1). Randomization will be performed using a central dynamic system with the following stratification factors including clinical stage, age and tumor location are considered.
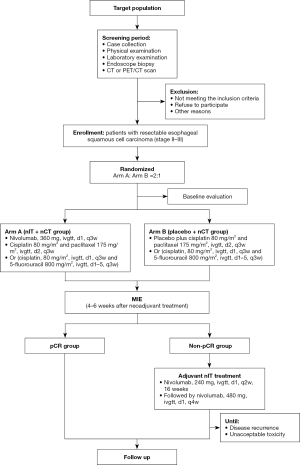
This study is a superiority trial, and we hypothesize that nivolumab/chemo is superior to placebo/chemo in locally advanced ESCC (cII–III). pCR rate in all per-protocol patients is deemed as the primary endpoint. pCR is defined as complete response of both primary tumor and lymph nodes in the resected specimen. The secondary endpoints are R0 resection rate, event-free survival (EFS), OS, AEs. Exploratory endpoints include quality of life (QOL) and biomarkers investigation. EFS is defined as the time duration from randomization to progression or death from any cause, and it is censored till the latest day when the patient is alive without progression. Disease progression except for distant metastasis during neoadjuvant therapy is not regarded as an event if R0 resection is achieved (37).
Study population
Eligibility criteria
Patients with locally advanced (stage II–III) ESCC who have been histologically-confirmed, reviewed by two experienced thoracic surgeons and considered surgically resectable.
Inclusion criteria
- The patient volunteers to participate in the study, signs a consent form, has good compliance, and obeys the follow-up, and is willing and able to follow the protocol during the study;
- Male or female, aged ≥18 and ≤75 years;
- The Eastern Cooperative Oncology Group (ECOG) performance status (PS) score is 0–1;
- Histologically-confirmed squamous cell carcinoma of the esophagus. Tumors of the esophagus are located in the thoracic cavity;
- Pre-treatment staging as stage II–III [cT2N0–1M0, cT3N0–1M0, cT1–3N2M0, American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) 8th edition];
- Expected lifetime >1 year;
- Adequate cardiac function. All patients should perform electrocardiogram (ECG), and those with a cardiac history or ECG abnormality should perform echocardiography with the left ventricular ejection fraction >50%;
- Adequate respiratory function with forced expiratory volume in 1 second (FEV1) ≥1.2 L, FEV1% ≥50% and lung diffusing capacity for carbon monoxide (DLCO) ≥50% shown in pulmonary function tests;
- Adequate bone marrow function (white blood cells >4×109/L, neutrophil >2.0×109/L, hemoglobin >90 g/L, platelets >100×109/L). Aspartate aminotransferase (AST), alanine aminotransferase (ALT) ≤3× upper level of normal (ULN) (if liver metastases exist, AST and ALT allow ≤5× ULN);
- Adequate liver function (total bilirubin <1.5× ULN, AST and ALT <1.5× ULN);
- Adequate renal function [glomerular filtration rate (GFR) >60 mL/min; serum creatinine (SCr) ≤120 µmol/L];
- Agree to provide hematology and histology samples.
Exclusion criteria
Patients who meet any of the following conditions will be excluded:
- Patients have previously received an anti-PD-1, PD-L1 or any other antibody or drug specifically targeting T-cell co-stimulation or checkpoint pathways.
Related to cancer:
- Patients with non-squamous cell carcinoma histology;
- Patients with advanced inoperable or metastatic EC (M1);
- Patients without qualified Pre-treatment stage;
- Patients with another previous or current malignant disease.
Others:
- Any patient with a significant medical condition which is thought unlikely to tolerate the therapies. Such as cardiac disease (e.g., symptomatic coronary artery disease or myocardial infarction within last 12 months), clinically-significant lung disease, clinically-significant bone marrow, liver, renal function disorder;
- Patients who have autoimmune diseases;
- Pregnant or lactating women and fertile women who will not be using contraception during the trial;
- Allergy to any drugs;
- Patients who have received or are receiving other chemotherapy, radiotherapy or targeted therapy;
- Patients who recently or currently taking hormones or immunosuppressive agents;
- Human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV) active infection or known HIV seropositivity; including HBV or HCV surface antigen positive (RNA).
Randomization and blinding
Patients will be randomly assigned in a 2:1 allocation ratio to receive nivolumab combined with chemotherapy followed by MIE (arm A, nIT + nCT group) or placebo + nCT followed by MIE (arm B, nCT group) and were stratified according to coordinating centers. Randomization will be assigned by the central dynamic, computer-generated random system. The stratification factors include age, tumor location and clinical staging. The research center will generate assignments in the random system online after enrollment.
The study will use an internally blinded double-blind approach. Nivolumab and normal saline were in the same package, and the corresponding labels were prepared and affixed on site to maintain blindness. Subjects, investigators and sponsor staff who participated in subject treatment or clinical evaluation were unaware of the assignments. Only the open-label pharmacist/nurse will obtain each subject’s study identification number and study drug assignment from the central randomization system and prepare the drugs.
Interventions
nIT
A fixed dose of 360 mg nivolumab will be administered intravenously on day 1, followed by cisplatin (80 mg/m2) and paclitaxel (175 mg/m2) on day 2, or cisplatin (80 mg/m2) on day 2 and 5-fluorouracil (800 mg/m2) on days 2–6, every 3 weeks (2 cycles). All given intravenously.
Dosage adjustment is not permitted for nivolumab, but are allowed for chemotherapy in case of severe febrile neutropenia or neutropenia, thrombocytopenia and anemia. Treatment will be interrupted or postponed in case of any serious AE (SAE), and could be resumed until the protocol-defined criteria for treatment resumption is met.
MIE
Four to 6 weeks after the second cycle of neoadjuvant therapy, MIE will be performed. The surgery contains esophagectomy plus two/three-field lymphadenectomy. The procedure in details is referred in previous article (38,39).
Adjuvant immunotherapy
For non-pCR patients, a postoperative immunotherapy would be applied. The treatment will begin 4–6 weeks after operation. Nivolumab 240 mg will be administered intravenously every 2 weeks for 16 weeks, followed by 480 mg intravenous infusion every 4 weeks till disease progress, unacceptable toxicity or 1 year after operation.
Research process
Screening period
- Tumor evaluation, including computed tomography (CT)/magnetic resonance imaging (MRI), positron emission tomography (PET)/CT, endoscopy and pathological evaluation should be performed within 14 days before enrollment;
- The following assessments should be performed within 14 days before enrollment: demographic data, concomitant diseases/treatment, full physical examination [including vital signs, Karnofsky performance score (KPS), height, weight, and physical examination of the nervous system], laboratory tests (blood routine/biochemical, fecal routine + occult blood, urine routine, coagulation function, tumor markers, thyroid function, hepatitis B and C markers, myocardial enzyme spectrum, T cell spot test (T-SPOT), HIV antibodies], ECG, echocardiography, and pregnancy tests (for all women with menopause less than 12 months);
- Pulmonary function tests will be performed on patients suspected or known to have severe respiratory disease or have significant respiratory symptoms unrelated to underlying cancer, including but not limited to spirometry tests and assessment of lung dispersion during the screening period to help determine if it is appropriate to participate in this study;
- After the completion of all screening items, the researcher must review the results/data, and the subjects can only be enrolled after passing the review.
Treatment period
- The baseline assessment is performed within 7 days after signing the informed consent, and treatment must be performed within 7 days after enrollment;
- Make the following assessments within 1 week before treatment: vital signs (temperature, blood pressure, heart rate), physical examination (including PS score, height, weight, physical examination of each system), blood routine/biochemical examination (including creatinine clearance calculation), coagulation function, fecal routine and occult blood, urine routine and pregnancy test, tumor markers, thyroid function, hepatitis B and C markers, myocardial enzyme spectrum, T-SPOT, HIV antibodies, ECG, cardiac ultrasound, lung function;
- After completing all screening activities and baseline assessments, eligible patients identified by the researcher will be treated with medications, and treatment options will not be allowed to change during the study. After the investigator’s first evaluation of disease progression based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, treatment can be continued if there is evidence of “pseudo progression” and the consent of the sponsor and the patient’s signing of the consent form again;
- Safety will be assessed throughout the study by monitoring AE/SAE [toxicity grades are assigned according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 (40)] and laboratory results. Vital signs, physical examination, changes in ECOG PS score, ECG results, and other tests will also be used for safety assessment.
Follow-up period after study treatment
Follow-up will be first performed 1 month after surgery. From then on, follow-up visits will be every 3 months in the first 2 years. The detailed examination items include standard laboratory tests (blood routine, tumor biomarker), a CT scan of thorax, an ultrasound of the neck and abdomen and QOL questionnaires [3-level version of the EQ-5D (EQ-5D-3L) and Functional Assessment of Cancer Therapy-Esophageal (FACT-E)] at the out-patients site of the institutions.
If the patient has signs of recurrence (such as related clinical manifestations), additional tumor evaluations are performed during the treatment; possible reoperations and/or further cancer treatments are also documented. During the follow-up period without tumor recurrence, other cytotoxic agents are not allowed. Patient recurrence and survival will be followed up to the patient’s death, the last date on which the patient is known to survive, or 1 year after the primary effectiveness analysis.
Sample size
The planned sample size is approximately 81 subjects. The calculations are based on the previous observational results of neoadjuvant therapy in our center (Department of Thoracic Surgery, Zhongshan Hospital Affiliated to Fudan University). In the past 2 years, about 85% of patients succeeded to undergo surgery and complete the entire treatment plan. As calculated based on an asymptotic method, 81 patients will be randomized at 2:1 ratio to either nivolumab/chemo or chemo group respectively, with 80% power to detect a 21% difference in the proportion of patients achieving a pCR at a one-sided α of 0.05, assuming a pCR rate of 25% in the nivolumab/chemo group and 4% in the chemo group based on our previous study. Considering a 10% drop rate, 90 patients will be enrolled.
A total of 10–20 more subjects may be added to each group after the evaluation of feasibility and safety as the justification.
Outcome definitions
Primary endpoints
- pCR rate: pCR rate is defined as the proportion of the subjects in the analysis population who have a complete response in postoperative pathology. It is assessed in the resected specimen following neoadjuvant therapy using standardized work up of the resection specimen in the pathology department and standardized histological criteria for tumor regression grading. The tumor regression grade (TRG) is categorized as follow: grade 1, no evidence of vital residual tumor cells (pCR); grade 2, less than 10% vital residual tumor cells; grade 3, 10 to 50%; grade 4, more than 50%.
Secondary endpoints
- R0 resection rate: no vital tumor is presented at the proximal, distal, or circumferential resection margin, then it is considered R0 resection. If a vital tumor is shown at 1 mm or less from the proximal, distal, or circumferential resection margin, it is then considered to be microscopically positive (R1);
- EFS: EFS refers to the time from enrollment to the occurrence of any event, including death, disease progression, change of chemotherapy regimen, change to chemotherapy, addition of other treatments, fatal or intolerable side effects and other events;
- OS: OS in the intent-to-treat population, which ends with the date of death of any causes since the date of randomization assessed up to 36 months. For patients alive at study closure, the survival time will be censored at time of last known survival status;
- Incidence of treatment-emergent AEs: all clinical AEs encountered during the clinical study will be recorded on the case report form (CRF)’s adverse reactions page. The severity of adverse reactions (except for surgery-related ones) will be graded according to the National Nomenclature Standard for Adverse Reactions (NCI-CTC AE v5.0) and recorded in CRF in detail.
Exploratory endpoints
- To explore potential biomarkers associated with clinical efficacy (EFS and OS) and/or incidence of AEs of nivolumab by analyzing biomarker measures within the tumor microenvironment and periphery (blood, plasma, serum and PBMCs) in comparison to clinical outcomes;
- To assess the subjects’ overall health status using the EQ-5D-3L index and visual analog scale;
- To assess the subjects’ cancer-related QOL using the FACT-E questionnaire and selected components, including the Esophageal Cancer Subscale (ECS) and 7-item version of the FACT-General (FACT-G7).
Data monitoring
The Data and Safety Monitoring Committee is consisted of five physicians independent from the sponsor and declare no competing interests. Active monitoring will be conducted every 2 months to ensure the safety of patients and integrity of data. Interim analysis is scheduled after half of the planned patients have been recruited. Data will be analyzed based on intention-to-treat (ITT) principle including all patients subjected to randomization, and per-protocol principle including all protocol-abiding patients. If the superiority (both oncologic and safety outcomes) of chemotherapy vs. nivolumab/chemotherapy is confirmed with an adjusted α level, the study will be stopped.
Quality control
To ensure quality of study, the trial launching conference will be hold for investigators before the first enrollment. Trainings of study protocol, CRF filling and AE/SAE reporting will be conducted. All the centers must comply with the standard operating procedures (SOPs) for the management of study drugs.
Statistical analysis
All statistical analysis will be calculated using SPSS (version 24.0, Chicago, IL, USA) statistical analysis software programming. All statistical tests are two-sided. P values less than 0.05 will be considered statistically significant. The confidence interval (CI) is 95%. Statistical analysis for primary endpoint: pCR rates will be calculated as the proportions of the subjects in the analysis population who have a complete response in postoperative pathology.
Statistical analysis for baseline variables and secondary endpoints: the 3-year OS rates in the two treatment arms will be calculated by the Kaplan-Meier method and compared by the log-rank test. The Cox proportional hazard model will be used to evaluate the survival-independent factors. Continuous variables were examined by independent sample t-test or Wilcoxon rank-sum test, and categorical variables were compared by Pearson chi-square test, Fisher’s exact test or CMH chi-square test as appropriate.
Analysis sets are consist of full analysis set (FAS) and per-protocol set (PPS). Based on ITT principle, the FAS is the primary analysis set for the trial efficacy analysis and is depend on the groups assigned by randomization. PPS is a subset of FAS, which is in accordance with the protocol.
Anticipated results
We expected that the pCR rate, R0 resection rate, EFS and OS of the study group (nivolumab/chemo) will be significantly better than those of control group, but there will be no difference for AEs, subjects’ overall health status (EQ-5D-3L index) or cancer-related QOL (FACT-E questionnaire).
Discussion
nCT has been practiced for decades in the treatment of EC. Since the OEO2 trial (6,7) as well as JCOG9907 trial successfully demonstrated the survival benefit of nCT in ESCC, it has been considered as standard of care in locally advanced ESCC, especially in Japan (9). A clear advantage to nCRT over nCT has not been established (5). Clinical trials from West countries mostly in EAC patients (41-43), as well as our own recent CMISG1701 trial in ESCC patients (11), have not detected significant survival difference between nCRT and nCT groups. The superiority of nCRT and nCT remains inconclusive so far. As matter of fact, doublet chemotherapy based on fluoropyrimidine and platin or according to the CROSS regimen remains the primary therapeutic option for ESCC (9,44).
However, although JCOG9907 demonstrated an OS benefit after nCT compared with adjuvant chemotherapy, there were no differences in progression-free survival, indicating limited improvement in the QOL. Additionally, patients at stage III received less benefit compared with stage II (9). A more effective therapy regimen is therefore demanded.
In recent years, ICIs have been implemented as a novel modality in tumor therapies. Nivolumab is a genetically engineered human immunoglobulin G4 (IgG4) monoclonal antibody specific against PD-1 (45), which possesses a high affinity for PD-1 and blocks binding of both PD-L1 and PD-L2 to PD-1 (45). Nivolumab also stimulates secretion of cytokines in vitro and the proliferation of tumor antigen-specific T cells (46,47). So far, nivolumab has been approved for various types of tumor therapy, including melanoma, advanced squamous and non-squamous non-small cell lung cancer, urothelial carcinoma, Hodgkin’s lymphoma, renal cell carcinoma, squamous cell carcinoma of the head and neck, microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer, hepatocellular carcinoma and EC (48-57).
First being implemented in unresectable advanced EC, anti-PD-1 monotherapy has demonstrated safety and efficacy in previously treated ESCC as well as gastroesophageal adenocarcinoma (GEA) patients (58). The ATTRACTION-3 (17) trial heralded the new era of immunotherapy for advanced ESCC patients, which was the first randomized phase 3 study to show a significant increase in OS [10.9 vs. 8.4 months, 95% CI: 9.2–13.3 vs. 7.2–9.9, hazard ratio (HR): 0.77, P=0.019] with nivolumab vs. chemotherapy in the second-line treatment of unresectable advanced or recurrent ESCC. 18% of patients in nivolumab group is associated with grade 3 or 4 treatment-related AEs (TRAEs), compared with 63% in the chemotherapy group. Based on the ATTRACTION-3 trial results, nivolumab monotherapy has become the standard second-line chemotherapy for metastatic or recurrent ESCC patients regardless of PD-L1 expression (17).
In the first-line chemotherapy for advanced ESCC, CheckMate 648 trial showed that OS was significantly longer with nivolumab plus chemotherapy than chemotherapy alone, both among patients with tumor-cell PD-L1 expression of 1% or greater (median OS 15.4 vs. 9.1 months, HR: 0.54, P<0.001) as well as in overall population (median OS 13.2 vs. 10.7 months, HR: 074, P=0.002). In addition, OS was also significantly longer with nivolumab plus ipilimumab than with chemotherapy among patients with tumor-cell PD-L1 expression of 1% or greater (median OS 13.7 vs. 9.1 months, HR: 0.64, P=0.001) and in the overall population (median OS 12.7 vs. 10.7 months, HR: 0.78, P=0.01). The incidence of TRAEs of grade 3 or 4 was 47% with nivolumab plus chemotherapy, and 36% with chemotherapy alone. The CheckMate 648 trial has convincingly demonstrated that both first-line treatment with nivolumab plus chemotherapy and nivolumab plus ipilimumab caused significantly longer OS than chemotherapy alone in patients with advanced ESCC, with no new safety signals identified (21).
Meanwhile, in the adjuvant setting, CheckMate 577 study proved that in non-pCR patients who had resected EC and gastroesophageal junction (GEJ) cancer after nCRT (regardless of PD-L1 expression), nivolumab adjuvant therapy was associated with a significantly longer DFS than placebo and the safety profile was similar to other types of solid tumors. In nivolumab group, the DFS was 22.4 months (95% CI: 16.6 to 34.0), compared with 11.0 months in placebo group (95% CI: 8.3 to 14.3). The results of this trial suggest that postoperative nivolumab monotherapy might be useful as a new standard treatment for patients with EC and GEJ cancer treated with preoperative chemoradiotherapy followed by surgery who have failed to achieve a pCR.
Moreover, pilot studies using anti-PD-1 ICI in combination with nCRT for locally advanced ESCC patients have raised our concerns regarding the safety profile as 5–12.5% mortality occurred during the therapy (29,30). By contrast, neoadjuvant therapy with anti-PD-1 ICI in combination with chemotherapy presented with favorable safety profile and efficacy (31-35). Notably, various ICIs and combination with chemotherapy caused drastically different short-term outcomes (pCR ranging from 18.8–39.2%) and safety profiles (grade 3 or more AEs rate, ranging from 10.7–56.7%), yet the optimal combination of anti-PD-1 ICIs and chemotherapy remains to be verified. In addition, the survival benefit of nIT is unknown. Meanwhile, CheckMate 577 has established the role of adjuvant ICI treatment in patients having residual tumor in the resected specimen after nCRT and R0 resection (36). However, it is unclear that whether adjuvant immunotherapy offers additional benefit in patients who have received nIT/nCT. This present trial is designed to answer the following questions: (I) whether combination of neoadjuvant nivolumab and chemotherapy (TP regimen) exerts better local tumor control compared with chemotherapy; (II) whether this combination brings survival benefit; (III) whether adjuvant nivolumab treatment offers additional survival benefit in non-pCR patients after neoadjuvant immune/chemotherapy; (IV) whether this combination has similar safety profile compared with chemotherapy.
To summarize, the NATION-2203 study is a multicenter prospective randomized controlled trial, comparing neoadjuvant nivolumab plus chemotherapy (TP regimen) vs. chemotherapy followed by surgery and adjuvant nivolumab for the treatment of locally advanced ESCC. It is hypothesized that perioperative immunotherapy in addition to nCT could prolong survival due to enhanced local tumor control and potential systemic restrain for micrometastases.
Acknowledgments
We would like to express gratitude to the participating patients and their family. We thank Fei Liang, PhD (Department of Biostatistics, Zhongshan Hospital, Fudan University), for the assistance in statistical analysis. We also want to thank all the participating centers in the trial.
Funding: The study was funded by Bristol-Myers Squibb (No. CA209-6KP), whose role is limited to provision of financial support and nivolumab for patients. The study protocol has been subjected to peer-review by the funding body.
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1789/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1789/coif). HC serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. LT reports that the study was funded by Bristol-Myers Squibb (CA209-6KP). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol has been reviewed and approved by the Ethics Committees of Zhongshan hospital of Fudan University (No. B2022-004R). In each participating institution, approval by the institutional review board will be obtained before patient accrual. All patients will provide written informed consent before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [Crossref] [PubMed]
- Turgeman I, Ben-Aharon I. Evolving treatment paradigms in esophageal cancer. Ann Transl Med 2021;9:903. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Wang H, Tang H, Fang Y, et al. Morbidity and Mortality of Patients Who Underwent Minimally Invasive Esophagectomy After Neoadjuvant Chemoradiotherapy vs Neoadjuvant Chemotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma: A Randomized Clinical Trial. JAMA Surg 2021;156:444-51. [Crossref] [PubMed]
- Tang H, Tan L, Shen Y, et al. CMISG1701: a multicenter prospective randomized phase III clinical trial comparing neoadjuvant chemoradiotherapy to neoadjuvant chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced resectable esophageal squamous cell carcinoma (cT(3-4a)N(0-1)M(0)) (NCT03001596). BMC Cancer 2017;17:450. [Crossref] [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1-24.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol 2018;36:61-7. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832-42. [Crossref] [PubMed]
- Shen L, Kato K, Kim SB, et al. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J Clin Oncol 2022;40:3065-76. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. [Crossref] [PubMed]
- Lu Z, Wang J, Shu Y, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 2022;377:e068714. [Crossref] [PubMed]
- Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022;40:277-88.e3. [Crossref] [PubMed]
- Antonia SJ, Özgüroğlu M. Durvalumab in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:869-70. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- van den Ende T, de Clercq NC, van Berge Henegouwen MI, et al. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT). Clin Cancer Res 2021;27:3351-9. [Crossref] [PubMed]
- Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 2021;144:232-41. [Crossref] [PubMed]
- Park SY, Hong MH, Kim HR, et al. The feasibility and safety of radical esophagectomy in patients receiving neoadjuvant chemoradiotherapy with pembrolizumab for esophageal squamous cell carcinoma. J Thorac Dis 2020;12:6426-34. [Crossref] [PubMed]
- Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [Crossref] [PubMed]
- Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg 2022;103:106680. [Crossref] [PubMed]
- Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e003497. [Crossref] [PubMed]
- Liu J, Li J, Lin W, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer 2022;151:128-37. [Crossref] [PubMed]
- He W, Leng X, Mao T, et al. Toripalimab Plus Paclitaxel and Carboplatin as Neoadjuvant Therapy in Locally Advanced Resectable Esophageal Squamous Cell Carcinoma. Oncologist 2022;27:e18-28. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Nakamura K, Shibata T, Takashima A, et al. Evaluation of three definitions of progression-free survival in preoperative cancer therapy (JCOG0801-A). Jpn J Clin Oncol 2012;42:896-902. [Crossref] [PubMed]
- Feng M, Shen Y, Wang H, et al. Thoracolaparoscopic esophagectomy: is the prone position a safe alternative to the decubitus position? J Am Coll Surg 2012;214:838-44. [Crossref] [PubMed]
- Shen Y, Feng M, Khan MA, et al. A simple method minimizes chylothorax after minimally invasive esophagectomy. J Am Coll Surg 2014;218:108-12. [Crossref] [PubMed]
- Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051-9. [Crossref] [PubMed]
- Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 2011;47:354-60. [Crossref] [PubMed]
- Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer 2017;81:183-90. [Crossref] [PubMed]
- von Döbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus 2019; [Crossref]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Hamanishi J, Mandai M, Ikeda T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol 2015;33:4015-22. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71.
- Okada M, Kijima T, Aoe K, et al. Clinical Efficacy and Safety of Nivolumab: Results of a Multicenter, Open-label, Single-arm, Japanese Phase II study in Malignant Pleural Mesothelioma (MERIT). Clin Cancer Res 2019;25:5485-92. [Crossref] [PubMed]
- Smyth EC, Gambardella V, Cervantes A, et al. Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann Oncol 2021;32:590-9. [Crossref] [PubMed]


