Importance of fractional exhaled nitric oxide in diagnosis of bronchiectasis accompanied with bronchial asthma
Introduction
Asthma and bronchiectasis (BE), which exhibit different clinical characteristics, are common diseases of airways and lungs. The complications of these diseases often lead to misdiagnosis. Currently, methods used to distinguish BE from BE with asthma mainly depend on clinical symptoms, signs, and pulmonary function test (PFT) results (1,2). Although these tests are valuable, they are expensive and time consuming and exhibit high risk of inducing severe bronchospasm (3). Nitric oxide (NO) is an important inflammatory mediator of biological functions in airways (4); the levels of NO in air exhaled by asthmatic patients is elevated (5). Thus, NO is a useful marker for evaluating responses to airway inflammation (6). However, the FeNO level of patients with BE is not significantly different from that of healthy controls (7,8). Moreover, FeNO level is reduced among patients with BE co-infected with nontuberculous mycobacteria infections (9). In this study, FeNO level was determined to differentiate BE accompanied with asthma from BE alone and evaluate the responses of these diseases to treatments.
Methods
Study populations
From February 2013 to February 2015, suspected patients with BE in the First Affiliated Hospital of Sun Yet-sen University were retrospectively enrolled in this study.
The inclusion criteria for patients with BE included the following: (I) presentation of clinical symptoms, such as chronic cough and mucopurulent or purulent sputum production, as well as chest examination with wheezing or crackles; and (II) high-resolution computed tomography (HRCT) scan for confirmation of BE diagnosis.
For the control groups, patients presenting chronic cough caused by asthma, COPD, pneumonia, and other respiratory diseases were enrolled in this study and were age matched with those in the BE group (18–80 years old). All the patients in the control group were excluded BE through HRCT. The diagnosis of allergic bronchopulmonary aspergillosis and eosinophilic lung diseases were also excluded clinically. The requirement for approval of ethics committee and a signed informed consent form was waived by the institutional review board due to no intervention trials and the retrospective nature of the study.
Designs
HRCT is the current “gold standard” used to confirm the diagnosis of BE. Bronchial challenge test (BCT) or bronchodilator test is also used to diagnose asthma. FeNO level was assessed using the receiver operating characteristic (ROC) curves, from which the optimal operating point was determined. Asthmatic patients were treated with budesonide (200 µg, bid) for 12 weeks to evaluate changes in FeNO level and assess their responses to the treatment.
Measurements
FeNO level was determined using the chemiluminescence analyzer NIOX MINO (Aerocrine AB, Solna, Sweden) according to the guidelines established by the American Thoracic Society (ATS) (10). Patients were asked to inhale the maximum amount of air and were then instructed to exhale the air into the valve connected to the analyzer. The flow rate (50 mL/s) was kept constant, and data was recorded after 90 s. All these procedures were followed up by the clinician.
Pulmonary function test
PFT was performed using a pneumotachograph-based system in accordance with the recommendations of the European Respiratory Society (11).
Statistical analysis
SPSS version 18.0 was used for data analysis. The results were expressed as mean ± standard deviation or case numbers/total patients (%). Student’s t-test was used to determine statistical differences in FeNO level between asthmatic and nonasthmatic patients with BE. The relationship between the PFT results and FeNO level was analyzed using Pearson correlation coefficients. Comparison between tests was performed by constructing ROC curves and measuring the area under the curve (AUC). The optimum cut-off points were determined. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated. P values <0.05 indicated statistical significance.
Results
Baseline characteristics of the study population
A total of 247 patients were initially enrolled in this study. Ninety-nine patients were diagnosed with BE, 52 with asthma, 22 with pneumonia, and 31 with COPD. The remaining 43 patients who visited outpatient clinics and presented chronic cough were diagnosed with various respiratory diseases, such as upper airway cough syndrome, gastroesophageal reflux disease, or unclassified chronic cough. Table 1 shows the final diagnosis in the study population.
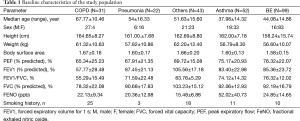
Full table
The measured pulmonary function values were presented as a percentage from the predictive.
Comparison of FeNO level between BE and non-BE groups
The FeNO level in patients with BE was 24.85±14.65 part per billion (ppb), which was significantly lower than that in the asthmatic group (52.02±40.73 ppb) (P<0.001). The FeNO level was also significantly higher in the asthmatic group compared with that in non-asthmatic groups (P<0.001). FeNO level was not significantly different among non-asthmatic groups (P>0.05) (Figure 1, Table 2).
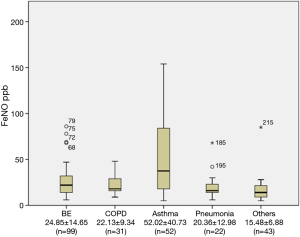
Full table
Characteristics of asthmatic patients with BE and asthmatic without BE
Ninety-nine patients (83 patients are female) satisfied the inclusion criteria for BE. Bronchial hyperreactivity and/or reversible airway obstruction was used to define asthma. Twenty patients were asthmatic (15 cases positive for BCT and five cases positive for bronchodilator reversibility test), and 79 patients were not asthmatic. Table 3 shows the demographic data, PFT values, and FeNO levels of all patients. The median (interquartile range) FeNO level in asthmatic patients with BE was 40.05±20.24 ppb, which was significantly higher than that in the non-asthmatic group (21.0±9.78 ppb) (P<0.05). Moreover, asthmatic and non-asthmatic groups were not significantly different in terms of height, body weight, and PFT results (P>0.05). Table 4 shows the demographic data, FeNO values, and PFT values in asthmatic patients with BE and asthmatic without BE. FeNO level was not significantly different among asthmatic groups (P>0.05).

Full table

Full table
Changes in the airways of asthmatic patients with BE over 12 weeks of ICS
Twelve asthmatic patients with BE underwent budesonide (inhaled corticosteroids) therapy for 12 weeks and conventional treatments. After 12 weeks of budesonide treatment, forced expiratory volume for 1 s (FEV1%) level significantly increased (P<0.05), whereas FeNO level significantly decreased (P<0.05) (Table 5).

Full table
Correlation analysis between FeNO and PC20
In asthmatic patients with BE, the association between FeNO level and PC20 was moderately strongly negative, with a Pearson correlation coefficient (r) of −0.631 (P=0.09) (Figure 2A,B). Correlation analysis was only performed in BCT positive subjects. In the asthmatic group, FeNO level and PC20 were positively but weakly correlated, with a Pearson correlation coefficient (r) of −0.539 (P<0.001) (Figure 2C,D). However, FeNO level and FEV1% were not correlated in both groups (Figure 2).
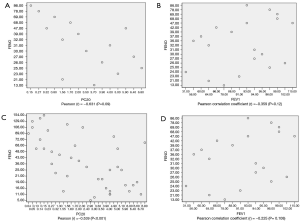
Optimal FeNO level to define BE with asthma
The constructed ROC curve (Figure 3) defines the optimal cut-off value for FeNO and was used to differentiate BE accompanied with asthma from BE only. The area under the ROC curve was estimated as 0.822 for FeNO. The optimal diagnostic cut-off point was 22.5 ppb (with 90% sensitivity and 62.5% specificity). Table 6 shows the sensitivity, specificity, PPV, and NPV values for different criteria tested.
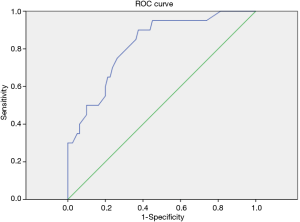
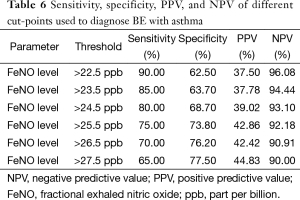
Full table
Discussion
We performed a retrospective study to define and used FeNO level to differentiate asthmatic and nonasthmatic patients with BE. This strategy was also compared with conventional diagnostic tests. Results showed that FeNO level was significantly reduced to alleviate airway inflammation. This study is the first to report the role of FeNO in asthmatic patients with BE.
Asthma is defined by the presence of reversible airway obstruction. Our study employed BCT or bronchodilator test to define the presence of asthma. Airway inflammation is also considered a key element of asthma. Studies showed that FeNO level is elevated in asthmatic patients and is correlated with airway inflammation (12-14). The ATS clinical practice guideline uses FeNO as a biomarker, a new tool used in traditional clinical methods to assess and manage asthma. Dupont et al. (15) used a cut-off value of 16 ppb FeNO to diagnose asthma, and the FeNO measurement indicated a specificity of 90% and a PPV of >90%. Furthermore, FeNO level was significantly higher in the asthmatic group than that in the non-asthmatic groups (P<0.001). Asthma and BE are common diseases with different clinical characteristics. BE is characterized by irreversible airway dilatation resulting from structural abnormality of the bronchial wall. Some patients presenting complications associated with BE are possibly misdiagnosed, leading to the administration of inappropriate treatments. Asthmatic patients with BE must undergo inhaled corticosteroid therapy, in addition to conventional treatments (16). Foley (17) reported low FeNO levels in patients with BE, especially those co-infected with NTM. The present results indicated that FeNO level was not significantly different among non-asthmatic groups, including the BE group. These results confirm the feasibility of applying FeNO measurement to differentiate BE accompanied with asthma from BE alone.
In this study, we also analyzed the correlation of FeNO with FEV1% and PC20 in asthmatic and nonasthmatic patients with BE. The association between FeNO level and PC20 was moderately strongly negative in both groups. Meanwhile, FeNO level was not correlated with FEV1%. Although the results were not statistically significant, FeNO level was found to be correlated with the inflammatory process. In the last decade, as an important biomarker, FeNO testing has an important role in the assessment and management of asthma. Donohue et al. (18) showed that FeNO has value for identifying patients with asthma who will and will not respond to corticosteroids. In this study, a 12-week treatment with anti-inflammatory drugs, such as budesonide, reduced FeNO level. Hence, FeNO offers additional advantages for patient care, including detection of airway inflammation and monitoring of responsiveness to corticosteroids in asthmatic patients with BE.
FeNO is a good predictor for differentiating BE accompanied with asthma from BE alone, with an ROC AUC of 0.822, which is not mentioned in previous studies. Moreover, the optimal combination of sensitivity and specificity in our cohort of patients was achieved at a cut-point of 22.5 ppb. In this study, the values are associated with high NPV, and a NPV of >90%, which means that the measurements can provide reliable clinical guidance, particularly when values are low (19).
Our study had several limitations. First, the diagnosis was confirmed by BCT or bronchodilator test, which may have yielded false positive and negative results. Second, we did not exclude ex-smokers, whose NO production may have been affected. However, Wagener (20) reported that smoking history does not affect FeNO level. Therefore, we assume that our results are not biased by this limitation. In addition, our study focused on FeNO level at the initial stage and after 12 weeks of treatment. Moreover, we could not regularly monitor the FeNO levels of the patients. Finally, although attempts have been made to standardize FeNO measurements, caution should be made in extrapolating absolute values obtained in one center to those obtained elsewhere.
In conclusion, we found that FeNO measurement is an alternative diagnostic tool to conventional lung function tests for differentiating BE accompanied with asthma from BE alone. FeNO level exhibits high sensitivity and specificity. FeNO measurement is a simple, reproducible, and noninvasive method of monitoring patient responsiveness to specific treatments. However, additional large-scale prospective studies must be performed to expand the clinical applications of FeNO measurement.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Clinical Practice Guidelines. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda (MD): National Heart, Lung, and Blood Institute. 2007.
- GINA executive committee. Global Strategy for Asthma Management and Prevention 2006. Available online: http://ginasthma.org/
- Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:309-29. [PubMed]
- Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem 1994;269:13725-8. [PubMed]
- Kim SH, Yoon HJ. Use of the exhaled nitric oxide for management of asthma and respiratory diseases. Korean J Med 2008;74:579-86. [Crossref]
- Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010;138:682-92. [Crossref] [PubMed]
- Tsang KW, Leung R, Fung PC, et al. Exhaled and sputum nitric oxide in bronchiectasis: correlation with clinical parameters. Chest 2002;121:88-94. [Crossref] [PubMed]
- Ho LP, Innes JA, Greening AP. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J 1998;12:1290-4. [Crossref] [PubMed]
- Cho YJ, Lim HJ, Park JS, et al. Measurement of fractional exhaled nitric oxide in stable bronchiectasis. Tuberc Respir Dis (Seoul) 2013;74:7-14. [Crossref] [PubMed]
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- al-Ali MK, Eames C, Howarth PH. Exhaled nitric oxide; relationship to clinicophysiological markers of asthma severity. Respir Med 1998;92:908-13. [Crossref] [PubMed]
- Jatakanon A, Lim S, Kharitonov SA, et al. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 1998;53:91-5. [Crossref] [PubMed]
- Lim S, Jatakanon A, Meah S, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax 2000;55:184-8. [Crossref] [PubMed]
- Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003;123:751-6. [Crossref] [PubMed]
- Kim C, Kim DG. Bronchiectasis. Tuberc Respir Dis (Seoul) 2012;73:249-57. [Crossref] [PubMed]
- Foley SC, Hopkins NO, Fitzgerald MX, et al. Airway nitric oxide output is reduced in bronchiectasis. Respir Med 2007;101:1549-55. [Crossref] [PubMed]
- Donohue JF, Jain N. Exhaled nitric oxide to predict corticosteroid responsiveness and reduce asthma exacerbation rates. Respir Med 2013;107:943-52. [Crossref] [PubMed]
- Taylor DR. Advances in the clinical applications of exhaled nitric oxide measurements. J Breath Res 2012;6:047102. [Crossref] [PubMed]
- Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015;70:115-20. [Crossref] [PubMed]


