
Ex-vivo exploration of an endobronchial sentinel lymph node procedure in lung cancer for optimizing workflow and evaluating feasibility of novel imaging tools
Highlight box
Key findings
• A navigation bronchoscopy mediated sentinel lymph node (SLN) procedure seems technically feasible. Several parameters and workflow settings were explored. While intratumoral injections seem successful in the majority of lesions, peritumoral injections were always successfully performed.
What is known and what is new?
• There is no standardized protocol to perform an SLN procedure in lung cancer, specifically endobronchially. Several parameters, e.g., injection method, injection volume and SPECT/CT-acquisition are not unambiguously reported.
• Peritumoral injections of 99mTc-nanocolloid performed with a Pioneer Plus catheter were always successful. The tumor and injection were visible on IVUS-imaging and SPECT/CT-imaging was able to identify most injection depots.
What is the implication, and what should change now?
• When implementing an endobronchial SLN procedure, the results of this study regarding injection method, injection volume and SPECT/CT-acquisition parameters need to be considered. Further implementation will determine the applicability and added clinical value of this procedure.
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of all diagnosed lung cancers. Its 5-year survival rate is highly depended on the stage of disease and ranges from >77% in stage IA disease to 10% in stage IVA disease (1). A minority of NSCLC patients are diagnosed at an early stage and these tumors are treated with curative intent, receiving surgery or stereotactic ablative radiation (2). Even in this early stage, 2 years after intended curative treatment, a recurrence rate of 9.4–28.3% is observed (3,4). Moreover, up to 23.1% of clinically N0-staged patients are upstaged to N1 or N2 lymph nodal involvement based upon routine pathological examination of surgically resected material (5). These results indicate that the presence of occult disease—albeit micro- or macro-metastatic—might be underestimated in early-stage NSCLC, resulting in undertreatment and inferior outcomes (6).
A technique that could improve the staging accuracy and that is increasingly used in other clinical fields is a sentinel lymph node (SLN) procedure. An injection of a radioactive, fluorescent, or paramagnetic tracer is administered in, or surrounding the tumor area which subsequently drains via the lymphatic system to the locoregional lymph nodes. These tracers are then visualized on imaging before or during surgery to help identify and retrieve the first draining (‘sentinel’) lymph node, allowing further specific evaluation. 99mTc-nanocolloïd and/or indocyanine green (ICG) are most often used, but other methods can also be applied (7). The literature on SLN procedures in lung cancer by radioactive tracers or ICG suggests an identification rate of around 90%, while varying approaches are reported (8-10). Despite this published success, an SLN procedure has not been implemented into routine surgical treatment, mainly due to the complexity and lack of clinical consequences since lobectomy still is the cornerstone of curative surgery (11). However, with the raised interest in sublobar resection and the upcoming interest in minimal invasive, image guided local therapy, the importance of research revolving around SLN is considerably increased.
Since an SLN procedure is not part of clinical practice for lung cancer, no standardized protocol has been established. The need for a robust and accurate protocol to standardize the injection, identification and dissection of SLN has been addressed by multiple authors in the past (10,12,13). This will be essential to determine the added value of an SLN procedure in lung cancer treatment. In NSCLC, both peri- and intratumoral tracer injections have been successfully performed before and during surgery to identify the SLN, but there is often no specific rationale for choosing one above the other (14,15).
Nowadays, patients with a peripheral pulmonary nodule suspected of lung cancer are increasingly often diagnosed through cone-beam computed tomography (CBCT)-based navigation bronchoscopy programs (16-19). These procedures may facilitate the opportunity to perform an SLN procedure in patients with small peripheral pulmonary lesions during diagnostic work-up (20). A pre-operative identification of the SLN could possibly decrease operation time and improve SLN localization, particularly in patients with atypical drainage patterns or with an SLN outside the surgical field (21). In this ex-vivo study, we aim to assess multiple parameters that are relevant for a standardized SLN procedure through transbronchial injection. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-984/rc).
Methods
Study design
This is a prospective, single-center study using ex-vivo lung resection specimens that examined the feasibility of an endobronchial SLN procedure in lung cancer patients. Multiple procedure parameters were varied in small subsets of explants and adjusted to optimize a protocol that could be implemented in clinical practice. Furthermore, a novel device was used to test its clinical value as an endobronchial injection tool.
Patients undergoing lung cancer resection, either by segmentectomy, lobectomy or pneumectomy, were eligible to participate in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research Ethics Committee of the Radboud University Medical Center agreed upon study design and confirmed that no formal ethical approval is required for this research (Reference No. 2020-6946). All patients gave informed consent for use of resected lung specimen within this feasibility study prior to study enrollment. A total of 10 patients were included. Patient demographic and nodule characteristics were collected.
Radioactive tracer
A dual-labelled radioactive-fluorescent tracer (99mTc-ICG-nanocolloid, GE Healthcare) was used for injection. For the purpose of this study, the fluorescent component of this tracer was not utilized. To test the retention of tracer volume (i.e., spillage) and repeatability of injection, we varied injection volume—previously described as feasible in literature—while taking tumor size into account by not injecting the largest volume in the smallest tumor (12,14). Volumes and dose activity were obtained from pooled commercially available single syringes pre-filled with 99mTc-ICG-nanocolloid. All used materials and the syringe were measured before and after the injections to calculate the total injected activity.
Tracer injection method
Tissues were injected with the tracer either in or around the tumor—intra- or peritumoral—to evaluate which method would be preferable in a clinical setting. Of 10 obtained specimens, 5 were injected intratumorally and 5 peritumorally, which was allocated in a non-randomized fashion to match lesion size in both groups. Since non-solid lesions are less dense and have a much more compressible compound than soft tissue, it was hypothesized that these lesion types could allow for better retention of tracer volume compared to solid tumors that are dense and compressed. Therefore, in addition, all non-solid lesions were allocated to intratumoral injections.
Tracer injection tool
There are currently no commercial endobronchial instruments that allow for needle placement and/or tracer injection under direct ultrasound (US)-visualization in the periphery of the lung. Precise placement and location confirmation are important in order to adequately inject tracers within the tumor area. For this reason, an intravascular ultrasound (IVUS) catheter, the Pioneer Plus (Philips, The Netherlands; manufactured by Viant, USA) was used endobronchially (Figure 1). The Pioneer Plus is a torquable device that combines US-guidance with an angulated 24-G needle that has an outer diameter of 2.1 mm (6-F sheath compatibility) (22). This could facilitate reaching multiple parts of the tumor in one procedure and allow for US-visualization of tumor location and needle placement.

Workflow
A syringe filled with a 99mTc-ICG-nanocolloid solution was attached to the Pioneer Plus and the working volume of around 0.3 mL (as measured previously) was filled up with tracer solution. The catheter was connected to the IVUS-console to obtain radial US-images. See Figure 1 for a visualization of the set-up. With the needle retracted in-sheath, the catheter was navigated through the bronchial tree to a location near the tumor. This was confirmed both on the US-imaging and by palpation, as the tumor and catheter were palpable in the soft lung tissue. The tumor was visualized on US-imaging and depending on the injection method, the needle was fully extended while the tumor was visible (intratumorally) or while the tumor was not visible (peritumorally). This was confirmed again by palpating the location of the tumor and catheter before attempting to inject the tracer, based on the defined dose escalation protocol.
Subsequently, the catheter was navigated to new locations near or in the tumor to place multiple tracer depots. Using this approach, an attempt to administer the tracer in or around four quadrants of the tumor was made (23). A flowchart of the study procedures can be found in Figure 2. The real-time US-images of lung tumor and tracer injection were recorded in addition to documentation on visibility of the tumor and successfulness of the injection. Unsuccessful injections were defined as injections where the plunger of the syringe could not be moved due to pressure at the needle tip, or, when there was visible leakage of tracer. Additionally, photo- and video-graphic material was collected during the experiment to capture difficulties and points of improvement.
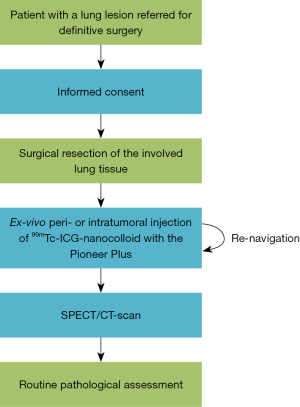
Single photon emission computed tomography/computed tomography (SPECT/CT)-scanning
Since ex-vivo lung tumor tissue was used, there was no drainage of the tracer to the lymph nodes. A SPECT/CT-scan was made to visualize the injection depots and optimize the imaging protocol. SPECT acquisition parameters included a 360° orbit with 180° detector geometry, 256×256 matrix size, and 6° angle step with 30 s/view and 20 views. After acquisition, an iterative reconstruction with 6 iterations, 16 subsets and a gaussian filter of 8.4 mm was used to obtain the final SPECT/CT-images.
Pathological assessment
All tissues were fixed with formalin after scanning. Histological sections were assessed by a pathologist to evaluate tumor size, origin and lymph nodal involvement, following routine clinical practice. These outcomes were collected.
Statistical analysis
This was an explorative feasibility study and descriptive analysis were performed in Excel (Microsoft version 16.0, Redmond, WA, USA).
Results
Table 1 visualizes experimental outcomes. Of the 10 included specimens, eight were solid tumors and two were non-solid lesions. Final pathology showed seven adenocarcinomas, two squamous cell carcinomas and one benign lesion. The median tumor size was 30 mm. An average total volume of 0.7 mL (range, 0.3–1.2 mL) was injected, with an average of 0.16 mL per depot. The injection speed ranged from 2 to 10 s, depending on volume and resistance during injection. Pathological assessment was not hindered by tracer injection, as scored by the pathologist. No samples or data were excluded from analysis.
Table 1
| Specimen number | Gender | Age (y) | Lesion type (on CT) | Lesion size (mm) | Intra- or peritumoral | Injected tracer volume (mL) | Number of injections (n) | Successful injections (n) | Injections visible on IVUS-images (n) | Pathological outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 74 | Solid | 41 | Intratumoral | 0.5 | 4 | 4 | 3 | Acinar AC |
| 2 | Male | 74 | Non-solid | 32 | Intratumoral | 0.3 | 3 | 3 | 3 | Lepidic AC |
| 3 | Female | 80 | Sub-solid | 30 | Peritumoral | 0.6 | 3 | 3 | 2 | Lepidic AC |
| Intratumoral | 1 | 0 | 1 | |||||||
| 4 | Female | 48 | Solid | 16 | Peritumoral | 0.6 | 4 | 4 | 1 | Benign |
| 5 | Female | 64 | Solid | 41 | Peritumoral | 0.5 | 4 | 4 | 2 | SCC |
| 6 | Male | 75 | Solid with cavitation | 53 | Peritumoral | 1.2 | 6 | 6 | 3 | SCC |
| 7 | Male | 73 | Solid | 66 | Intratumoral | 0.4 | 3 | 3 | 3 | AC |
| Peritumoral | 1 | 1 | 0 | |||||||
| 8 | Female | 48 | Non-solid | 42 | Intratumoral | 0.9 | 4 | 4 | 4 | AC |
| 9 | Male | 65 | Solid | 27 | Peritumoral | 0.8 | 4 | 4 | 4 | Solid AC |
| 10 | Male | 72 | Solid | 26 | Intratumoral | 1.1 | 6 | 2 | 3 | Acinar AC |
y, years; CT, computed tomography; IVUS, intravascular ultrasound; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Tracer injection method
Intratumoral injections were performed in two non-solid and three solid tumors and were successful in 7 out of 7 (100%) and 9 out of 14 injections (64.3%), respectively. Peritumoral injections were performed in five solid tumors and all 22 performed injections were successful (100%). A total of 5 injections were unsuccessful; 3 were due to visible tracer leakage during injection and 2 were due to an inability to inject, because the plunger of the syringe could not be moved when the needle was extended in the tissue.
Tracer injection tool
The Pioneer Plus catheter and console were able to visualize all tumors, both solid and non-solid. Figure 3 shows a tumor on US-imaging. The needle always exits at the 12 o’clock position in the US-image and because the catheter is torsionally rigid, rotation of the catheter will change the orientation of the image and therefore the needle location when extended. Based on the distance indicator presented in mm on the image (see Figure 3), the desired needle depth—up to 7 mm—can be determined. Controlling the Pioneer Plus was considered easy and no training additional to reviewing the manual was necessary. A short instruction to familiarize with the imaging console was completed, but the control panel is largely self-explanatory.
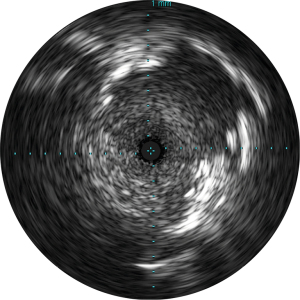
Injections were visible in 17/21 intratumoral injections and 12/22 peritumoral injections. When the catheter was placed peritumoral, the overall US-visibility was inferior due to air in the parenchyma whereas an intratumoral catheter location provided better overall visibility. When the injection is visible on the US-image, the location and possible leakage can be monitored.
SPECT/CT-scanning
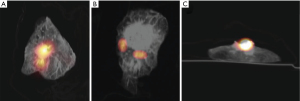
An average total radioactivity of 89.5 MBq was injected. The radioactivity in the tissues ranged from 35.4 to 188.0 MBq as a dose escalation protocol was used and the tracer had a fixed radioactive concentration. The scanning protocol with a SPECT-scan of around 17 min and a low-dose CT-scan provided enough resolution to locate individual injection depots. Of all injected radioactive depots, 76.7% (33/43) could be individually identified on SPECT/CT-images (see also Figure 4). The amount of radioactivity did not affect the visibility of tracer depots.
Discussion
Our ex-vivo results suggest that performing an SLN imaging procedure by navigation bronchoscopy is feasible when using a novel hybrid imaging and injection device and a radiotracer. While intratumoral injections seem successful in the majority of lesions, peritumoral injections were always successfully applied. Several parameters and workflow settings were explored.
There are a range of tracer types available to perform an SLN procedure. Magnetic tracers for example, have been explored in the past, but the identification rate of SLN during surgery has remained between 73% and 84%. Visible dyes on the other hand report an identification rate of only 22–50% as it is difficult to visualize dyes in anthracotic nodes. Additionally, CT lymphography can provide exceptional spatial resolution to distinguish SLN from an injection depot but cannot be used intraoperatively (24). During this experiment, we used 99mTc-ICG-nanocolloid, but the ICG was not used for visualization purposes as there was no drainage in this ex-vivo experiment. Depending on the use of the tracer after injection, either pre- or perioperatively, these tracers could also be used individually or in combination with other tracer types.
In breast cancer, where an SLN procedure is guideline recommended clinical practice, there is still debate on the site of tracer injection. Intracutaneous injections can be placed successfully subareolar, intra- or peritumorally (25). In a systematic review and meta-analysis on SLN procedures in lung cancer, Taghizadeh Kermani et al. in 2013 found peritumoral injection of a tracer to perform best in terms of sensitivity and detection rate when compared to intratumoral injections (9). This resonates with our results, where 100% of peritumoral injections were successful compared to 76% of all intratumoral injections. Tumor characteristics on CT have not yet been correlated to injection successfulness. We do however hypothesize that choosing an injection method pre-procedurally based on CT-characteristics could be difficult and that peritumoral injections would be a safer option to establish successful injections.
Injections were only determined to be unsuccessful when leakage was seen at the injection site or when intratumoral resistance was too high and the plunger of the syringe could not be moved. Leakage into the bronchi when injecting in a peripheral tumor could therefore not be properly assessed during the procedure.
The intended number of injections was adjusted periprocedural in three specimens because the tumor area was already covered (specimen 2), because more injections were needed to cover more possible drainage patterns (specimen 3) or because multiple injections failed (specimen 10). Additionally, the peritumoral region proved to be difficult to reach in a large and more centrally located tumor (specimen 3) and we have therefore performed an intratumoral injection to cover more possible drainage patterns. Uribe-Etxebarria Lugariza-Aresti et al. in 2017 could also not complete 4 peritumoral injections in all quadrants in two very central tumors, although they injected transpleurally (26). Adjusting the protocol per-procedurally seems inevitable and should be considered when the injections are not considered to cover the tumor and possible drainage patterns adequately. A formal volume escalation protocol is difficult to realize due to heterogeneous tumor characteristics (i.e., size, solidity, density), and the protocol used can therefore be described as a feasibility protocol.
One specimen that was assigned to the intratumoral injection group (specimen 7) was injected peritumorally once by accident, as the actual needle location was discovered after injection of the tracer.
The Pioneer Plus was able to image all tumors and is therefore considered to be a valuable tool when performing endobronchial tracer injections. The handling of the device and imaging quality are up to standard when compared to clinically used endobronchial US-devices. The standardized orientation in which the needle always deploys in the 12 o’clock position, makes determining needle position easy and possibly safer, even if the needle or injection itself are not visible. Moreover, the angulated needle seems essential in enabling the placement of injections in multiple places in or around the tumor. Therefore, the use of this catheter should be further investigated in a clinical setting to determine its added value as an endobronchial catheter to perform injections.
The lung was deflated, and no ventilation was performed, which makes it difficult to evaluate possible injection diffusion in the tissue in a clinical setting. Gilmore et al. in 2012 found that lymphatic migration was unreliable when injecting ICG intraoperatively into deflated lung parenchyma (12). They were able to improve the consistency of lymphatic migration and SLN identification by ventilating the lung for <3 min. This should therefore be considered when developing a clinical protocol.
There was limited attenuation of the radioactivity during scanning, as would be the case when the tissue is surrounded by the thorax wall. In a patient, the resolution of SPECT-imaging could therefore be inferior when scanning the same amount of radioactivity and injection depot volumes. Additionally, when considering the time the tracer needs to drain to the lymph nodes, the half-life of 99mTc should be considered. This could result in the use of more activity or a longer scan time to be able to detect the amount of tracer drained to the SLN in a clinical setting. Future clinical studies will determine if the SLN would be visible using these doses and imaging settings.
Conclusions
An SLN procedure carried out in a minimally invasive, endobronchial manner seems feasible. The Pioneer Plus is a suitable catheter to perform injections endobronchially at multiple locations, while being able to view the injected area. Peritumoral injections seem more successful than intratumoral injections, while a SPECT/CT-scan is able to visualize all radioactive injection depots. Future research should determine the applicability, lymphatic tracer drainage and visibility for SLN detection and its added clinical value in a large patient cohort.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-984/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-984/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-984/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-984/coif). RLJV reports unrestricted research grants from Philips Medical, Astra Zeneca, Pentax Medical and Johnson & Johnson, consulting fees from Johnson & Johnson and Intuitive Surgical, speaker fees from Medtronic, Travel arrangements from Johnson & Johnson and Intuitive Surgical, is a board member of the Dutch Society of Technical Physicians (NVvTG) and has patents issued, pending and planned in the field of advanced and navigation bronchoscopy; EHFMvdH reports unrestricted research grants from Philips Medical, Astra Zeneca, Pentax Medical and Johnson & Johnson, consulting fees from Johnson & Johnson and Intuitive Surgical, speaker fees from Janssen-Cilag and Pentax Medical, Travel arrangements from Johnson & Johnson and Intuitive Surgical, is a board member of European Association for Bronchology and Interventional Pulmonology (ECBIP) and a board of regents member of the World Association for Bronchology and Interventional Pulmonology (WABIP) and has patents issued, pending and planned in the field of advanced and navigation bronchoscopy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol 2017;7:170070. [Crossref] [PubMed]
- Isaka M, Kojima H, Takahashi S, et al. Risk factors for local recurrence after lobectomy and lymph node dissection in patients with non-small cell lung cancer: Implications for adjuvant therapy. Lung Cancer 2018;115:28-33. [Crossref] [PubMed]
- van den Berg LL, Klinkenberg TJ, Groen HJM, et al. Patterns of Recurrence and Survival after Surgery or Stereotactic Radiotherapy for Early Stage NSCLC. J Thorac Oncol 2015;10:826-31. [Crossref] [PubMed]
- Beyaz F, Verhoeven RLJ, Schuurbiers OCJ, et al. Occult lymph node metastases in clinical N0/N1 NSCLC; A single center in-depth analysis. Lung Cancer 2020;150:186-94. [Crossref] [PubMed]
- He Z, Xia Y, Tang S, et al. Detection of occult tumor cells in regional lymph nodes is associated with poor survival in Pn0 non-small cell lung cancer: a meta-analysis. J Thorac Dis 2016;8:375-85. [Crossref] [PubMed]
- Deken MM, van Doorn HC, Verver D, et al. Near-infrared fluorescence imaging compared to standard sentinel lymph node detection with blue dye in patients with vulvar cancer – a randomized controlled trial. Gynecol Oncol 2020;159:672-80. [Crossref] [PubMed]
- Karamustafaoglu YA, Yoruk Y, Yanik F, et al. Sentinel lymph node mapping in patients with operable non-small cell lung cancer. J Thorac Dis 2013;5:317-20. [Crossref] [PubMed]
- Taghizadeh Kermani A, Bagheri R, Tehranian S, et al. Accuracy of sentinel node biopsy in the staging of non-small cell lung carcinomas: systematic review and meta-analysis of the literature. Lung Cancer 2013;80:5-14. [Crossref] [PubMed]
- Sun WYL, Dang JT, Modasi A, et al. Diagnostic accuracy of sentinel lymph node biopsy using indocyanine green in lung cancer: a systematic review and meta-analysis. Gen Thorac Cardiovasc Surg 2020;68:905-13. [Crossref] [PubMed]
- Chao YK. Deciding to trust, coming to believe: sentinel lymph node assessment in lung cancer. J Thorac Dis 2018;10:S3978-80. [Crossref] [PubMed]
- Gilmore DM, Khullar OV, Colson YL. Developing intrathoracic sentinel lymph node mapping with near-infrared fluorescent imaging in non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:S80-4. [Crossref] [PubMed]
- Caso R, Jones GD, Jones DR. Sentinel lymph node mapping in lung cancer: a step forward? J Thorac Dis 2018;10:S3254-6. [Crossref] [PubMed]
- Gilmore DM, Khullar OV, Jaklitsch MT, et al. Identification of metastatic nodal disease in a phase 1 dose-escalation trial of intraoperative sentinel lymph node mapping in non-small cell lung cancer using near-infrared imaging. J Thorac Cardiovasc Surg 2013;146:562-70; discussion 569-70. [Crossref] [PubMed]
- Lafuente-Sanchis A, Estors-Guerrero M, Zúñiga Á, et al. Clinical Significance of Molecular Micrometastasis in the Sentinel Lymph Node of Early-stage Non-Small Cell Lung Cancer Patients. Am J Clin Oncol 2018;41:1106-12. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Verhoeven R, Fütterer J, Schampaert S, et al. Real-time 3D cone beam CT imaging guidance for advanced navigation bronchoscopy to centimeter sized lesions. European Respiratory Journal 2019;54:PA3117.
- Verhoeven RLJ, Fütterer JJ, Hoefsloot W, et al. Cone-Beam CT Image Guidance With and Without Electromagnetic Navigation Bronchoscopy for Biopsy of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2021;28:60-9. [Crossref] [PubMed]
- Verhoeven RLJ, van der Sterren W, Kong W, et al. Cone-beam CT and Augmented Fluoroscopy-guided Navigation Bronchoscopy: Radiation Exposure and Diagnostic Accuracy Learning Curves. J Bronchology Interv Pulmonol 2021;28:262-71. [Crossref] [PubMed]
- Cicenia J, Avasarala SK, Gildea TR. Navigational bronchoscopy: a guide through history, current use, and developing technology. J Thorac Dis 2020;12:3263-71. [Crossref] [PubMed]
- Abele JT, Allred K, Clare T, et al. Lymphoscintigraphy in early-stage non-small cell lung cancer with technetium-99m nanocolloids and hybrid SPECT/CT: a pilot project. Ann Nucl Med 2014;28:477-83. [Crossref] [PubMed]
- Philips. Pioneer Plus IVUS-guided re-entry catheter. Available online: https://www.philips.nl/healthcare/product/HCIGTDPPLUS/pioneer-plus-ivus-guided-re-entry-catheter
- Celikoglu F, Celikoglu SI, Goldberg EP. Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J Pharm Pharmacol 2010;62:287-95. [Crossref] [PubMed]
- Gregor A, Ujiie H, Yasufuku K. Sentinel lymph node biopsy for lung cancer. Gen Thorac Cardiovasc Surg 2020;68:1061-78. [Crossref] [PubMed]
- Gommans GMM. Radiopharmaceutical and clinical aspects of sentinel lymph node procedures in breast cancer patients. 2009.
- Uribe-Etxebarria Lugariza-Aresti N, Barceló Galíndez R, Pac Ferrer J, et al. Biopsy of the sentinel node in lung cancer. Med Clin (Barc) 2017;148:257-9. [Crossref] [PubMed]


