
The role of the RAPID score in surgical planning for empyema
Highlight box
Key findings
• Patients diagnosed with pleural infections who had early surgery and low RAPID scores experienced better outcomes compared with late surgery and low RAPID scores including decreased length of stay and organ failure.
What is known and what is new?
• The RAPID score is a validated clinical score that predicts mortality in empyema.
• This manuscript suggests that the RAPID score may have a role in determining ideal surgical timing empyema.
What is the implication, and what should change now?
• Prospective data is needed to validate the RAPID score’s usefulness in surgical timing for pleural infections.
Introduction
The RAPID score [Renal (urea), Age, fluid Purulence, Infection source, Dietary (albumin)] is a validated scoring system which allows risk stratification in patients with pleural infection at presentation (1). The score stratifies patients in low [0–2], medium [3–4], and high [5–7] risk groups, with increasing score associated with increased 3- and 12-month mortality and increased length of hospitalization (2-4).
Surgical intervention plays a key role in the management of stage 2 (fibropurulent) and stage 3 (organizing) empyema (5,6). Even when risk-adjusted, surgical management of pleural infections is associated with lower risk of early mortality (7). Deciding early surgical intervention or trial of medical therapy with chest tube drainage and intrapleural fibrinolytics is controversial. Some groups recommend surgical intervention as first-line therapy, while others recommend trial of medical management (6,8). Rahman et al. hypothesized that risk stratification utilizing the RAPID score may identify patients who would benefit from early surgical intervention (1). To date, there are no published studies which have tested this hypothesis. This study aims to evaluate if the RAPID score can aid surgical decision making regarding pleural infections. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-747/rc).
Methods
This is a retrospective, multicenter, observational study on patients with complicated pleural effusions and/or empyema undergoing thoracoscopic or open surgical management of pleural infection from September 2014 to September 2018 in multiple North and Central Texas hospitals. We evaluated demographic and clinical data from the electronic health record (EHR). Patients aged 18 and older with International Classification of Diseases (ICD)-9 and ICD-10 diagnostic codes for empyema and complicated pleural effusion as well as Current Procedural Terminology (CPT) codes for thoracoscopy, resection, decortication, and intrapleural fibrinolytic therapy were included. From the initial cohort of patients selected based on ICD codes, patients were excluded if they did not have surgery or the diagnosis of empyema was not confirmed. Our research team then recorded the following categories of patient data into a secure Health Insurance Portability and Accountability Act (HIPAA) compliant electronic data capture tool called REDCap (9): demographics, comorbidities as measured by the Charlson Comorbidity Index (CCI), presentation, management, RAPID score components on day 0 of the hospitalization, and outcomes. The individual components of the REDCap online collection form can be found in the Table S1.
Patient outcomes
The primary study outcome was all-cause 90-day mortality. Secondary outcomes included organ failure [as measured by: need for noninvasive or invasive mechanical ventilation, vasopressors, and/or development of acute renal failure defined per Kidney Disease: Improving Global Outcomes (KDIGO) guidelines which includes increase in serum creatinine (SCr) by ≥0.3 mg/dL within 48 hours or increase in SCr by 50% from baseline within 7 days], length of stay (defined as days from diagnosis of empyema to hospital discharge) and 30-day readmission rate (10). In a subset analysis, empyema stage, type of surgery and length of stay (days from surgery to hospital discharge) were also evaluated. In this analysis, 22 patients were excluded from the total cohort due to inability to access their charts after that hospital switched their EHR system.
These outcomes were compared between the following groups: (I) early surgery and late surgery; (II) low vs. high RAPID; (III) low vs. high RAPID in early surgery group; (IV) low vs. high RAPID in late surgery group. Early surgery was defined as ≤3 days from diagnosis and late surgery was defined as >3 days. As noted by Touray et al., there is no consensus on the definition of early versus late surgical timing with reported values between 48 hours and 2 weeks in previous literature (3,11-13). Also, we chose 3 days to allow for the typical dose regimen of tissue plasminogen activator (tPA) and deoxyribonuclease (DNase) as reported in the MIST2 trial (14). Low RAPID score was defined as ≤3 and high RAPID was defined as >3. This study was not adequately powered to stratify patients into the typical low [0–2], medium [3–4], and high [5–7] risk groups while assessing the relationship between surgical timing and RAPID score on mortality. The surgical practices varied between different hospitals and the timing of surgery was not decided based on any algorithm but clinician judgement.
Statistical analysis
Statistical analysis was performed using SAS 9.4 (Cary, NC, USA). Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Means and standard deviations (or medians and ranges where appropriate) were used to describe continuous variables. A chi-square test (or Fisher’s exact test when low cell counts are present) was used to test for associations in bivariate comparisons. A logistic regression was used to assess multivariate relationships with RAPID score, surgical timing and categorical outcomes. A negative binomial regression was used to assess multivariate relationships with RAPID score, surgical timing and categorical variables. Statistical significance is set at P<0.05 unless otherwise noted.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Baylor Scott & White Institutional Review Board (Project No. 018-784) and individual consent for this retrospective analysis was waived.
Results
Patient characteristics
We reviewed 411 patient medical records and after removing patients who failed to meet inclusion criteria (Figure 1), 182 unique patients were analyzed. Sixty-nine percent of patients were male with mean age of 58 years old [standard deviation (SD), 15.5 years old] (Table 1). The mean age of patients who had low RAPID score was 54 years old while the mean age of patients with high RAPID scores was 70 years old. The most common comorbidities in the study population were heart disease, type 2 diabetes mellitus and history of solid and/or hematologic cancer, excluding lung cancer. Of note, both heart disease (P=0.0073) and cancer (P=0.0240) were significantly associated with higher RAPID scores. Most patients developed empyema from community acquired pneumonia, however the presence of hospital-acquired pneumonia was more common in patients with RAPID score ≥4 (P=0.0002).
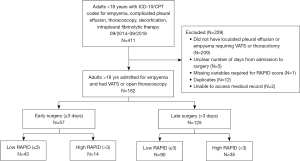
Table 1
| Characteristic | Total (N=182) | Early (N=57) | Late (N=125) | P value |
|---|---|---|---|---|
| Age (years), mean (SD) | 57.88 (15.5) | 57.96 (17.6) | 57.85 (14.5) | 0.9051 |
| Male, n (%) | 126 (69.2) | 44 (77.2) | 82 (65.6) | 0.116 |
| Comorbidities, n (%) | ||||
| None | 64 (35.2) | 22 (38.6) | 42 (33.6) | 0.5126 |
| Heart disease | 61 (33.5) | 20 (35.1) | 41 (32.8) | 0.7617 |
| Diabetes | 54 (29.7) | 17 (29.8) | 37 (29.6) | 0.9755 |
| History of cancer | 42 (23.1) | 12 (21.1) | 30 (24.0) | 0.6616 |
| COPD | 32 (17.6) | 9 (15.8) | 23 (18.4) | 0.6679 |
| Lung disease | 20 (11.0) | 5 (8.8) | 15 (12.0) | 0.5184 |
| Non-small cell lung cancer | 11 (6.0) | 5 (8.8) | 6 (4.8) | 0.3239 |
| Intravenous drug use | 4 (2.2) | 0 (0.0) | 4 (3.2) | 0.3108 |
| Source of infection, n (%) | 0.8742 | |||
| Community acquired pneumonia | 119 (65.4) | 38 (66.7) | 81 (64.8) | |
| Postsurgical infection | 27 (14.8) | 10 (17.5) | 17 (13.6) | |
| Hospital acquired pneumonia | 21 (11.5) | 5 (8.8) | 16 (12.8) | |
| Health care associated pneumonia | 6 (3.3) | 2 (3.5) | 4 (3.2) | |
| Unknown | 9 (4.9) | 2 (3.5) | 7 (5.6) | |
| End-organ failure, n (%) | ||||
| None | 78 (42.9) | 31 (54.4) | 47 (37.6) | 0.0338 |
| Noninvasive/invasive ventilation | 80 (44.0) | 18 (31.6) | 62 (49.6) | 0.0231 |
| Renal failure | 65 (35.7) | 15 (26.3) | 50 (40.0) | 0.074 |
| Vasopressors | 39 (21.4) | 9 (15.8) | 30 (24.0) | 0.2106 |
| Empyema stage, n (%) | ||||
| Stage 2 | 9 (5.6) | 6 (11.3) | 3 (2.8) | 0.0605 |
| Stage 3 | 115 (71.9) | 36 (67.9) | 79 (73.8) | 0.4341 |
| Mixed stage | 36 (22.5) | 11 (20.8) | 25 (23.4) | 0.7098 |
| Type of surgery, n (%) | ||||
| VATS | 101 (63.1) | 30 (56.6) | 71 (66.4) | 0.2289 |
| Thoracotomy | 111 (69.4) | 39 (73.6) | 72 (67.3) | 0.4162 |
| VATS converted to thoracotomy | 52 (32.5) | 16 (30.2) | 36 (33.6) | 0.6604 |
VATS, video-assisted thoracoscopic surgery; COPD, chronic obstructive pulmonary disease.
The median number of days from diagnosis of empyema to surgical management was 5 (IQR, 3–9 days). One hundred sixty-four (90%) patients received chest tube drainage and 45 (25%) received intrapleural fibrinolytics (tPA/DNase). All patients included in the study underwent surgical management [video-assisted thoracoscopic surgery (VATS) and/or open thoracotomy]. Nearly every patient (153/160, 95.6%) required decortication while only 7 patients needed a simple washout and drainage. Ninety-day mortality for patients undergoing VATS, thoracotomy and VATS converted to thoracotomy was 6% (6/101), 8% (9/111), and 8% (4/52) respectively. There were no statistically significant associations between surgery type (VATS, thoracotomy, VATS converted to thoracotomy) and primary or secondary outcomes, including mortality, length of stay, readmission rate, and organ failure.
Overall mortality at 90 days was 6.0% (11 of 182), 106 patients (58.2%) developed new organ failure (i.e., ventilator use, vasopressor use, acute renal failure), median length of stay was 14 days (IQR, 10–22 days), and 41 (22.5%) patients were readmitted within 30 days of discharge. Most patients (115/160) had stage 3 empyema, which was also significantly associated with 90-day mortality in bivariate analysis (3.5% vs. 15.6%, P=0.0120).
Early (≤3 days) vs. late (>3 days) surgery
Fifty-seven (31%) patients had surgery ≤3 days from diagnosis. In bivariate analyses, late surgery was associated with increased organ failure, CCI, length of stay (diagnosis to hospital discharge) and with receiving intrapleural fibrinolytics before surgical management (Tables 2,3). Surgical timing was not associated with increased mortality, 30-day readmission or length of stay, defined as surgery to hospital discharge.
Table 2
| Variables | Early (≤3 days) vs. late (>3 days) surgery | Low (≤3) vs. high (>3) RAPID score | Low (≤3) vs. high (>3 days) RAPID score in early surgery | Low (≤3) vs. high (>3) RAPID score in late surgery | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early (n=57) | Late (n=125) | P value | Low RAPID (n=133) | High RAPID (n=49) | P value | Low RAPID (n=43) | High RAPID (n=14) | P value† | Low RAPID (n=90) | High RAPID (n=35) | P value† | ||||
| Primary outcome | |||||||||||||||
| 90-day mortality, n (%) | 3 (5.3) | 8 (6.4) | 1.0000 | 3 (2.3) | 8 (16.3) | 0.0014 | 0 (0) | 3 (21.4) | 0.0124 | 3 (3.3) | 5 (14.3) | 0.0385 | |||
| Secondary outcomes | |||||||||||||||
| Organ failure‡, n (%) | 26 (45.6) | 80 (64.0) | 0.0197 | 66 (49.6) | 40 (81.6) | 0.0001 | 15 (34.9) | 11 (78.6) | 0.0044 | 51 (56.7) | 29 (82.9) | 0.0062 | |||
| 30-day readmission, n (%) | 14 (24.6) | 27 (21.6) | 0.6574 | 26 (19.5) | 15 (30.6) | 0.1130 | 7 (16.3) | 7 (50.0) | 0.0272 | 19 (21.1) | 8 (22.9) | 0.8313 | |||
| Length of stay (diagnosis to discharge) (days), median (SD) | 10.0 (13.7) | 16.0 (16.2) | <0.0001 | 13.0 (12.3) | 17.0 (21.1) | 0.0054 | 9.0 (8.4) | 16.0 (21.2) | 0.0064 | 16.0 (13.2) | 17.0 (21.3) | 0.1587 | |||
| Other outcomes | |||||||||||||||
| Charlson Comorbidity Index, median (SD) | 2.0 (3.2) | 3.0 (2.4) | 0.1545 | 2.0 (2.8) | 6.0 (2.7) | <0.0001 | 2.0 (3.0) | 6.0 (2.3) | <0.0001 | 3.0 (2.7) | 5.0 (2.8) | 0.0010 | |||
| Received intrapleural fibrinolytics, n (%) | 8 (14.0) | 37 (29.6) | 0.0240 | 38 (28.6) | 7 (14.3) | 0.0475 | – | – | – | – | – | – | |||
| ICU admission, n (%) | 30 (52.6) | 87 (69.6) | 0.0267 | 78 (58.6) | 39 (79.6) | 0.0089 | – | – | – | – | – | – | |||
| Positive blood cultures, n (%) | 7 (12.3) | 17 (13.6) | 0.8073 | 10 (7.5) | 14 (28.6) | 0.0002 | – | – | – | – | – | – | |||
†, the significance level for this section has been adjusted to 0.025 due to low patient sample size; ‡, composite of: need for noninvasive or invasive ventilation, vasopressors, and/or development of acute renal injury. SD, standard deviation; RAPID, Renal (urea), Age, fluid Purulence, Infection source, Dietary (albumin); ICU, intensive care unit.
Table 3
| Secondary outcome | Early (≤3 days) vs. late (>3 days) surgery | Low (≤3) vs. high (>3) RAPID score | Low (≤3) vs. high (>3 days) RAPID score in early surgery | Low (≤3) vs. high (>3) RAPID score in late surgery | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early (n=52) | Late (n=108) | P value | Low RAPID (n=120) | High RAPID (n=40) | P value | Low RAPID (n=40) | High RAPID (n=12) | P value† | Low RAPID (n=80) | High RAPID (n=28) | P value† | ||||
| Length of stay (surgery to discharge)‡, median (SD) | 7.0 (9.2) | 8.0 (12.8) | 0.503 | 7.0 (11.2) | 11.0 (12.8) | 0.0017 | 6.5 (7.9) | 13.5 (10.7) | 0.0009 | 7.0 (12.48) | 10.0 (13.7) | 0.119 | |||
†, the significance level for this section has been adjusted to 0.025 due to low patient sample size; ‡, sub-set analysis with 160/182 patients as noted in patient outcome section. SD, standard deviation; RAPID, Renal (urea), Age, fluid Purulence, Infection source, dietary (albumin).
In multivariate models, late surgery was associated with organ failure [odds ratio (OR) 2.048, 95% confidence interval (CI): 1.012–4.145, P=0.0463] (Table 4) and longer length of stay (diagnosis to hospital discharge) [incidence rate ratio (IRR) 1.23, 95% CI: 1.04–1.45, P=0.015].
Table 4
| Effect | Odds ratio | 95% CI | P value |
|---|---|---|---|
| RAPID score: high (RAPID >3) vs. low (RAPID ≤3) | 4.332 | 1.872–10.027 | 0.0006 |
| Surgical timing: late (>3 days) vs. early (≤3 days) | 2.048 | 1.012–4.145 | 0.0463 |
| Heart disease | 2.860 | 1.383–5.915 | 0.0046 |
| Intrapleural fibrinolytics | 1.986 | 0.916–4.308 | 0.0823 |
CI, confidence interval; RAPID, Renal (urea), Age, fluid Purulence, Infection source, dietary (albumin).
Low (≤3) vs. high (>3) RAPID score
One hundred thirty-three (73%) patients had RAPID scores on admission of ≤3 days. In bivariate analyses, a high RAPID score was associated with 90-day mortality, organ failure, higher CCI, increased length of stay (diagnosis to hospital discharge and surgery to discharge). It was not associated with 30-day readmission (Tables 2,3).
In a multivariate model, high RAPID score was associated with increased 90-day mortality (OR 4.028, 95% CI: 1.001–18.695, P=0.0479) (Table 5) and organ failure (OR 4.332, 95% CI: 1.872–10.027, P=0.0006) (Table 4). In a separate multivariate model, it was also associated with increased length of stay (diagnosis to hospital discharge) (IRR 1.34, 95% CI: 1.098–1.636, P=0.0040).
Table 5
| Effect | Odds ratio | 95% CI | P value |
|---|---|---|---|
| RAPID score: high (RAPID >3) vs. low (RAPID ≤3) | 4.028 | 1.001–18.695 | 0.0479 |
| Surgical timing: late (>3 days) vs. early (≤3 days) | 1.179 | 0.230–7.294 | 0.8423 |
| History of COPD | 4.987 | 1.265–20.918 | 0.0206 |
| Need for invasive mechanical ventilation | 3.746 | 0.884–22.313 | 0.0848 |
| Stage 3 empyema | 2.962 | 0.665–13.806 | 0.1355 |
| History of cancer | 3.477 | 0.871–15.057 | 0.0732 |
CI, confidence interval; RAPID, Renal (urea), Age, fluid Purulence, Infection source, dietary (albumin); COPD, chronic obstructive pulmonary disease.
Low (≤3) vs. high (>3) RAPID score with early surgery
Forty-three (24%) patients who underwent early surgery had low RAPID scores. High RAPID scores were associated with 90-day mortality, higher CCI, increased length of stay (diagnosis to hospital discharge and surgery to discharge) and developing organ failure (Tables 2,3). It was not associated with 30-day readmission.
Low (≤3) vs. high (>3) RAPID score with late surgery
Ninety (49%) patients who underwent late surgery had low RAPID scores. High RAPID scores were associated with higher CCI and development of organ failure (Tables 2,3). They were not associated with 90-day mortality, 30-day readmission, or increasing length of stay.
Discussion
The ideal timing of surgery for pleural infections is variable in the literature and in practice it is often dependent on the individual surgeon’s expert opinion (14). Lim et al. took an overall aggressive approach to surgical intervention after 48 hours of failing intrapleural fibrinolytics (13). However, surgery has been prolonged up to 2 weeks after hospitalization in some studies with good outcomes (11). Touray et al. hypothesized that earlier intervention in patients who are good surgical candidates with a high RAPID score could decrease the risk of mortality (3).
Previous studies have examined how the RAPID score and surgical timing, independently, have affected outcomes (1-3,11,12,15,16). To our knowledge, ours is the first multicenter study that examines RAPID score in association with surgical timing. After accounting for demographics and comorbidities the regression analyses suggested that late surgery and RAPID score >3 were independently associated with new organ failure and that RAPID score >3 was associated with increased mortality. Those with low RAPID scores and early surgery had much lower rates of organ failure and mortality than those with low RAPID scores and late surgery. This suggests the RAPID score can be useful to predict those who may benefit from early surgery in order to avoid increased rates of new organ failure. Due to its retrospective nature, however, this study does not provide definitive evidence of the RAPID score’s potential usefulness in deciding when to perform surgery for empyema.
RAPID score was directly associated with mortality and organ failure (Table 4). The strong association between mortality and RAPID score is unsurprising based on prior studies (1-4). The overall mortality of our patient population was lower than previous studies examining the RAPID score (1-4). This may be because our study only included patients who underwent surgery and therefore excluded patients who were not surgical candidates due to comorbidities or severity of illness.
Among the early surgical intervention group, RAPID score was directly associated with length of stay (LOS). This relationship does not exist among the late surgical intervention group and may indicate a dependent relationship between RAPID score and surgical timing on length of stay. Part of this may stem from the inherent differences between surgical timing groups: the late intervention group added on 3 days. The post-hoc length of stay analysis measuring surgery to discharge tried to address this weakness. It similarly indicated RAPID scores helped stratify those who would have longer post-operative stays in the early surgery group, but not in the late surgery group (Tables S2,S3).
No significant associations were found between RAPID scores or surgical timing and 30-day readmission. However, it is interesting to note the 30-day readmission rates for those with high RAPID scores and early surgery were very high, 50%, even higher than both RAPID groups in those with late surgery.
Chest tube drainage with intrapleural fibrinolytic therapy has been recommended to avoid surgical intervention, but there is some debate regarding its use to treat empyema (8). For example, the American Association for Thoracic Surgery recommends against routine use of intrapleural fibrinolytics to treat empyema (6). This may explain why intrapleural fibrinolytic rates in our study were relatively low (25%) since the decision to use intrapleural fibrinolytics was pragmatic and often at the discretion of the primary surgical team. The typical dose of tPA and DNase given was 10 mg/5 mg respectively two times daily for 3 days (14).
Our study has several limitations inherent to retrospective study design. First, the four separate groups were not evenly matched. Timing of surgery was at the discretion of the surgeon and varied across the hospitals in our study. However, this makes the study more applicable to clinical practice since there is no consensus on a standard algorithm to determine surgical timing. Accounting for this limitation would likely require a multi-center prospective study with the creation of a standard algorithm for the medical and surgical treatment of empyema. Second, the secondary analysis was affected by low numbers of patients and therefore we were unable to categorize RAPID scores into the usual three groups (low, medium, high risk). Future prospective studies should examine the validity of our results to determine which patients would benefit from expedited surgery.
Conclusions
We found a significant association between RAPID scores and surgical timing with new organ failure. The RAPID score was more strongly associated with mortality and organ failure than surgical timing. Patients with complicated pleural effusions who had early surgery and low RAPID scores experienced better outcomes including decreased length of stay and organ failure compared with those who had late surgery and low RAPID scores. This suggests that using the RAPID score may help identify those who would benefit from early surgery. The RAPID tool remains an easily applicable bedside tool to provide prognostication in patients with pleural infections.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-747/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-747/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-747/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-747/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Baylor Scott & White Institutional Review Board (Project No. 018-784) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rahman NM, Kahan BC, Miller RF, et al. A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest 2014;145:848-55. [Crossref] [PubMed]
- White HD, Henry C, Stock EM, et al. Predicting Long-Term Outcomes in Pleural Infections. RAPID Score for Risk Stratification. Ann Am Thorac Soc 2015;12:1310-6. [Crossref] [PubMed]
- Touray S, Sood RN, Lindstrom D, et al. Risk Stratification in Patients with Complicated Parapneumonic Effusions and Empyema Using the RAPID Score. Lung 2018;196:623-9. [Crossref] [PubMed]
- Corcoran JP, Psallidas I, Gerry S, et al. Prospective validation of the RAPID clinical risk prediction score in adult patients with pleural infection: the PILOT study. Eur Respir J 2020;56:2000130. Erratum in: Eur Respir J 2020 Dec 17;56(6):2050130. [Crossref] [PubMed]
- Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg 2015;48:642-53. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Chaddha U, Agrawal A, Feller-Kopman D, et al. Use of fibrinolytics and deoxyribonuclease in adult patients with pleural empyema: a consensus statement. Lancet Respir Med 2021;9:1050-64. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [Crossref] [PubMed]
- Wilson H, Mohite P, Hall A, et al. Timing and Efficacy of VATS Debridement in the Treatment of Parapneumonic Empyema. Arch Pulmonol Respir Care 2016;2:016-019.
- Pereira RR, Alvim CG, Andrade CR, et al. Parapneumonic pleural effusion: early versus late thoracoscopy. J Bras Pneumol 2017;43:344-50. [Crossref] [PubMed]
- Lim TK, Chin NK. Empirical treatment with fibrinolysis and early surgery reduces the duration of hospitalization in pleural sepsis. Eur Respir J 1999;13:514-8. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Wilshire CL, Jackson AS, Meggyesy AM, et al. Comparing Initial Surgery versus Fibrinolytics for Pleural Space Infections: A Retrospective Multicenter Cohort Study. Ann Am Thorac Soc 2022;19:1827-33. [Crossref] [PubMed]


