
Differentiating between pulmonary adenocarcinoma and squamous cell carcinoma by spectral CT volumetric quantitative analysis: a comparative study with conventional spectral analysis
Highlight box
Key findings
• The spectral CT parameters of pulmonary ADC and SQCC that can be used in the interpretation of lung cancer diagnoses differ.
What is known and what is new?
• A conventional spectral CT analysis, as an auxiliary means, cannot comprehensively represent whole lesions, but has been applied to distinguish between pulmonary ADC and SQCC.
• This was the first study to explore the value of the spectral CT volumetric quantitative analysis in differentiating between 2 pathological types of pulmonary ADC and SQCC.
What is the implication, and what should change now?
• The volumetric quantitative analysis of spectral CT may be invaluable in the differential diagnosis of pulmonary ADC and SQCC.
Introduction
According to the annual report results of the cancer registry released by the Chinese National Cancer Center, primary lung cancer is one of the most common malignant tumors in China (1). Additionally, its incidence and mortality have increased significantly all over the world in the past 50 years, and it has become a serious threat to human life and health. In the last decade, the application of molecule-targeted drugs in the treatment of non-small cell lung cancer (2,3), especially adenocarcinoma (ADC), has become widespread. An individualized approach, including immunotherapy and targeted therapy, is the standard treatment strategy for patients with lung adenocarcinoma and lung squamous cell carcinoma (SQCC). Specific therapies can be offered to patients based on the histological and molecular status of primary tumors. Therefore, an accurate differentiation between lung ADC and SQCC has become increasingly indispensable (4,5).
In clinical practice, the gold standard for the qualitative diagnosis of such patients is histopathology. However, histopathological results may not be available for certain patients who cannot tolerate invasive methods. Non-invasive imaging strategies can provide an effective auxiliary diagnosis for such patients.
Following the rapid development of medical imaging technology, computed tomography (CT), magnetic resonance imaging, and positron emission tomography-CT have come to play an increasingly important role in disease diagnosis (6). A qualitative diagnosis still largely depends on a pathological biopsy; however, CT imaging is now the preferred non-invasive method for the preoperative diagnosis and staging of lung cancer. We sought to examine whether the newly established spectral CT imaging could be used to identify the potential histopathological types of lung cancer lesions.
Recently, tissue can be quantitatively and qualitatively analyzed by spectral CT imaging, which opens up a new field for CT imaging. At present, throughout the country and abroad, spectral CT imaging was trending toward the identification of parenchymal organ tumors. It can quantitatively measure iodine concentration and water concentration by material decomposition images to reflecting tumor blood flow. So spectral CT multi-parameter and quantitative imaging technology is expected to further improve the accuracy of the preoperative pathological classification of lung cancer (7). Under the conventional spectral CT observation method for lesion parameters, the region of interest (ROI) is displayed in an axial image to obtain the relevant data. However, this method cannot fully represent the whole lesion. This was the first study to investigate the value of the spectral CT volume quantitative analysis in differentiating between the 2 pathological types of ADC and SQCC and to compare it with the conventional spectral analysis to determine the sufficient imaging reference for the preoperative evaluation of lung cancer pathological types. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-115/rc).
Methods
Clinical data
The data of 57 patients with lung cancer who underwent chest plain and enhanced-CT scans with gemstone spectral imaging (GSI) at The Third Affiliated Hospital of Chongqing Medical University (Gener Hospital) from July 2018 to July 2019 were collected, and their spectral CT imaging data and clinical data were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of the Third Affiliated Hospital of Chongqing Medical University (Gener Hospital) (No. 2019-26-01). Individual consent for this retrospective analysis was waived. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have undergone no therapeutic intervention before the spectral CT examination; (II) have no allergy to iodine and no contraindications, such as hyperthyroidism; (III) have the ability to cooperate with the CT examination, and have scans with an image quality that met the research needs; and (IV) have pathological surgery or biopsy results.
Among the 57 patients, 35 had ADC and 22 had SQCC. The 35 ADC patients comprised 16 males and 19 females with a mean age of 63.7±7.8 years (range, 49 to 77 years), and a maximum lesion diameter of 3.3±1.9 cm. The 22 SQCC patients comprised19 males and 3 females with a mean age of 63.6±7.4 years (range, 49 to 75 years), and a maximum lesion diameter of 3.9±1.7 cm.
Method of inspection
Multi-slice spiral CT (Revolution CT, GE Healthcare, Milwaukee, Wisconsin, USA) was performed of each patient in the supine position. Chest imaging from the lung tip to the costophrenic angle, plain, and enhanced-CT scanning were performed with GSI. The scanning parameters were as follows: Tube voltage: 80–140 KV; automatic switching; automatic milliampere second; detector width: 80 mm; pitch: 0.992:1; layer thickness and layer spacing: 5 mm, adaptive statistical iterative reconstruction iterative reconstruction: 50%; and thickness of reconstruction layer: 1.25 mm. The contrast agent was Eurapic (350 mgI/mL), and an Ulrich high-pressure syringe was used for the injection, which was made through the median cubital vein. The dosage was 1.0 mL/kg of Eurapic + 30 mL of normal saline, and the injection rate was 3.5 mL/s. The delay time of the arterial phase (AP) scan was 30 s.
Image analysis and data measurement
The spectral data were imported into the GE AW4.7 post-processing workstation, and 2 experienced physicians used GSI special post-processing software to independently conduct the image post-processing and measurements. In case of disagreement, the average value was determined after discussion, and a consensus was reached.
Under the quantitative analysis method, volume rendering (VR) software was first used to avoid the obvious blood vessels and necrotic foci, delineate the lesion contour layer by layer on the original lung window (window width: 1,600 HU, window level: −600 HU), and reconstruct the whole VR image of the lesion to determine the volume of the lesion. Based on the VR images, the plain scan (PS), calcium concentration, effective-Z (Eff-Z), AP iodine concentration, and water concentration were each respectively measured in the whole lesion.
The conventional spectral quantitative analysis was used to select the solid component of the largest 3 adjacent layers of the lesion in the axial image and delineates the same ROI, determine its average value, avoid the obvious blood vessels and necrotic areas, and ensure that the size and location of the ROI placement in the PS was consistent with that in the AP. The PS calcium concentration, Eff-Z, AP iodine concentration, and water concentration were each respectively measured and recorded.
Statistical analysis
SPSS 21.0 social science statistical software was used for the statistical analysis. The normally distributed measurement data are presented as the . The non-normally distributed measurement data are presented as the median [interquartile range (IQR)]. The between group comparisons were performed using the t-test or rank-sum test. A P value <0.05 was considered statistically significant. A receiver operating characteristic (ROC) curve analysis was used to calculate the areas under the curves (AUC), and the diagnostic accuracy of the volumetric spectral analysis was compared to that of the conventional spectral analysis, the DeLong test was used to compare the differences in AUC. An AUC of 0.5–0.7 indicated a low diagnostic accuracy; an AUC of 0.7–0.9 indicated a medium diagnostic accuracy. The Youden index was used to determine the optimal diagnostic threshold, and the sensitivity and specificity of the volumetric spectral analysis and the conventional spectral analysis were calculated respectively.
Results
Spectral CT analysis of the 2 types of lung cancer
The volumetric spectral analysis results showed that the PS calcium concentration, Eff-Z, and AP iodine concentration of ADC were higher than those of SQCC, and the AP water concentration was lower than that of SQCC, and the comparisons of the various parameters between the 2 were statistically significant (P<0.05) (Table 1 and Figures 1,2). Similar to the results for the volumetric spectral analysis, the conventional spectral analysis results showed that the PS calcium concentration, Eff-Z, and AP iodine concentration of ADC were all higher than those of SQCC, while the water concentration of ADC was lower than that of SQCC. The conventional spectral analysis the 2 types of lung cancer, only the AP water concentration was no statistically significant (P>0.05) (Table 1 and Figures 1,2).
Table 1
| Pathological type | PS | AP | |||
|---|---|---|---|---|---|
| Concentrations of calcium (mg/cm3) | Eff-Z | Concentrations of iodine (mg/cm3) | Concentration of water (mg/cm3) | ||
| Volumetric spectral analysis | |||||
| ADC (n=35) | 6.97±2.83 | 7.90±0.14 | 1.42 (0.84) | 995.00 (38.70) | |
| SQCC (n=22) | 5.14±2.39 | 7.80±0.10 | 1.16 (0.65) | 1,007.00 (14.38) | |
| t or Z | 2.513* | 2.860* | –2.246** | –2.082** | |
| P | 0.015 | 0.006 | 0.025 | 0.037 | |
| Conventional spectral analysis | |||||
| ADC (n=35) | 8.51±4.28 | 7.97±0.20 | 1.33 (0.80) | 1,026.15 (14.00) | |
| SQCC (n=22) | 5.96±2.50 | 7.86±0.13 | 0.94 (0.63) | 1,029.28 (10.49) | |
| t or Z | 2.534* | 2.475* | –2.524** | –1.885** | |
| P | 0.014 | 0.016 | 0.012 | 0.059 | |
*, Student t-test; **, rank-sum test. CT, computed tomography; M, median; IQR, interquartile range; PS, plain scan; AP, arterial phase; Eff-Z, effective-Z; ADC, adenocarcinoma; SQCC, squamous cell carcinoma.
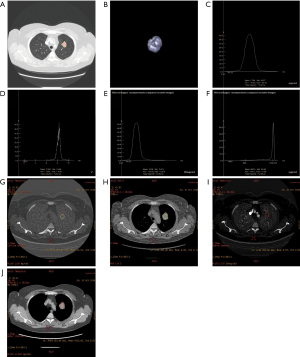
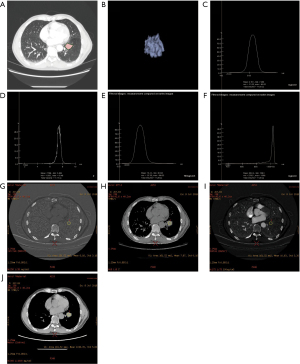
Observation results of lung cancer using the 2 spectral CT analysis methods
In both ADC and SQCC, the PS calcium concentration, Eff-Z, and AP water concentration of the volumetric spectral analysis were significantly lower than those of the conventional spectral analysis, and the comparisons revealed that the differences were statistically significant (P<0.05). Additionally, in both ADC and SQCC, the concentration of iodine in the AP of the volumetric spectral analysis was significantly higher than that in the conventional spectral analysis, and the difference in the concentration of iodine in the AP of SQCC was statistically significant (P<0.05) (Table 2 and Figures 1,2).
Table 2
| Spectral parameters | PS | AP | |||
|---|---|---|---|---|---|
| Concentrations of calcium (mg/cm3) | Eff-Z | Concentrations of iodine (mg/cm3) | Concentration of water (mg/cm3) | ||
| ADC (n=35) | |||||
| Volumetric spectral analysis | 6.97±2.83 | 7.90±0.14 | 1.42 (0.84) | 995.00 (38.70) | |
| Conventional spectral analysis | 8.51±4.28 | 7.97±0.20 | 1.33 (0.80) | 1,026.15 (14.00) | |
| t or Z | –4.357* | –4.340* | –1.900** | –5.159** | |
| P | 0.000 | 0.000 | 0.057 | 0.000 | |
| SQCC (n=22) | |||||
| Volumetric spectral analysis | 5.14±2.39 | 7.80±0.10 | 1.16 (0.65) | 1,007.00 (14.38) | |
| Conventional spectral analysis | 5.96±2.50 | 7.86±0.13 | 0.94 (0.63) | 1,029.28 (10.49) | |
| t or Z | –2.328* | –3.796* | –3.036** | –4.107** | |
| P | 0.030 | 0.001 | 0.002 | 0.000 | |
*, Student t-test; **, rank-sum test. CT, computed tomography; M, median; IQR, interquartile range; PS, plain scan; AP, arterial phase; Eff-Z, effective-Z; ADC, adenocarcinoma; SQCC, squamous cell carcinoma.
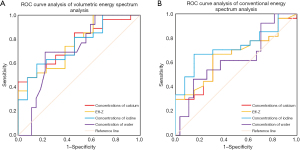
Differential diagnosis efficiency of the 2 spectral CT analysis methods for ADC and SQCC
The optimal thresholds of calcium concentration, Eff-Z, iodine concentration, and water concentration in the differential diagnosis of ADC and SQCC were 6.54 mg/cm3 and 6.00 mg/cm3, 7.92 and 7.86, 1.24 mg/cm3 and 1.08 mg/cm3, 1,006.50 mg/cm3 and 1,021.41 mg/cm3, respectively. The sensitivity, specificity, and AUC of all the parameters of the volumetric spectral analysis were greater than those of the conventional quantitative parameters (Table 3, Figure 3). The conventional spectral analyses and the volumetric quantitative analysis of spectral CT for ADC and SQCC, the AUC of the PS calcium concentration, Eff-Z were statistically significant (Z=2.770, 2.880, P=0.006, 0.004), but the AP iodine and water concentration were no statistically significant (Z=1.041, 0.860, P=0.298, 0.389).
Table 3
| Spectral parameters | Concentrations of calcium (mg/cm3) | Eff-Z | Concentrations of iodine (mg/cm3) | Concentration of water (mg/cm3) |
|---|---|---|---|---|
| Optimal threshold | ||||
| Volumetric spectral analysis | 6.54 | 7.92 | 1.24 | 1,006.50 |
| Conventional spectral analysis | 6.00 | 7.86 | 1.08 | 1,021.41 |
| AUC | ||||
| Volumetric spectral analysis | 0.76 | 0.76 | 0.75 | 0.71 |
| Conventional spectral analysis | 0.65 | 0.66 | 0.73 | 0.63 |
| Sensitivity | ||||
| Volumetric spectral analysis | 66.7% | 66.7% | 66.7% | 85.2% |
| Conventional spectral analysis | 44.4% | 48.1% | 59.3% | 66.7% |
| Specificity | ||||
| Volumetric spectral analysis | 92.3% | 84.6% | 86.9% | 69.2% |
| Conventional spectral analysis | 69.2% | 69.2% | 84.6% | 53.8% |
ROC, receiver operating characteristic; ADC, adenocarcinoma; SQCC, squamous cell carcinoma; CT, computed tomography; Eff-Z, effective-Z; AUC, the area under the curve.
Discussion
In this study, spectral CT volumetric quantitative analysis parameters were used for the differential diagnosis of ADC and SQCC for the first time, and the results showed that the differential diagnosis value of the spectral volumetric analysis parameters was better than that of the conventional spectral analysis parameters. The results of the volumetric spectral analysis showed that the concentrations of calcium, Eff-Z, and iodine were all higher in ADC than SQCC, while the concentration of water was lower in ADC than SQCC, which is consistent with the results of Li et al.’s (8) single conventional spectral analysis. However, the differences between the volumetric spectral analysis parameters and the conventional spectral analysis parameters in this study were statistically significant. Moreover, the sensitivity, specificity and AUC of the volumetric spectral analysis parameters for the differential diagnosis of ADC and SQCC were better than those of the conventional spectral analysis parameters.
As previous studies have reported, the calcium concentration of ADC is higher than that of SQCC (8,9). Thus, we are of the view that ADC is more prone to calcification than SQCC, and the main mechanisms by which this occurs are as follows: (I) original calcification foci in the lung; (II) calcification of the scar in the cancer foci; (III) dystrophic calcification in the necrotic area of the cancer foci; (IV) calcification as a result of related cytokines secreted by tumor cells; and (V) transformation of tumor mesenchymal cells into osteoblasts.
The Eff-Z of ADC is larger than that of SQCC. Pathologically, ADC is the result of adenoid differentiation and can secrete mucus, while SQCC is mainly a keratosis and an intercellular bridge, and the chemical composition and density of the two differ, which cause the Eff-Z difference between the two (10).
In relation to iodine concentration, it may be that ADC is higher than SQCC because the cancer tissue of ADC is loose and has more interstitial components and abundant small blood vessels, while the cancer tissue of SQCC is dense, and has fewer interstitial components and blood vessels (11,12). Thus, the iodine content in ADC tissue during the arterial enhancement scan will be higher than that in SQCC tissue.
The water concentration of ADC is lower than that of SQCC, which may be related to its growth mode. As ADC has more interstitial components and fewer cellular components than SQCC, its overall water content of intracellular and extracellular matrix is less. However, SQCC has a pileup growth style and a high cell density per unit volume, resulting in a higher overall water content of cancer tissues (8).
Our results showed that the differential diagnosis of ADC and SQCC by volumetric spectral analysis was superior to that by conventional spectral analysis, which may be due to the difference in the observation range for the lesions between the 2 methods. Conventional spectral analysis selects the maximum cross-section of the solid components of the lesion from the axial image, delineates areas of interest on the 3 adjacent layers, avoids blood vessels and necrosis, and takes the average value. However, the observation of a lesion based on a 2-dimensional sectional image cannot represent the whole tumor, and the measured results cannot objectively reflect the biological characteristics of the tumor (13).
This study also showed that the lesions in the upper part of the mediastinal window were smaller than those in the upper part of the pulmonary window, which may be because when the lesions invade the surrounding area, the tumor cells grow along the alveolar wall in a scaly manner (14), and the tumor tissues at the lesion margin are sparsely distributed with a ground-glass density, which is not enough to be displayed on the mediastinal window.
According to the 8th tumor, node, metastasis (TNM) staging system of lung cancer, previous studies have shown that the solid part of lung cancer has the greatest relationship with tumor invasion, and the presence of ground glass in the lesion indicates a good prognosis for lung cancer patients (15,16). In the observation of lesions in the conventional spectral analysis, the selection of the sensitive area of interest completely missed the tumor margin, resulting in a bias in the evaluation results. The observation method of energy volume spectrometry includes the complete outline of the whole lesion (including the edge part) on the lung window, and only avoids the visible blood vessels and necrotic components. Thus, the observation results may more truly reflect the biological characteristics of the tumor.
This study showed that the concentrations of calcium, Eff-Z, and water in the volumetric spectral analysis of ADC and SQCC were all lower than those in the conventional spectral analysis. This may be because the observation area of the volumetric spectral analysis was the whole tumor and covered the incomplete solid part of the lesion margin, while the ROI selected by the conventional spectral analysis only comprised the solid region of the lesion. This is probably the main reason for the apparent difference between the 2 methods of analysis. The concentration of iodine measured by the volumetric spectral analysis was higher than that measured by the conventional spectral analysis, which may also be related to the difference between the 2 spectral analysis methods in selecting the observation range of the lesions.
In our study, we found that the enhancement degree of the lesion edge during the AP of the enhanced scanning was often higher than its intrinsic part, indicating that the iodine concentration at the lesion edge was higher than its intrinsic component. The observation area of the volumetric spectral analysis included the entire lesion edge, while the conventional spectral analysis only included the intrinsic part of the lesion. As a result, the iodine concentration measured by the volumetric spectral analysis was higher than that of the conventional spectral analysis. This phenomenon has also been confirmed by previous research results. Liu et al. (17) found that the iodine concentration in the AP of a pulmonary ground glass nodule was 23.8±8.3 mg/cm3, while Fehrenbach et al. (18) found that the concentration of iodine in the AP of ADC and SQCC was only 2.01±1.05 and 1.29±0.69 mg/cm3, respectively.
From the perspective of differential diagnosis efficiency, the sensitivity, specificity and AUC of the volumetric spectral analysis parameters were higher than those of the conventional spectral analysis parameters, especially the AUC of the PS calcium concentration, Eff-Z were statistically significant, which shows that the volumetric spectral analysis has high clinical value in the differential diagnosis of ADC and SQCC.
This study conducted a preliminary study on the application of volumetric spectral analysis in lung cancer and obtained meaningful research results; however, it also had some limitations. First, the sample size of the study was small, which may have resulted in a statistical bias and may have affected the research results, and the sensitivity and specificity obtained were not sufficiently high. Thus, it is necessary to further expand the sample size to obtain more reliable research results. Second, the pathological types of lung cancer collected in this study only comprised ADC and SQCC; thus, it is necessary to expand the various pathological types of lung cancer examined in future studies to further verify the advantages and disadvantages of volumetric spectral analysis.
In summary, this study preliminarily discussed the value of quantitative spectral CT volumetric analysis in the differential diagnosis of ADC and SQCC and found that the spectral volumetric analysis was superior to the conventional spectral analysis. Future studies should expand the sample size and examine more pathological types, and even apply it to clinical studies of tumors and diseases in other organs.
Conclusions
In conclusion, the volumetric quantitative analysis of spectral CT imaging is a good non-invasive technology that can be used in the differential diagnosis of ADC and SQCC in clinical practice. Compared to the conventional spectral analysis parameter-based diagnosis, the volumetric spectral analysis parameter-based diagnosis had a better sensitivity and specificity for the differential diagnosis of ADC and SQCC. Notably, there were significant differences in the concentrations of calcium, water, Eff-Z, and iodine between ADC and SQCC. Given its advantages in the observation range of whole lesions, the volumetric spectral analysis may be invaluable in the diagnosis of different pathological types of cancers (i.e., ADC and SQCC), and is worthy of clinical recommendation. However, further in-depth studies with larger sample sizes need to be conducted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-115/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-115/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-115/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-115/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of The Third Affiliated Hospital of Chongqing Medical University (Gener Hospital) (No. 2019-26-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center 2022;2:1-9.
- Marks S, Naidoo J. Antibody drug conjugates in non-small cell lung cancer: An emerging therapeutic approach. Lung Cancer 2022;163:59-68. [Crossref] [PubMed]
- Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:586-97. [Crossref] [PubMed]
- Qi Y, Yang L, Liu B, et al. Highly accurate diagnosis of lung adenocarcinoma and squamous cell carcinoma tissues by deep learning. Spectrochim Acta A Mol Biomol Spectrosc 2022;265:120400. [Crossref] [PubMed]
- Chen JW, Dhahbi J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci Rep 2021;11:13323. [Crossref] [PubMed]
- Mikla VI, Mikla VV. Medical imaging technology. Elsevier. 2014, 23-38.
- Liang Y, Li Q, Luo T. Quantitative analysis of spectral CT in differential diagnosis of peripheral lung cancer and tuberculosis in unenhanced phase. Chinese Journal of Medical Imaging Technology 2017;33:1206-10.
- Li Q, Luo T, Lv F, et al. The value of quantitative analysis with spectral CT imaging in the diagnosis of non-small cell lung cancer with different pathological types. Chinese Journal of Radiology 2017;51:257-61.
- Johnson TR, Krauss B, Sedlmair M, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol 2007;17:1510-7. [Crossref] [PubMed]
- Wang X, Shi H, Yang L, et al. Value of Spectral CT Imaging in Differentiating Pulmonary Squamous Cell Carcinoma and Adenocarcinoma. Journal of Ningxia Medical University 2013;35:415-8.
- Cohen JG, Reymond E, Jankowski A, et al. Lung adenocarcinomas: correlation of computed tomography and pathology findings. Diagn Interv Imaging 2016;97:955-63. [Crossref] [PubMed]
- Yu Y, Zhu H, Hu S, et al. The value of spectral CT imaging in the differential diagnosis of adenocarcinoma, squamous carcinoma and inflammatory myofibroblastic tumor. Chinese J Radiology 2017;51:756-60.
- Revel MP, Bissery A, Bienvenu M, et al. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable. Radiology 2004;231:453-8. [Crossref] [PubMed]
- Lu T, Chen Y, Liu X. Comparative analysis of shape features and histopathology in pulmonary ground-glass nodules. Journal of Medical Imaging 2017;27:2098-2101+2195.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Aokage K, Miyoshi T, Ishii G, et al. Clinical and Pathological Staging Validation in the Eighth Edition of the TNM Classification for Lung Cancer: Correlation between Solid Size on Thin-Section Computed Tomography and Invasive Size in Pathological Findings in the New T Classification. J Thorac Oncol 2017;12:1403-12.
- Liu G, Li M, Li G, et al. Assessing the Blood Supply Status of the Focal Ground-Glass Opacity in Lungs Using Spectral Computed Tomography. Korean J Radiol 2018;19:130-8. [Crossref] [PubMed]
- Fehrenbach U, Kahn J, Böning G, et al. Spectral CT and its specific values in the staging of patients with non-small cell lung cancer: technical possibilities and clinical impact. Clin Radiol 2019;74:456-66. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


